-
Medical journals
- Career
CURRENT OPTIONS IN PHARMACOLOGICAL INTERVENTIONS FOR MICROVASCULAR ANASTOMOSIS PATENCY: REVIEW
Authors: Š. Pohanka; J. Šimek
Authors‘ workplace: Tomas Bata Regional Hospital Zlín, Department of Oral and Maxillofacial Surgery, Zlín, Czech Republic
Published in: ACTA CHIRURGIAE PLASTICAE, 62, 1-2, 2020, pp. 40-44
INTRODUCTION
The scope of reconstructive techniques using microsurgical anastomosis continues to expand. The surgical approach and materials were mostly standardized. Nevertheless, the patency of microvascular anastomosis and prevention of thrombosis of peripheral vessels of the flap remain the key points of a successful reconstruction. Although most specialists agree that the surgeon’s experience, quality operative techniques and well-chosen and prepared patient lead to major reduction of ischemic complications in the first place, and some authors consider this to be the only relevant factor, there are still various drugs applied to prevent clot formation in the anastomosis site and inside the flap.
Despite the long history of microvascular reconstructive procedures, no consensus on a single perioperative pharmacological approach exists between the authors. The literature offers mostly retrospective studies, presentation of individual experiences or laboratory experiments. Strong factors influencing potential research are interindividual differences in vessel quality and individual coagulation status among patients. Most protocols are based on the creation of iatrogenic hypocoagulation or hypoaggregation condition in perioperative care for prevention of thrombotic complications, yet the used doses of drugs differ. Minority of protocols use spasmolytics, promote faster epithelization and minimize expression of pro-inflammatory and pro-coagulative factors in the anastomose site.
PHARMACOLOGICALLY INFLUENCEABLE CAUSES OF FLAP ISCHEMIA
Beginning with general conditions, flap could be ischemic due to the alteration of blood circulation, which can be caused by low cardiac output, hypotension, hypothermia or low haematocrit, high blood viscosity or excessive blood loss. Locally may occur peripheral vasoconstriction or in 5–10% cases spasm of flap nutrition vessel1. Low blood flow with damaged endothelium and hypercoagulopathy lead potentially to thrombus formation2.
Blood clot in anastomosis site is the most frequent local event resulting in flap failure3. That is the reason why increased effort is necessary during perioperative period to prevent this condition and also microthrombotisation of peripheral flap vessels. Venous thrombus is more frequent than arterial. 90% of arterial thrombi are formed usually within the first 24 hours. Conversely, the venous thrombus occurs mostly between the first 24–48 hours after surgery4. Other unfavourable local conditions as vessel kinking, strangulation or compression by haematoma should be eliminated by proper surgical technique.
PERIOPERATIVE FLUID AND PHARMACOLOGICAL MANAGEMENT
Former recommendations aimed at prevention of the flap vessels spasm by administering vasodilators and usage of peripheral vasoconstrictors for mean arterial pressure (MAP) stabilisation was considered risky5. MAP around 100mmHg is now recommended and considered appropriate for sufficient perfusion of the flap. Recent studies have not demonstrated that administration or omission of peripheral vasoconstrictors e.g. phenylephrine, ephedrine, dopamine, dobutamine, noradrenalin in perioperative period is important for flap survival6. Some studies differentiate between drugs influencing blood pressure and circulation; for example Suominen prefers usage of Dobutamine that increases stroke volume and decreases systemic vascular resistance more than dopamine, which only increases stroke volume7. German society for microsurgery of peripheral nerves and vessels recommends to use norepinephrine rather than dobutamine8. Mokatef reported better flap perfusion with norepinephrine or dobutamine9. (Figure 1.)
1. The surgeon´s experience and quality operative technique are major factors of success (archive of the author) 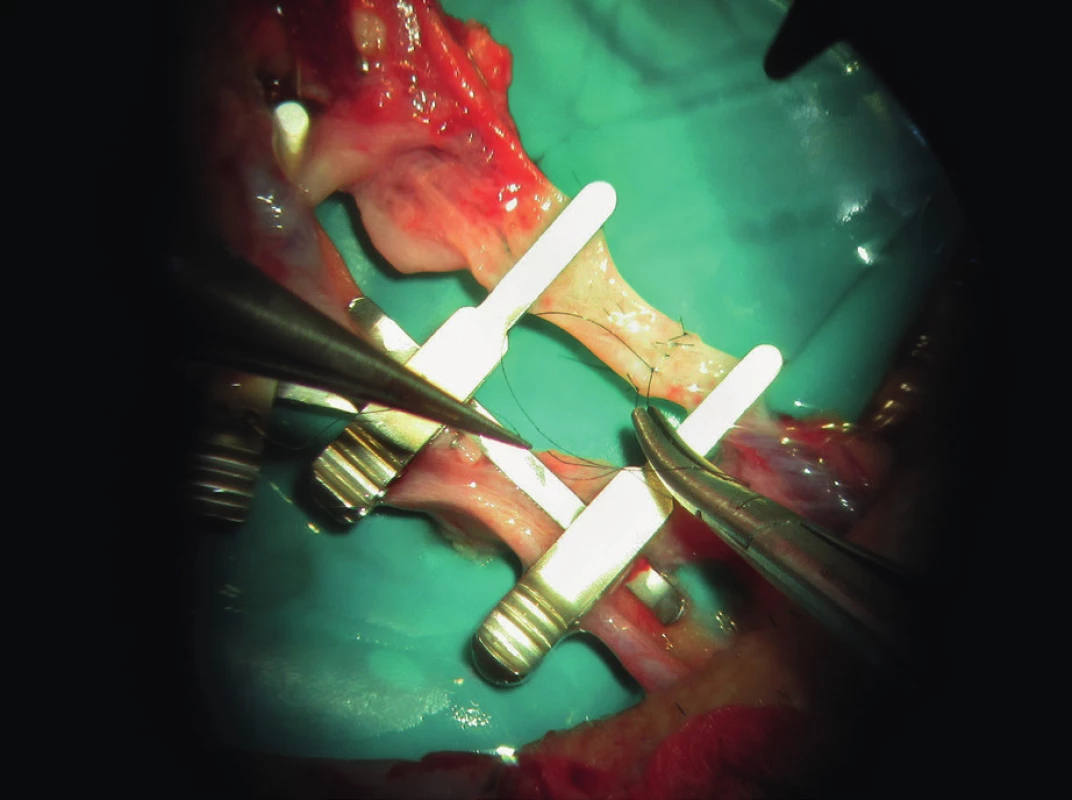
It is recommended to maintain normal partial pressure of O2 a CO2 during general anaesthesia (GA). Hyperoxemia and hypokapnia as well as hypothermia and insufficient postoperative pain control can lead to peripheral vasoconstriction5. Drugs used in GA often lead to systemic hypotension. Sevoflurane is the most commonly used volatile anaesthetic agent. It has documented protection for endothelium against ischemic damage, it promotes healing of vessels and in comparison with Propofol it decreases capillary filtration coeficient9. As prevention of hypotension during GA, it is recommended to reduce doses of anaesthetics and to increase circulating fluid volume. It is necessary to balance pros and cons. The benefit is elevation of MAP, but it may cause oedema of tissues within the flap, worsening of microcirculation, haemodilutive coagulopathy or heart failure10.
Ševčíková found that 20–30% haemodilution by crystalloids can lead to hypercoagulative condition, caused by dilution of coagulation inhibitors and by lowering threshold for positive feedback, which is a component of coagulation cascade11. Administration of more that 130 ml/kg crystalloids per day or more that 7 litres during the operation correlates significantly with perioperative complications including thrombosis and flap loss. The recommended dose of crystalloids in the first perioperative 24 hours is 3.5 to 6.0 ml/kg per hour9.
Infusion of colloids as hydroxyethyl starch or gelatine can help to maintain reasonable MAP, and also cause mild prolongation of activated partial thromboplastin time (aPTT) and prothrombin time (PT). Five percent human albumin influences coagulation less than starch solutions9.
No data support dependency of haematocrit level on the flap loss16. Also, an association between blood transfusion administration and more surgical complications and flap failure is disapproved. Complications occur more often with more administered transfusions, but increased frequency of complications depends more on polymorbidity of the patient.
Although corticosteroids promote pro-coagulative state and release of platelets, their administration is supported as an anti-swelling agent and for prevention of postoperative nausea and vomiting8. Surgical complications were observed more frequently with longer duration of GA12.
Pentoxifylline increases red blood cells deformability and lowers blood viscosity. Vasodilation effect was observed too1. Statins prevent endothelial dysfunction, release of nitrogen monoxide (NO) from endothelium, and reduce inflammatory response and by that act anti-thrombotically. Some studies on animals also support vasodilatation1,13.
SYSTEMIC ANTICOAGULATION AND ANTIAGGREGATING
The most common way how to minimize the risk of thrombus formation is to create hypocoagulation or hypoaggregation of blood. The most commonly used drugs are heparin, low molecular weight heparins (LMWH) and acetylsalicylic acid14.
Heparin is an anticoagulant and it prevents both arterial and venous thrombosis acting on various systems: it inactivates or reduces activation of coagulation factors15, lowers the recruitment of platelets and fibrin deposition and in higher doses increases vasodilation probably by releasing NO from endothelium3. Effect of Heparin is measurable by activated Partial Thromboplastin Time (aPTT), the normal range of which is 22–35 s2. Measurement with thromboelastography (TEG) is more dynamic, but aPTT is considered the standard16. As heparin cannot dissolve an existing clot, it should be administered systemically preoperatively in a bolus, or before the division of flap vessels in the harvest site. Some protocols recommend administration before clamp release after suturing the anastomosis17. However, flap is not presaturated with heparin in these cases. Heparin medication is often prolonged after surgery.
Heparin can also be used locally, when anastomosing vessels are irrigated by heparin solution, or flap is totally flushed with heparin solution. Pressure of irrigation solution should not be more than 100mmHg, because of the risk of intimal damage4.
The use of heparin can lead to complications. The most common is haematoma formation and bleeding, less commonly heparin induced thrombocytopenia may occur18.
Low molecular weight heparin (LMWH) is a derivate of heparin, with more specific effect on factor X. Easier administration is the major benefit as well as higher biological availability and lower risk of thrombocytopenia17. However, monitoring of the activity of LMWH with anti-Xa is not as precise as in heparin with aPTT and the effect on arterial thrombosis prevention is doubtfull3.
Acetylsalicylic acid (ASA) irreversibly inhibits cyclooxygenase in platelets. By this, the transformation of arachidonic acid to thromboxane and prostacyclin is blocked13. Thromboxane is a vasoconstrictor and platelet aggregator; the effect of prostacyclin is the opposite. ASA also interferes with thrombin formation13. ASA is commonly used in vascular surgery. Dosage and time of administration is important. The vessel patency is better if ASA is administrated 10 hours before a surgery. The dose of 5mg/kg sufficiently blocks synthesis of thromboxane and maintains synthesis of prostaglandins4,17. ASA is usually used in combination with heparin or LMWH. Most common complication is higher risk of perioperative bleeding and haematoma formation, renal dysfunction and bleeding from the gastrointestinal tract19. Because of relevant interindividual pharmacokinetic differences and inhibition of only external path of coagulation cascade, warfarin is not used in free flap surgery. Also, usage of ticlopidine and clopidogrel is only experimental in free flap surgery20.
Dextrans as plasmaexpanders lower blood viscosity and improve rheology of blood and act antithrombotically by increasing electronegativity of red blood cells, platelets and endothelium13. Anaphylactic reaction, acute respiratory distress syndrome, risk of heart failure and kidney damage can occur as a negative complication3,4. In prospective randomized studies dextran does not have an effect on flap survival in comparison with the group without any antithrombotic medication. Systemic complications were observed in both cases9.
Zhou pointed out in 2018 in a prospective randomized double-blinded controlled clinical trial that the use of antithrombotic agents in head and neck microvascular surgery does not decrease the risk of thrombosis formation and may increase the risk of haematoma formation. He also summarized that postoperative antithrombotic agents should not be used routinely, but administration should be based on an individual risk assessment21.
We follow the procedure to measure the actual aPTT, PT, red blood cells and platelets just before the flap division and administer usually 10,000 IU bolus of heparin systemically. Since the end of surgery, we administer an infusion of 1000ml of normal saline + 10ml of 1% Mesocain + 10ml of 10% MgSO4 + 5ml of pentoxifylline (Agapurin) continually for 24 hours. Heparin is administered continually with an effort to maintain aPTT between 42–45s for the following week.
TOPICAL AND LOCAL DRUGS
The idea of the vasodilator administration locally onto or into the sutured vessel as a prevention of spasm and subsequent prevention of systemic effect have led to local administration of drugs. A survey of plastic surgeons in the United Kingdom revealed that although 94 percent routinely used vasodilators intraoperatively, 99 percent of surgeons used them topically, with 19 percent additionally irrigating the vessel lumen1. There are two major ways to smooth muscle relaxation: increase of NO synthesis and blockage of Ca2+ channels. Well documented effect and simple application has lidocaine as a local anaesthetic. Increased release of NO, blockage of Na2+ channels and sympathetic innervation of vessel produces vasodilatation1,22. Lidocaine is administered topically and intraluminary onto the sutured vessel in 4% concentration4,9,23. Vasospasm was observed in the concentration of 1% or less. Although the total dose administered this way exceeded DMS, no signs of intoxication were observed24. Hyža proved vasodilatation effect of 1% trimecaine and 10% MgSO425.
Other drugs for local administration such as pentoxifylline, calcium channel blockers, papaverine, sodium nitroprusside, amrinone, phenolamin, chlorpromazine, botulotoxin, vascular endothelial growth factor (VEGF) and prostaglandins are documented mostly in vitro and on animal models1,15, 26, 27, 28, 29. Use of a blunt endodontic cannula with side hole has showed in our practice as a smart solution for internal irrigation of sutured vessels. We irrigate vessels topically and intraluminary during suturing using a solution consisting of mesocain, Agapurin, MgSO4 and heparin. (Figure 2, 3.)
2. Blunt double side vented irrigation tip cannula for root cannal irrigation during endodontic treatment can be well used for intraluminary application of solutions (Endo/Tech™, Halifax, Canada) Double Side Vented Irrigation Tips [internet] Endo/Tech™, Halifax, Canada [cited 2020 May1] Available from: https://endo-tech-com.3dcartstores.com/Double-Side-Vented-Irrigation-Tips_p_205.html ![Blunt double side vented irrigation tip cannula for root cannal irrigation during endodontic treatment can be well used for intraluminary application of solutions (Endo/Tech™, Halifax, Canada) Double Side Vented Irrigation Tips [internet] Endo/Tech™, Halifax, Canada [cited 2020 May1] Available from: https://endo-tech-com.3dcartstores.com/Double-Side-Vented-Irrigation-Tips_p_205.html](https://www.prolekare.cz/media/cache/resolve/media_object_image_small/media/image_pdf/7fae2304e57d24cd1beb322d185922ac.jpeg)
3. Solution for intraluminary irrigation during microvascular suture used at our department. Solution is consisting of: 10ml of Mesocain 1%, 10ml MgSO4 10%, 5ml of Agapurin (Pentoxifyllinum 100 mg). Then 19ml of this solution is mixed with 1ml of Heparin 5000 IU/ml (archive of the author) 
MONITORING
The following techniques should be mentioned as standard methods of flap assessment: visual inspection of skin or mucous island colour, flap temperature, invasive blood pressure (IBP) with MAP, Doppler ultrasound for flap vessel blood flow detection, pin-prick test in soft tissue flaps with fresh bleeding up to 5 seconds. Promising seems to be near infrared spectroscopy (NIRS) for continual monitoring of flap perfusion. In case of patients with artificially altered coagulation or aggregation, it is possible to use thromboelastography/metry (TEG/ROTEM) besides the standard measurements of aPTT and antiXa. (Figure 4, 5.)
4. Near infrared spectroscopy (NIRS) sensor for children is suitable for skin flap measurement because of its size (archive of the author) 
5. Trend is more important than absolute value. Recommended ranges for different flaps are also investigated (archive of the author) 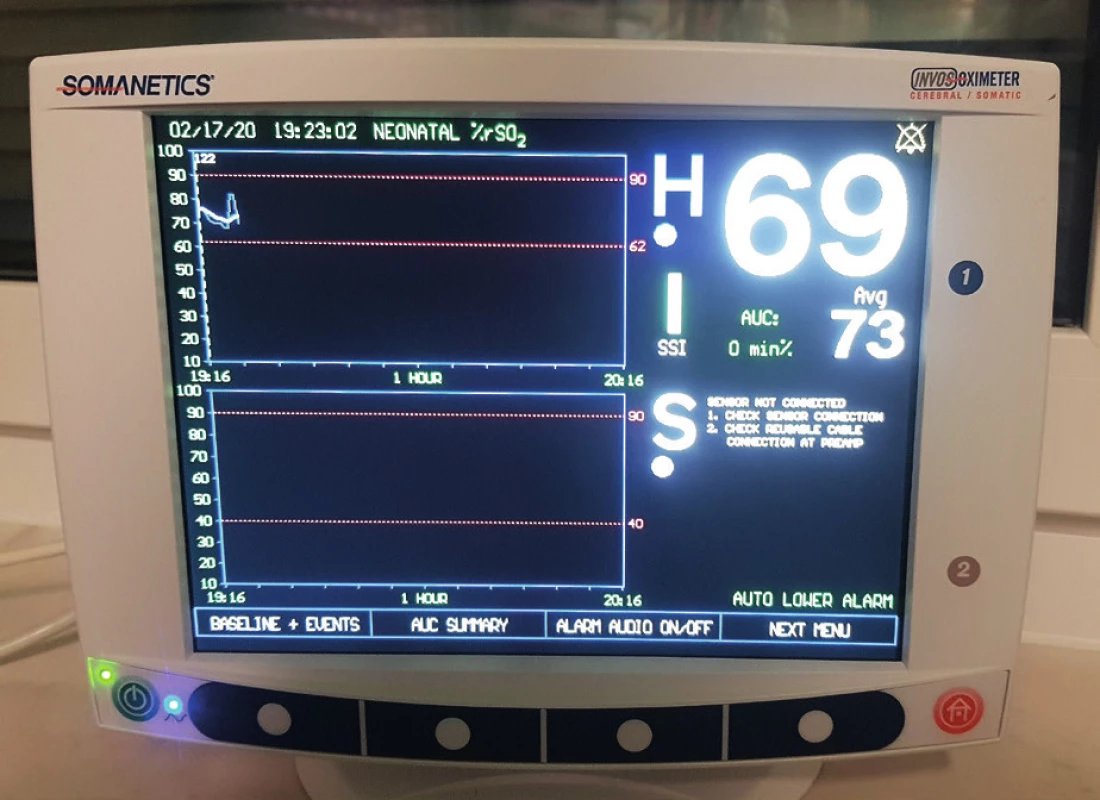
SALAVGE PROCEDURES
Beside revisional surgery, medical leeches can be applied on a venostatic flap also after successful revision of anastomosis. Venostasis can be reduced by bleeding and by hirudin released in tissues. Infection of a wound by bacteria from leeches’ digestive tract can occur as a major complication3.
Thrombolytics such as streptokinase, urokinase, t-PA are enzymes dissolving a thrombus. Their use is experimental in anastomosis revisions and in replantation surgery because of a serious risk of bleeding4.
CONCLUSION
Despite the wide range of drugs in investigation, mainly heparin, LMWH, acetylsalicylic acid and various local agents are in fact used nowadays. Other drugs have a potential for future research rather than for an actual daily administration in free flap surgery. Moreover, recent well-documented studies do not justify routine use of antithrombotic agents in head and neck free flap surgery. Good message is the dismissed concern of blood transfusions and vasoconstrictor administration and also more options for perioperative monitoring. The perioperative pharmacological management evolves from special interventions based on theoretic pathophysiology to less complex care similar to other comparable surgical procedures as a result of research. Nevertheless, surgeon should definitely stay focused on a meticulous operative technique.
List of abbreviations
MAP – mean arterial pressure
GA – general anaesthesia
aPTT – activated partial thromboplastin time
PT – prothrombin time
NO – nitrogen monoxide
TEG – thromboelastography
LMWH – low molecular weight heparin
ASA – acetylsalicylic acid
VEGF – vascular endothelial growth factor
MgSO4 – magnesium sulphate
Role of authors: Štěpán Pohanka, MDDr – research, assessment of publications and writing of manuscript, corresponding author. Jiří Šimek, MD, PhD – consultant of resources and final manuscript.
Conflict of interest: Authors declare no conflict of interest.
Disclosure: We declare that this study has received no financial support. All procedures performed in this study involving human participants were in accordance with ethical standards of the institutional and/or national research committee and with the Helsinki declaration and its later amendments or comparable ethical standards.
Corresponding author:
Štěpán Pohanka, MDDr
Department of Oral and Maxillofacial Surgery
Tomas Bata Regional Hospital
Havlíčkovo nábřeží 600
762 75 Zlín, Czech Republic
E-mail: stepan.pohanka@seznam.cz
Sources
1. Vargas ChR, Matthew LI, Bernard TL. A Systematic Review of Topical Vasodilators for the Treatment of Intraoperative Vasospasm in Reconstructive Microsurgery. Plastic and Reconstructive Surgery. 2015, 136 : 411–22.
2. Mačák J., Mačáková J., Dvořáčková J. Patologie. 2., dopl. vyd. Praha: Grada, 2012. ISBN 978-80-247-3530-6.
3. Kaciulyte J., Losco L., Maruccia M., Delia G., Lo Torto F., Di Taranto G., Caputo GG., Berchiolli R., Ribuffo D., Cigna E. Postsurgical antithrombotic therapy in microsurgery: our protocol and literature review. Eur Rev Med Pharmacol Sci. 2019, 23 : 4448–57.
4. Froemel D., Fitzsimons S-J, Frank J, Sauerbier M, Meurer A, Barker JH. A Review of Thrombosis and Antithrombotic Therapy in Microvascular Surgery. European Surgical Research. 50, 2013, p. 32-43.
5. Aps C, Cox RG., Mayou BJ. et al. The role of anaesthetic management in enhancing peripheral blood flow in patients undergoing free flap transfer. Annals of the Royal College of Surgeons of England. 1985, 67 : 177–9.
6. Goh CSL., Ng MJM., Song DH., Ooi ASH. Perioperative Vasopressor Use in Free Flap Surgery: A Systematic Review and Meta-Analysis. J Reconstr Microsurg. 2019; 35(7): 529–40.
7. Suominen S., Svartling N., Silvasti M., Niemi T., Kuokkanen H., Asko-Seljavaara S. The Effect of Intravenous Dopamine and Dobutamine on Blood Circulation during a Microvascular TRAM Flap Operation. Annals of Plastic Surgery. 2004, 53 : 425–31.
8. Kremer T., Bauer M., Zahn P. et al. Perioperatives Management in der Mikrochirurgie – Konsensus-Statement der Deutschsprachigen Arbeitsgemeinschaft für Mikrochirurgie der peripheren Nerven und Gefäße. Handchirurgie Mikrochirurgie Plastische Chirurgie. 2016, 48 : 205–11.
9. Motakef S., Mountziaris PM., Ismail IK., Agag RL., Patel A. Emerging Paradigms in Perioperative Management for Microsurgical Free Tissue Transfer. Plastic and Reconstructive Surgery. 2015, 135 : 290–9.
10. Dort JC., Farwell DG., Findlay M. et al. Optimal Perioperative Care in Major Head and Neck Cancer Surgery With Free Flap Reconstruction. JAMA Otolaryngology–Head & Neck Surgery. 2017, 143 : 292–303.
11. Ševčíková S., Durila M., Vymazal T. Vliv infuzních roztoků na krevní srážlivost. Anaesthesiology & Intensive Medicine/Anesteziologie a Intenzivni Medicina, 2018, 29 : 258–64.
12. Brady JS., Desai SV., Crippen MM., Eloy JA., Gubenko Y., Baredes S., Park RCh. Association of Anesthesia Duration With Complications After Microvascular Reconstruction of the Head and Neck. JAMA Facial Plastic Surgery. 2018, 20 : 188–95.
13. Pršić A., Kiwanuka E., Caterson SA., Caterson EJ. Anticoagulants and Statins as Pharmacological Agents in Free Flap Surgery: Current Rationale. Eplasty. 2015, 15:e51 : 478–93.
14. Abraham M. et al. Thromboprophylaxis in head and neck microvascular reconstruction. Craniomaxillofacial Trauma & Reconstruction, 2018, 11(2):85–95.
15. Huby M., Rem K., Moris V., Guillier D., Revol M., Cristofari S. Are prostaglandins or calcium channel blockers efficient for free flap salvage? A review of the literature. Journal of Stomatology, Oral and Maxillofacial Surgery. 2018, 119 : 297–300.
16. Zavlin D., Chegireddy V., Jubbal K., Agrawal N., Spiegel A. Management of Microsurgical Patients using Intraoperative Unfractionated Heparin and Thromboelastography. Journal of Reconstructive Microsurgery. 2019, 35 : 198–208. DOI: 10.1055/s-0038-1670683.
17. Askari M., Fisher Ch., Weniger FG., Bidic S., Lee AWP. Anticoagulation Therapy in Microsurgery: A Review. The Journal of Hand Surgery. 2006, 31 : 836–46.
18. Kroll SS., Miller MJ., Reece GP. et al. Anticoagulants and Hematomas in Free Flap. Plastic and reconstructive surgery, 1995, 96(3):643–7.
19. Enajat M., Aziz Mohammadi M., Debeij J., Van der Hulst R., Mureau M. Effect of Acetylsalicylic Acid on Microvascular Thrombosis in Autologous Breast Reconstruction. Journal of Reconstructive Microsurgery. 2014, 30 : 65–70.
20. Nayak VK., Deschler DG. Clopidogrel Use for Reducing the Rate of Thrombosis in a Rat Model of Microarterial Anastomosis. Archives of Otolaryngology–Head & Neck Surgery. 2005, 131 : 800–3.
21. Zhou W., Zhang WB., Yu Y. et al. Are antithrombotic agents necessary for head and neck microvascular surgery? International Journal of Oral and Maxillofacial Surgery. 2019, 48 : 869–74.
22. Cummins TR. Setting up for the block: the mechanism underlying lidocaine’s use-dependent inhibition of sodium channels. The Journal of Physiology [online]. 2007, 582(1), 11–11 [cit. 2019-07-15]. DOI: 10.1113/jphysiol.2007.136671.
23. Newton DJ., McLeod GA., Khan F., Belch JJF. Mechanisms influencing the vasoactive effects of lidocaine in human skin. Anaesthesia [online]. 2007, 62(2), 146–50 [cit. 2019-07-15]. DOI: 10.1111/j.1365-2044.2006.04901.x.
24. Johnestone RE., Vax MK., Bishop DJ. et al. Large doses of topical lidocaine during microvascular surgery are not associated with toxic blood concentrations. Anesthesiology: The Journal of the American Society of Anesthesiologists, 1995, 82.2 : 593–6.
25. Hýža P., Streit L., Schwarz D., Kubek T., Veselý J. Vasospasm of the Flap Pedicle. Plastic and Reconstructive Surgery. 2014, 134 : 574e–84e.
26. Segreto F., Marangi GF., Signoretti M., Cazzato V., Giorgino R., Alessandri-Bonetti M., Persichetti P. The Use of Botulinum Toxin in Flap Surgery: A Review of the Literature. Surgical Innovation. 2019, 26 : 478–84.
27. Infanger M., Shakibaei M., Kossmehl P. et al. Intraluminal Application of Vascular Endothelial Growth Factor Enhances Healing of Microvascular Anastomosis in a Rat Model. Journal of Vascular Research. 2005, 42 : 202–13.
28. Crabb DJMcK., Niall M., Kenneht R. et al. Topical use of prostacyclin in microvascular surgery. British Journal of Plastic Surgery, 1985, 38 : 383–8.
29. Leung PC., Chan MY., Roberts MB. The use of prostaglandins as local antithrombotic agents in microvascular surgery. British Journal of Plastic Surgery. 1981, 34 : 38–40.
Labels
Plastic surgery Orthopaedics Burns medicine Traumatology
Article was published inActa chirurgiae plasticae

2020 Issue 1-2-
All articles in this issue
- AN OVERVIEW AND OUR APPROACH IN THE TREATMENT OF MALIGNANT CUTANEOUS TUMOURS OF THE HAND
- ENHANCED RECOVERY PROTOCOL FOLLOWING AUTOLOGOUS FREE TISSUE BREAST RECONSTRUCTION
- SKIN SUBSTITUTES IN RECONSTRUCTION SURGERY: THE PRESENT AND FUTURE PERSPECTIVES
- GUNSHOT INJURIES OF THE OROFACIAL REGION
- INDICATION AND IMPORTANCE OF RECONSTRUCTIVE SURGERIES OF FACIAL SKELETON IN MAXILLOFACIAL SURGERY: REVIEW
- Editorial
- CURRENT OPTIONS IN PHARMACOLOGICAL INTERVENTIONS FOR MICROVASCULAR ANASTOMOSIS PATENCY: REVIEW
- ACCIDENTAL FINDING OF SYNCHRONOUS BILATERAL DUCTAL CARCINOMA IN SITU IN A YOUNG MAN REFERRED TO MASTECTOMY DUE TO GYNECOMASTIA – AND WHAT IF LIPOSUCTION HAVE BEEN USED? CASE REPORT
- RED BREAST SYNDROME (RBS) ASSOCIATED TO THE USE OF POLYGLYCOLIC MESH IN BREAST RECONSTRUCTION: A CASE REPORT
- Acta chirurgiae plasticae
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- RED BREAST SYNDROME (RBS) ASSOCIATED TO THE USE OF POLYGLYCOLIC MESH IN BREAST RECONSTRUCTION: A CASE REPORT
- AN OVERVIEW AND OUR APPROACH IN THE TREATMENT OF MALIGNANT CUTANEOUS TUMOURS OF THE HAND
- GUNSHOT INJURIES OF THE OROFACIAL REGION
- SKIN SUBSTITUTES IN RECONSTRUCTION SURGERY: THE PRESENT AND FUTURE PERSPECTIVES
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

