-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
The benefit of PET/CT in the diagnosis and treatment of esophageal cancer
Přínos PET/CT v diagnostice a léčbě karcinomu jícnu
Úvod:
Posouzení významu PET/CT pro iniciální staging karcinomu jícnu s důrazem na metastatické postižení uzlin a průkaz vzdálených metastáz. Dále bylo cílem práce ověření významu PET/CT vyšetření při hodnocení efektu neoadjuvantní léčby.Metody:
V prospektivní studii byl sledován soubor 354 nemocných s karcinomem jícnu, kteří byli vyšetřeni na I. chirurgické klinice LF UP a FN Olomouc v letech 2006–2012. Vstupní PET/CT vyšetření bylo provedeno u 349 nemocných. Analyzovali jsme přínos vyšetření ke stanovení stagingu onemocnění a v závislosti na něm jsme stanovovali strategii léčby. Na základě vstupního PET/CT byl u 102 nemocných zjištěn různý stupeň generalizace a byli indikovaní k paliativní či symptomatické terapii. U 247 pacientů bylo zjištěno omezení nádoru na jícen ev. regionální uzliny. Po posouzení celkového stavu a dle přání pacienta byla dále u 188 nemocných indikovaná neoadjuvantní radiochemoterapie (RCHT), léčbu nedokončilo 32 nemocných. U 156 pak bylo v průměrném odstupu 8,4 týdnů od ukončené neoadjuvantní terapie provedeno kontrolní vyšetření PET/CT. Na základě tohoto vyšetření bylo kompletní vymizení známek nádoru (complete response - CR) zjištěno u 38 vyšetřených (24,4 %), regrese u 89 (57,0 %), stacionární nález u 10 (6,4 %) a progrese u 19 (12,2 %). K chirurgické léčbě bylo indikováno 97 pacientů, z toho resekci jícnu bylo možné provést u 85 a u 12 byl výkon pouze paliativní resp. šlo o exploraci.Výsledky:
Vstupní PET/CT vyšetření u 349 pacientů u naprosté většiny správně posoudilo rozsah onemocnění v souhlase s histologicky ověřenou diagnózou. Falešně negativní výsledek byl jen u 5 nemocných (1,43 %).
Při hodnocení efektu neoadjuvantní léčby byli pacienti rozděleni do skupin dle nálezu při kontrolním PET/CT po neoadjuvanci a bylo vyhodnocováno jejich přežívání. Byl zjištěn signifikantní rozdíl (p=0,0004) v přežívání mezi skupinami s rozdílnou reakcí na neadjuvantní léčbu (CR (n=38), regrese (n=89), stacionární nález (n=10), progrese (n=19)) bez ohledu na další léčbu po neoadjuvanci, ve prospěch pacientů s lepší reakcí na neadjuvantní léčbu. Signifikantní rozdíl v přežívání byl zaznamenán i mezi skupinou pacientů po neoadjuvanci radikálně operovaných (n=85) a neoperovaných (n=59) ve prospěch operovaných (p=0,003). Nejdelšího průměrného přežívání 38,6 měsíců (medián 29,0 měsíců) bylo dosaženo ve skupině operovaných po neoadjuvanci, kde kontrolní PET/CT ukázalo CR. Nebyl však prokázán signifikantní rozdíl (p=0,587) ve výsledcích mezi skupinami operovaných s rozdílnou reakcí na neadjuvantní léčbu (regrese, stacionární nález). Počet případů v jednotlivých hodnocených skupinách není zatím natolik velký, abychom získané výsledky mohli považovat za jednoznačně průkazné a ve sledování a zařazování dalších nemocných do studie budeme dále pokračovat.Závěr:
V práci je dokumentován význam a přínos PET/CT v iniciálním stagingu karcinomu jícnu, zejména v průkazu metastatického onemocnění, a to jak v postižení uzlin, tak v odhalení vzdálených metastáz. PET/CT má velký význam pro stanovení léčebné strategie. Dále byl ověřen význam PET/CT vyšetření při hodnocení efektu neadjuvantní léčby.Klíčová slova:
karcinom jícnu −PET/CT − neoadjuvantní terapie − ezofagektomie
Authors: K. Vomackova 1; C. Neoral 1; R. Aujeský 1; R. Vrba 1; M. Stasek 1; M. Myslivecek 2; R. Formánek 2
Authors place of work: I. Department of Surgery, Medical Faculty at Palacky University in Olomouc, Department Head: prof. C. Neoral, M. D., CSc. 1; Department of Nuclear Medicine, Medical Faculty at Palacky University in Olomouc, Department Head: assoc. prof. P. Koranda, M. D., Ph. D. 2
Published in the journal: Rozhl. Chir., 2015, roč. 94, č. 1, s. 8-16.
Category: Původní práce
Summary
Introduction:
To evaluate the significance of PET/CT for the initial staging of esophageal cancer with emphasis on metastatic lymph node affection and detection of distant metastases. Furthermore, the aim of the work was to analyze the significance of PET/CT examination when evaluating the effect of neoadjuvant therapy.Methods:
A set of 354 patients with esophageal cancer treated at the 1st Department of Surgery, University Hospital Olomouc and Medical Faculty at Palacky University in Olomouc between the years 2006−2012 were analyzed in a prospective study. The initial PET/CT examination was performed in 349 patients. We analyzed the benefit of this examination in regard to disease staging and based on the result, therapeutic strategy was determined. The initial PET/CT showed varying degrees of disease generalization in 102 patients, these patients were indicated for palliative or symptomatic therapy. In 247 patients, the disease was limited only to the esophagus and /or regional lymph nodes. After considering the patient’s overall condition and taking into account the wishes of the patient, 188 patients were indicated for neoadjuvant chemoradiotherapy (CRT); 32 patients did not complete this treatment. In 156 patients a follow-up PET/CT scan was performed after an average of 8.4 weeks following completion of neoadjuvant therapy. Based on this examination, a complete response - CR, was observed in 38 patients (24.4%), regression of the tumor in 89 (57.0%), stationary findings were seen in 10 (6.4%), and progression in 19 (12.2%). Ninety-seven patients were indicated for surgical resection; however, esophagectomy was only possible in 85 patients, in the remaining 12 patients only an explorative laparotomy was performed due to disease progression.Results:
The initial PET/CT examination performed in 349 patients correctly described the extent of the disease in accordance with the histologically confirmed diagnosis in virtually all patients. A false positive result was seen in only 5 patients (1.43%).
When evaluating the effect of neoadjuvant therapy, the patients were divided into groups based on the findings of the follow-up PET/CT after neoadjuvant therapy and their overall survival was evaluated. A significant difference (p=0.0004) in survival was observed between the groups based on the different reactions to neoadjuvant therapy (CR (n=38), regression (n=89), stationary findings (n=10), progression (n=19)) without taking into account the following treatment the patient received after neoadjuvant therapy. Patients who had a better response to neoadjuvant therapy had better survival results. There was also a significant difference in survival between the group of patients who completed neoadjuvant therapy and underwent radical surgical resection (n=85) versus those patients who completed neoadjuvant therapy but did not undergo subsequent surgery (n=59). The operated group had a significantly higher overall survival (p=0.003). The longest mean survival, 38.6 months (median 29.0 months), was achieved by the group of patients who completed neoadjuvant therapy, showed a complete response on the follow-up PET/CT, and underwent surgical resection. However, a significant difference was not observed (p=0.587) between the groups who underwent surgical resection and whose follow-up PET/CT results differed (regression or stationary findings). To date, the number of cases in the individual groups is not great enough to consider the obtained results conclusive, and we will continue to include more patients into the study and continue with the analysis.Conclusion:
The work documents the significance and benefit of PET/CT in the initial staging of esophageal cancer, especially in detecting metastatic disease - positive lymph nodes as well as distant metastases. PET/CT has great importance in determining therapeutic strategy. Furthermore, the significance of PET/CT in evaluating the effect of neoadjuvant therapy was also studied.Key words:
esophageal cancer − PET/CT − neoadjuvant therapy − esophagectomyIntroduction
Malignant tumors of the esophagus represent approximately 1% of all solid malignant tumors in the Czech Republic, and have an incidence of 5.3 per 100 000 inhabitants. Although the incidence in males is 8.4 per 100 000 inhabitants, in women the incidence is much lower, only 1.7. When evaluating the prevalence (number of patients living with this disease before or after treatment, at the end of 2008, the prevalence in the Czech Republic was 791 patients, or 7.6 per 100 000 inhabitants. The severity of this disease is reflected by its poor prognosis. Based on an analysis of 2004−2007 by the Czech National Oncological Registry, five-year survival of patients with esophageal cancer is only 15.2%, and worse results are seen only in patients with cancer of the lungs, liver, biliary tree and pancreas [1,2,3].
In addition to our currently not very successful attempts at earlier diagnosis, our aim is to determine an optimal treatment plan. This necessitates precise preoperative staging as well as evaluating the significance of neoadjuvant therapy. The aim of this work was to include the new examination method PET/CT into the diagnostic algorithm in patients with esophageal cancer and evaluate its significance in perfecting the diagnosis and preoperative staging as well as restaging after completion of neoadjuvant concomitant chemo-radiotherapy. Then, based on the results of PET/CT, other classical examination methods and the overall condition of the patient, an optimal treatment plan should be set. We assumed that this examination would aid in correctly indicating the patient for esophagectomy as well as evaluate the significance of neoadjuvant therapy.
PET/CT is also important in the follow-up of patients after treatment, to detect recurrence and generalization. However, this was not a subject of study in this work.
Methods
Treatment of patients with diseases of the esophagus has a longstanding tradition at the 1st Department of Surgery in Olomouc, founded by the first Head of Department, Professor Vladislav Rapant, M.D., DrSc. According to the published bibliography of the works of the Olomouc surgeons, between the years 1948−2012, 305 works were published regarding esophageal diseases and 72 (24%) of these were about esophageal tumors [2]. Esophageal surgeons were always very aware of the significance of precise preoperative disease staging for the determination of the therapeutic plan, and paid great attention to this subject.
Until the first half of the 20th century, contrast X-ray barium swallow was the only method useful in determining the length and extent of stenosis. How advanced a tumor was, or its relationship to surrounding structures was difficult to determine precisely. Therefore the introduction of pneumomediastinum in the 1960’s was a great advancement which enabled more precise determination of the stage of the disease than was possible with only a barium swallow. The published work about pneumomediastinum by Olomouc surgeons [4] created significant international acclaim. This examination method was later completely replaced by the more sophisticated computer tomography (CT). After the introduction of CT examination, the Olomouc surgeons were once again pioneers in using this examination method in patients with esophageal cancer and their initial experiences were published in 1989 [5], similarly to their first experiences with endoscopic ultrasound [6]. In the past years, more precise diagnosis has been achieved due to the introduction of PET/CT, whose significance is evaluated presently and thus we are continuing in past traditions and reflecting on the possibilities of this new imaging method [7,8].
The first PET/CT examinations were performed in Olomouc in 2006, and PET/CT has been routinely used in patients with esophageal cancer since 2007. As part of disease staging in the diagnostic algorithm, an initial whole body (from the base of the skull to the proximal third of the femurs) 18F-FDG PET/CT scan is performed, and then a follow-up PET/CT is performed after the completion of neoadjuvant therapy. The examination is performed at the Department of Nuclear Medicine at the University Hospital in Olomouc using PET/CT scanner Biograph Sensation 16, Siemens. Interactive data reconstruction was used. One liter of oral contrast was administered one hour prior to the examination, i.v. application of 18F-FDG ensued. The patients were in the supine position during examination. First an initial spiral contrast CT scan was performed followed by the PET phase, also in the supine position. The intensity and accumulation of 18F-FDG was evaluated, both visually and semi-quantitatively with determination of SUV (standardized uptake value).
Between the years 2006 and 2012, 354 patients diagnosed with esophageal cancer were treated at the 1st Department of Surgery at the University Hospital and Medical Faculty in Olomouc. The set included 303 males (85.6%) and 51 females (14.4%). The average age of these patients was 61.2 years (ranging from 33 to 93 years). Histologically, the most common type was squamous cell cancer seen in 193 patients (55.9%), the second most common type was adenocarcinoma, seen in 138 patients (39.0%). The remaining patients had a more rare histological type of cancer, or the precise histological type was not determined.
The collected data from our patient set was statistically analysed. A Kaplan-Meier curve was constructed to evaluate survival, and the Log Rank Test (Mantel-Cox) was used to compare survival among the studied groups.
Results
Of the 354 patients with esophageal cancer treated at our department between the years 2006 and 2012, an initial PET/CT examination was performed in 349 patients. Of these patients, 109 (31.2%) had findings of only the esophageal tumor, 133 patients (38.1%) had findings of esophageal tumor with regional lymph node involvement, and in 5 patients (1.4%) with histologically confirmed esophageal cancer, the PET/CT findings were negative. In the remaining 102 patients (29.2%), PET/CT showed metastatic disease (Tab. 1).
Tab. 1. Initial PET/CT in our set of 349 patients 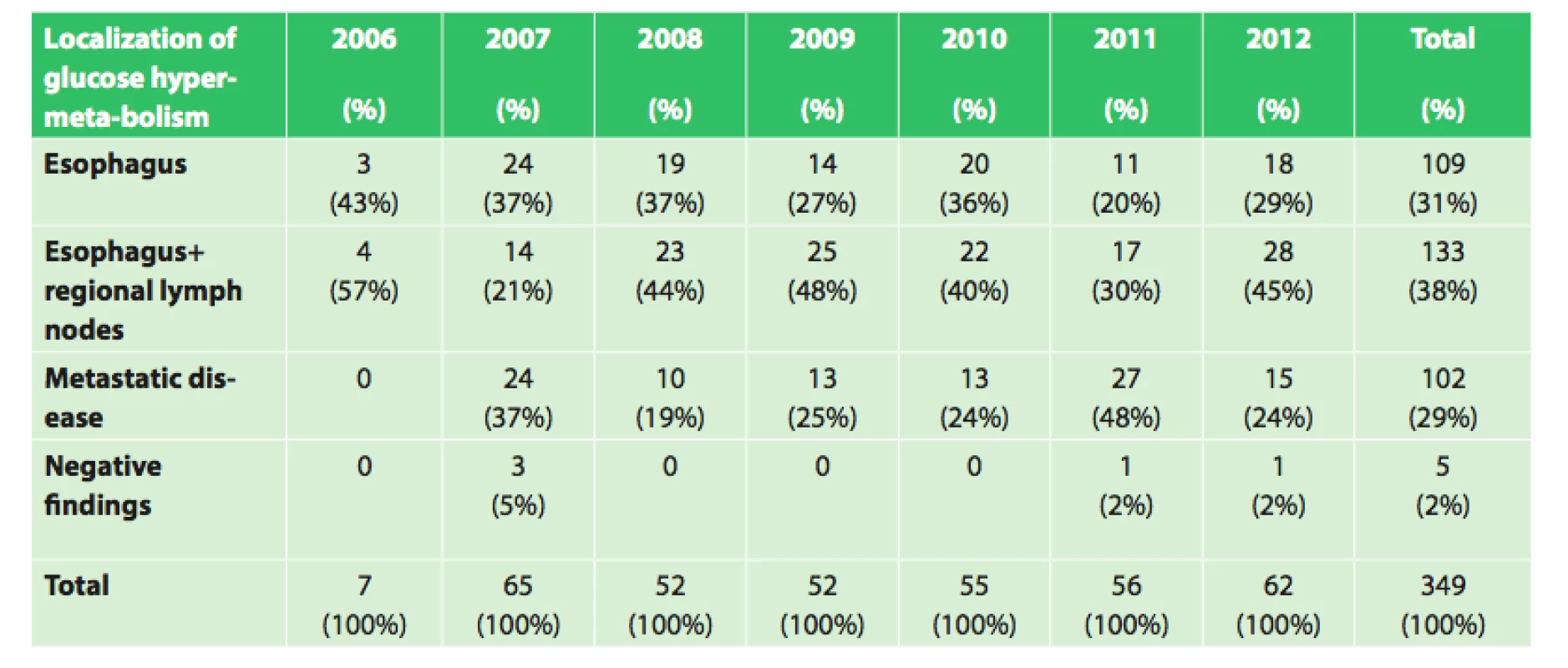
Of the 247 patients (109+133+5), where the tumor was limited to only the esophagus, or esophagus and regional lymph nodes, or where PET/CT findings were negative, 188 patients were indicated for neoadjuvant oncological therapy; in the remaining 59 patients neoadjuvant treatment with subsequent surgical procedure was contraindicated for overall poor medical condition or refusal of the patient. These patients received palliative therapy. Therefore of the total number of 349 patients with esophageal cancer who underwent initial PET/CT examination, neoadjuvant therapy was only indicated in 188 (53.9%) patients. The diagnostic-therapeutic algorithm used in our patients with esophageal cancer is depicted in Fig. 1.
Fig. 1: Diagnostic-therapeutic algorithm of our 349 patients with esophageal cancer treated from 2006−2012 
Neoadjuvant CRT consisted of radiation of the primary tumor and regional lymph nodes with a total radiation dose of 50 Gy applied in 25 fractions of 2 Gy during a course of 5 weeks. Together with the radiotherapy, 2 cycles of chemotherapy consisting of cisplatin and fluorouracil were administered; the third cycle was applied three weeks after the completion of radiotherapy. In several patients with a poor general condition and patients with squamous cell cancer, cisplatin was applied once a week during the course of radiotherapy.
In the remaining 102 patients, initial PET/CT examination revealed a varying extent of disease generalization and palliative or symptomatic therapy was indicated. In certain patients indicated for neoadjuvant therapy, due to severe dysphagia or even aphagia, nutrition had to be ensured by performing a jejunostomy (53x) or gastrostomy (4x). After completing neoadjuvant therapy, a follow-up PET/CT was performed in 156 patients to evaluate the effect of neoadjuvant therapy by comparing the results with the initial PET/CT. Follow-up PET/CT showed complete response (CR) in 38 patients, regression of the tumor in 89 patients, stationary findings in 10 patients and disease progression or generalization 19 patients (Tab. 2). Overall survival was evaluated in these four groups, based on the different findings on follow-up PET/CT after completion of neoadjuvant therapy (CR, regression, stationary findings, progression), regardless of subsequent treatment.
Tab. 2. Results of the follow-up PET/CT following neoad- juvant therapy in 156 patients 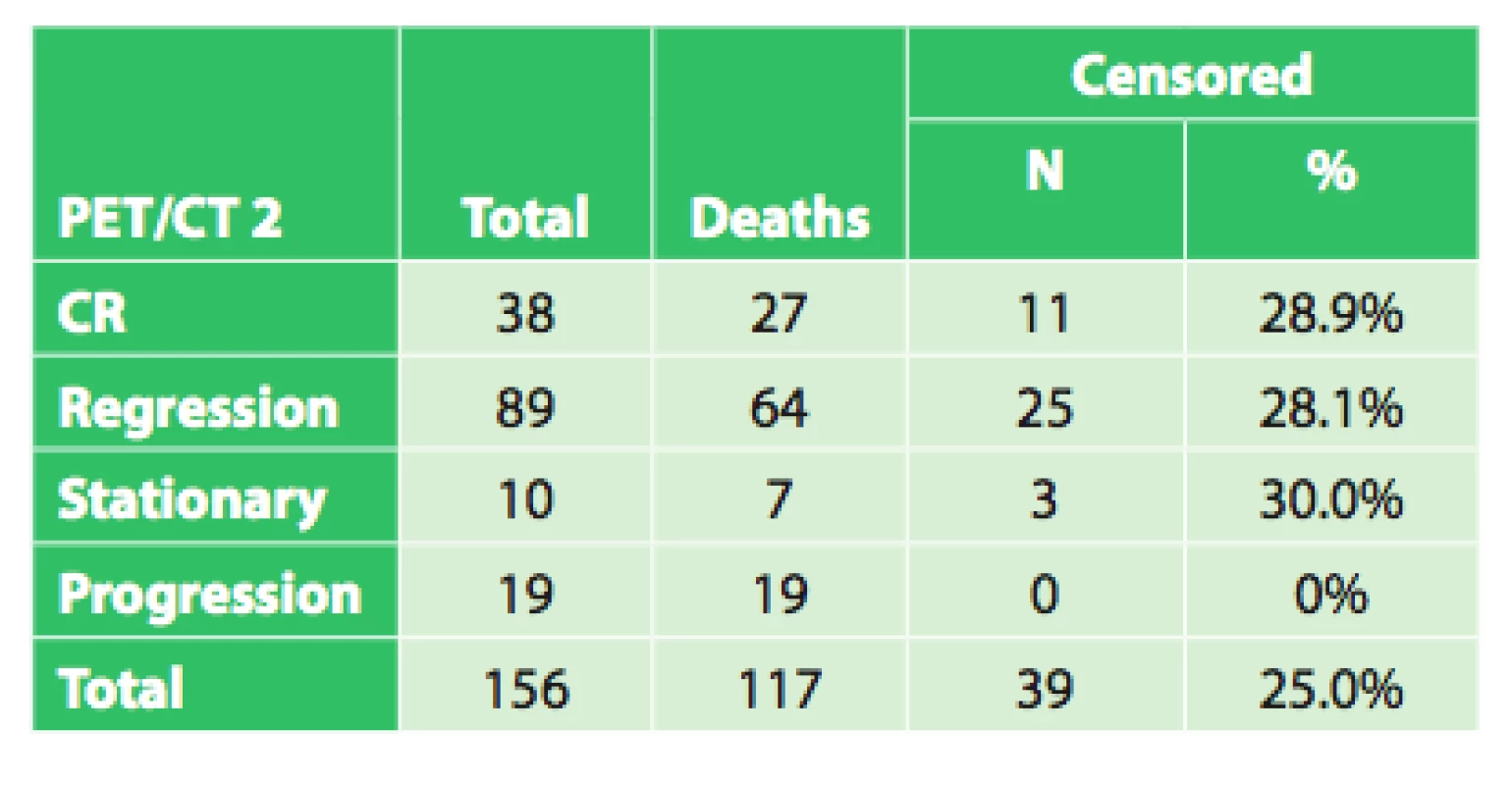
The average survival in the group with CR was 35.4 months (95%CI: 26.6–44.1 months). The median survival was 26.3 months (95%CI: 21.8–30.7 months).
The average survival in the group with regression was 28.2 months (95%CI: 22.5–34.0 months). The median survival was 15.4 months (95%CI: 11.1–19.8 months).
The average survival in the group with stationary findings was 33.1 months (95%CI: 14.3–52.0 months). The median survival was 12.0 months (95%CI: 0.0–34.7 months). (There were only 10 patients with stationary findings, therefore the estimates are inaccurate).
The average survival in the group with progression was 15.1 months (95%CI: 9.0–21.2 months). The median survival was 10.3 months (95%CI: 9.5–11.0 months).
Log rank test showed significant differences in survival based on the results of follow-up PET/CT (significance of Log rank test p = 0.0004).
As seen in the diagram in Fig. 1, of the 156 patients who completed neoadjuvant treatment, only 97 patients were subsequently operated. Only 19 of the 38 patients with CR on follow-up PET/CT were operated, 68 of the 89 patients with regression on follow-up PET/CT were operated, 9 out of 10 patients with stationary findings were operated and one patient from the group of patients with progression on follow-up PET/CT was operated. The indication for operation was established not only based on the results of follow-up PET/CT findings but also on the overall condition of the patient and their cooperation. Thus of the 156 patients who completed neoadjuvant therapy, only 97 underwent a subsequent operation (62.2% of the patients who completed neoadjuvant therapy). However, of these 97 operated patients, only an exploration was performed in 12 - inoperability was discovered in 11 patients and in 1 patient with CR findings on the esophagus, an esophagectomy was not performed but only an adrenalectomy for metastasis was performed, which was described on follow-up PET-CT. In the remaining 85 patients, esophagectomy with gastroplasty or coloplasty was performed (87.6% of patients who completed neoadjuvant treatment).
The set of 85 radically operated patients comprised of 72 males and 13 females, the average age was 57.8 years (33.9–75.8), and median was 58 years. Histologically 44 patients had adenocarcinoma (51.8%), 37 (43.5%) had squamous cell cancer and 4 (4.7%) had other types of malignant tumor. Based on precise TNM classification, the patients were divided into clinical stages as seen in Tab. 3. An interesting difference when analyzing the grading of the tumor is seen when comparing the initial grading and grading of the resected specimen (Tab. 4).
Tab. 3. Clinical stages of operated patients 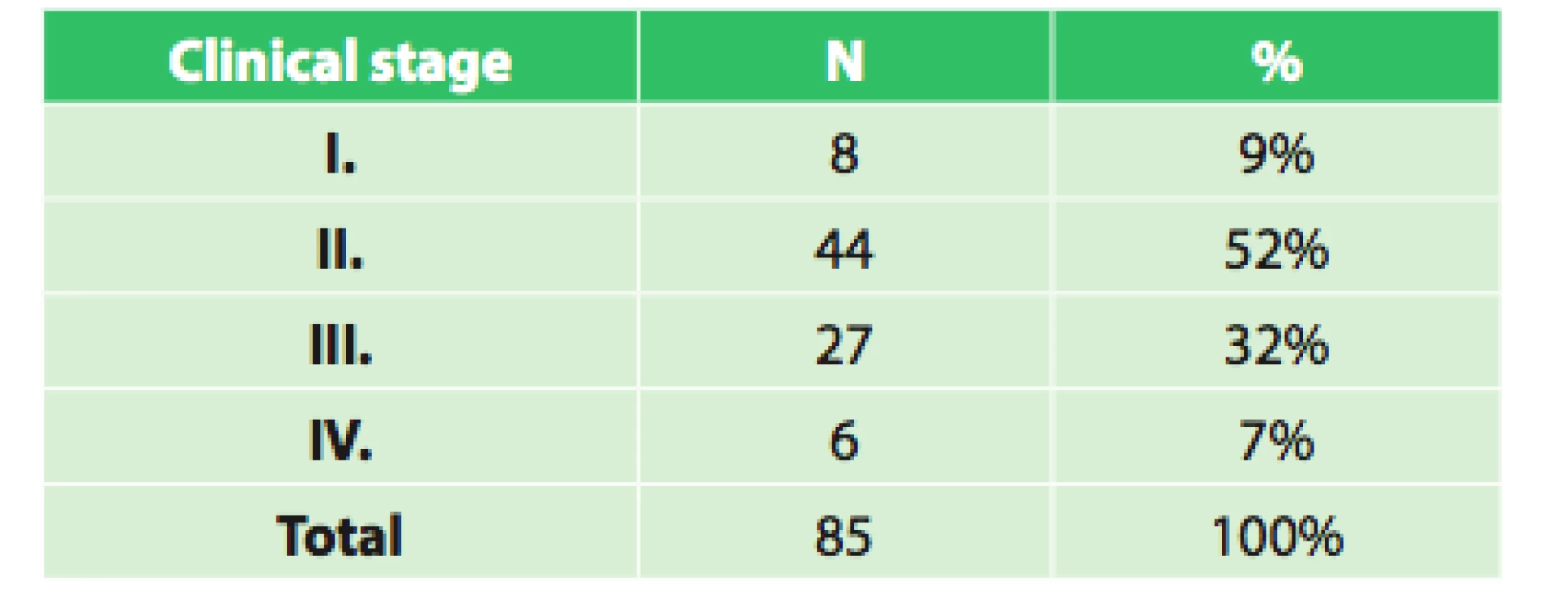
Tab. 4. Grading at diagnosis and of the resected specimen in operated patients 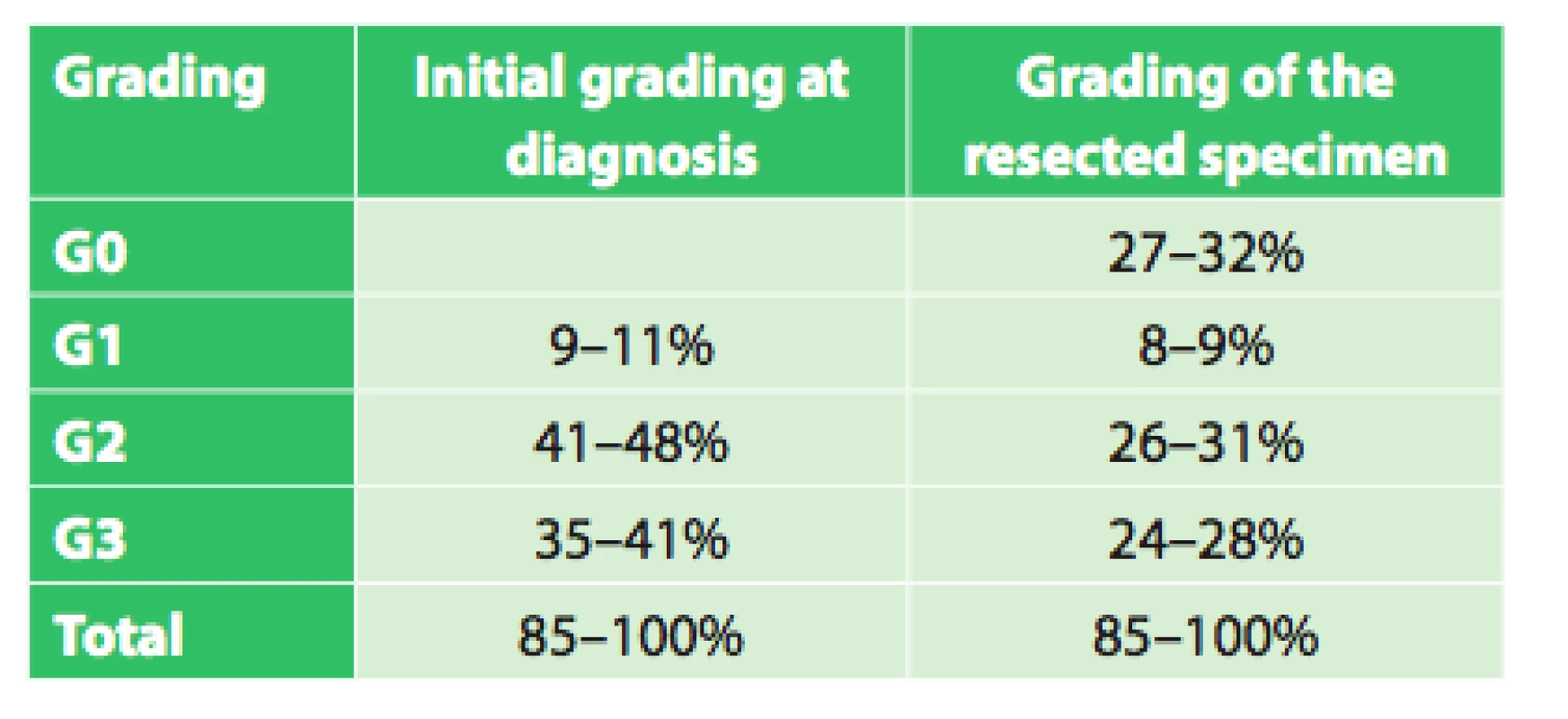
The topographical localization is important when determining therapeutic procedure. None of the operated patients had cancer of the cervical esophagus, 2 patients (2%) had cancer of the upper thoracic esophagus, 33 (39%) had cancer of the middle thoracic esophagus, and 50 (59%) of patients had cancer localized in the lower thoracic and abdominal esophagus. Postoperative mortality (≤30days) was seen in 7 patients, which is 8.2 %.
Table 2 shows differences in survival between the groups of patients based on their reaction to neoadjuvant therapy, regardless of subsequent treatment. First, survival of the entire group of operated patients was statistically evaluated. In addition, survival in the group of radically operated patients was evaluated.
At the time of analysis, out of the 85 operated patients, 53 had died and 32 were living (in living patients, only censored data are available regarding survival up to the time of analysis).
The average survival after operation was 29.1 months (95%CI: 22.2–36.0 months). The median survival was 15.7 months (95%CI: 8.3–23.1 months).
Subsequent evaluation compared the differences in survival in operated patients based on their reaction to neoadjuvant therapy. These included the group with CR, regression and stationary findings. One operated patient in the group with progression could not be included in statistical evaluation.
The average survival in patients with CR was 38.6 months (95%CI: 27.5–49.7 months). The median survival was 29.0 months (95%CI: 6.6–51.5 months).
The average survival in patients with regression was 36.4 months (95%CI: 28.2–44.5 months). Median survival was 18.3 months (95%CI: 8.8–27.9 months).
The average survival in patients with stationary findings was 35.4 months (95%CI: 13.0–57.9 months). Median survival was 8.8 months (95%CI: 7.7–9.9 months).
Log rank test did not show a statistically significant difference in survival based on findings on follow-up PET-CT (significance of Log rank test p = 0.587).
We also compared survival between the group of radically operated patients and group of unoperated patients, all of which completed neoadjuvant treatment.
The average survival in the group of unoperated patients was 23.3 months (95%CI: 17.5–29.0 months). Median survival was 13.9 months (95%CI: 12.7–15.2 months).
The average survival in the group of radically operated patients was 37.4 months (95%CI: 30.3–44.4 months). Median survival was 24.9 months (95%CI: 15.8–34.0 months).
Log rank test showed significantly better survival in the group of radically operated patients (p = 0.003).
In addition, survival based on the clinical stage of the operated patients was evaluated (Tab. 5).
Tab. 5. Overall survival of radically operated patients based on clinical stage 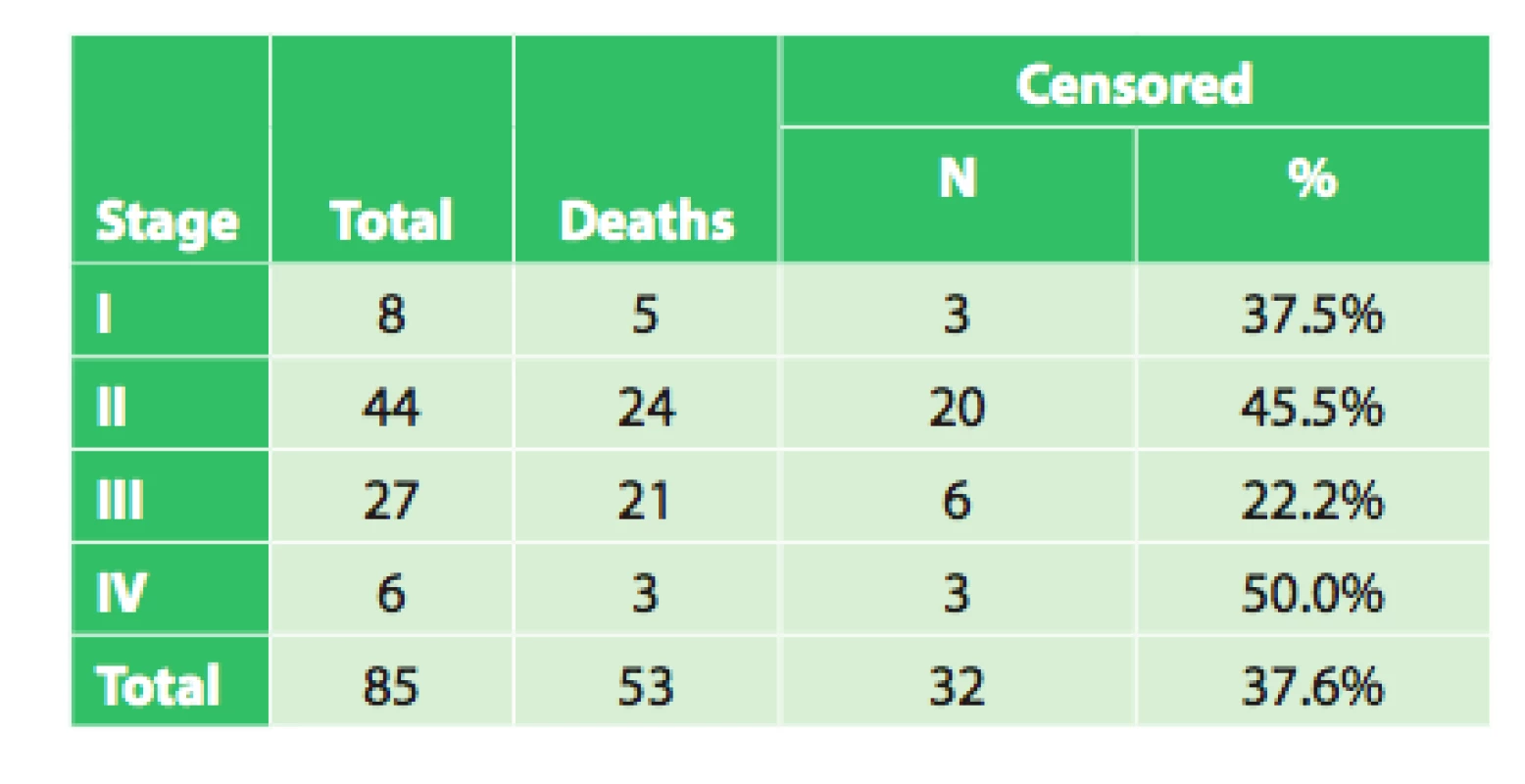
The average survival of patients in stage I was 30.1 months (95%CI: 17.9–42.3 months). Median survival was 39.9 months (95%CI: 5.1–74.7 months). However, there were only 8 patients in the group!
The average survival of patients in stage II was 40.5 months (95%CI: 30.6 – 50.4 months). Median survival was 27.9 months (95%CI: 23.6–32.2 months).
The average survival of patients in stage III was 21.0 months (95%CI: 13.4–28.6 months). Median survival was 15.4 months (95%CI: 9.9–21.0 months).
The average survival of patients in stage IV was 44.1 months (95%CI: 19.2–68.9 months). Median survival was 17.7 months. However, there were only 6 patients in the group!
The test of the equality of the survival distributions of the studied patient groups, Log rank test, did not demonstrate a significant correlation between survival and disease stage (p = 0.099). This result is not in accordance with repeatedly confirmed dependence of treatment results on disease stage. This dependence was also demonstrated on an extensive set of patients from the Czech NOR and presented in publications analyzing this topic in the Czech Republic [1,2,3]. The reasons for this discrepancy may be explained by the following:
Patients in stage IV are not usually indicated for surgical treatment. An exception was made in the case of our 6 patients in stage IV, because they were younger patients with a locally operable tumor, in overall very good health and therefore surgery was indicated and in certain cases even extirpation of a distant metastasis was performed.
Due to these reasons, this statistical comparison cannot be considered to be representative, because it is an error of small numbers. On the other hand, these results show that esophagectomy may, in certain specific cases, be beneficial even for patients with stage IV esophageal cancer. The problem with our statistical analysis is also the fact that there were only 8 patients in stage I and 6 patients in stage IV. The Kaplan-Meier test is therefore only able to provide very inaccurate estimates of average survival or median of survival and the same is true for the Log rank test. Thus results for such small patient groups may be opposite from those expected. For these reasons we also performed a comparison between patients in stages I and II compared with patients in stage III, which is more suitable for statistical analysis since both groups comprise of more than 25 patients. In general, there is no rule regarding a minimal number of patients in each group. However, the larger the patient group the better (the statistical evaluation will be more accurate). When evaluated as such, the results are in accordance with the repeatedly confirmed significance of lower disease stages having a more favorable survival.
Kaplan-Meier analysis with Log rank test showed significantly better survival in the group of patients in Stage I or II compared to Stage III (median survival was 29 months vs. 15 months, p = 0.021).
In the group of operated patients, the time intervals between neoadjuvant therapy, follow-up PET/CT and operation were evaluated. The interval between completion of neoadjuvant therapy and operation was 3.5 months on average (0.7–14.7, median 2.7), the interval between completion of neoadjuvant therapy and follow-up PET/CT was 8.4 weeks on average (1.1–54.6, median 6.7) and the interval between follow-up PET/CT and operation was 6.7 weeks on average (0.3–57.1, median 5.0).
Discussion
The role of PET and PET/CT in the diagnosis of esophageal cancer and the expected benefit of this examination method, especially its role in primary disease staging and its significance in comparison to other examination methods has been analyzed in a number of studies. Special attention has been paid to the detection of regional lymph node involvement and distant metastases and evaluating the significance of PET/CT in the therapeutic response [9,10,11,12,13].
In the detection of metastatic disease, both lymph node involvement and distant metastases, PET has a higher sensitivity compared to CT or EUS. This has been demonstrated in numerous published studies [14, 15,16,17,18,19,20]. In a meta-analysis from 2008, Van Vliet et al. compared the sensitivity and specificity of PET and CT examination in the detection of distant metastases [20]. They discovered that PET had a sensitivity and specificity of 71% and 93% respectively, and CT only 52% and 91%, resp. A study by Weber et al. stated that PET showed distant metastases in 20% of patients with esophageal cancer, who were deemed surgically resectable based on other examination methods [21]. PET also enables the identification of various rare types of metastases in a single examination, for example into muscles of the lower extremities, as was shown in the data from our patient set [21].
Tab. 6. Overall survival of patients with esophageal cancer, evaluation based on various criteria 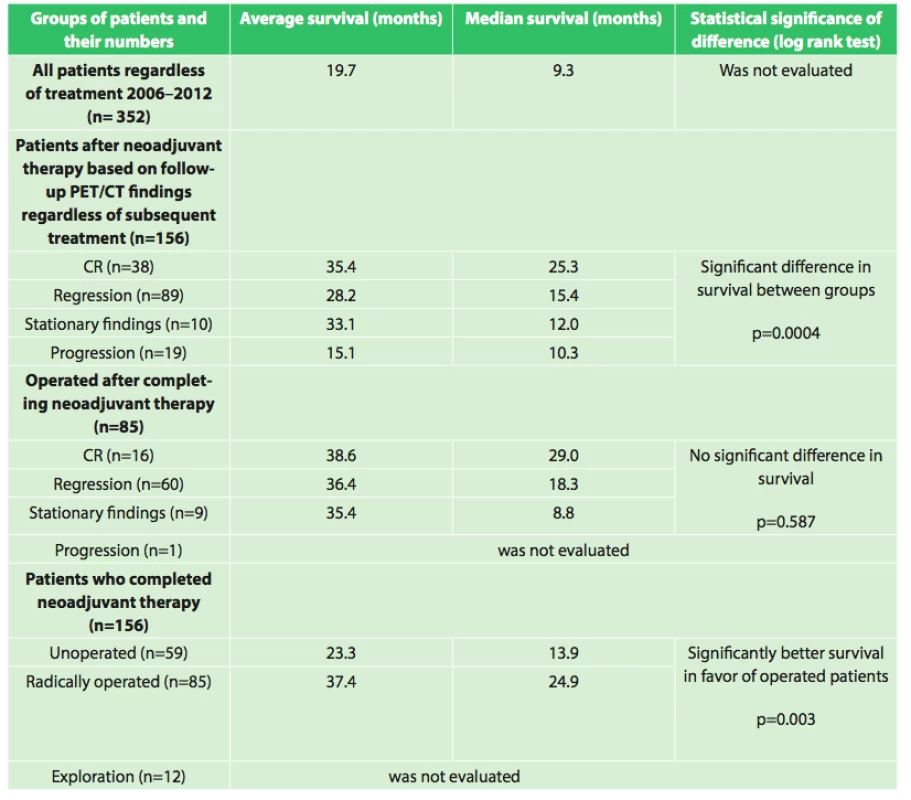
PET/CT examination, which combines a metabolic and morphologic view of the tumor, is therefore a significant examination method to use in the initial staging of esophageal cancer. According to some of the aforementioned studies, the implementation of PET/CT has an important impact on the surgical management of patients and affects it in up to 20%. In our set of patients, initial PET/CT was performed in 349 patients. The great majority of our patients had PET/CT findings which corresponded with the histologically confirmed diagnosis and with the correct extent of the disease. A false negative result was seen in only 5 patients (1.43%). The sensitivity of PET/CT in the detection of the tumor was 95.5 % (95% confidence interval for sensitivity = 96.6%–99.5%). The specificity could not be evaluated due to the fact that all patients had histologically confirmed esophageal cancer prior to the PET/CT examination; therefore false positive results were not possible.
PET measures the metabolic activity of the tumor in SUV (standard uptake value) units, and a number of studies have analyzed the significance of these values with regards to survival prognosis. A higher initial SUV is considered to be a factor indicating a worse prognosis. A meta-analysis by Pan et al. summarizing the results of 7 studies confirmed this conclusion [22]. However, other studies did not confirm this association. Contrarily Rizk et al. showed that there is no correlation between SUV and survival, and demonstrated that a high SUV correlated with a better response to neoadjuvant therapy [23]. Evaluation of the significance of SUV was performed in 82 patients from our patient set treated between the years 2006-2010 and did not reach a definite conclusion [24].
PET/CT is especially significant in evaluating the effect of neoadjuvant therapy. The purpose of neoadjuvant therapy in the treatment of esophageal cancer is the subject of many controversial debates and has been evaluated repeatedly in the past decades. In the last several years, there is an increasing number of extensive and qualified studies which attest to the significance of neoadjuvant therapy in improving survival, even in cases of primarily resectable esophageal tumors [25,26]. Follow-up PET/CT after completion of neoadjuvant therapy may detect new metastases in up to 8% of patients, as discovered by Bruzzi et al [27]. PET/CT plays a key role in monitoring the effect of neoadjuvant therapy [28,29,30] and should also be useful in determining which patient will have the greatest benefit from ensuing surgery [31,32,33]. A study performed on 81 patients with esophageal cancer at the Moffit Cancer Center who had a PET/CT scan before and after neoadjuvant chemotherapy with subsequent surgical resection analyzed whether negative PET/CT findings on follow-up PET/CT after chemotherapy was a predictor of complete pathological response [32]. Positive predictive values in the prediction of pathological CR and residual disease were discovered on negative follow-up PET/CT in 35% and in 70% of patients with positive findings. Thus this study showed that negative PET/CT findings had a low probability of predicting pathological CR. The significance of subsequent surgical resection in PET/CT negative patients after CRT was studied by Monjazeb et al [31]. In their analysis of 163 patients who completed neoadjuvant CRT, 88 (54%) underwent surgical resection. Patients with negative findings on PET (defined as SUV less than 3) had a better 2-year survival (71% versus 11%, p<0.01). They came to the conclusion that patients with negative findings on PET do not benefit from subsequent surgical resection. Lomas et al analyzed 77 patients with esophageal cancer, who had PET scans before and after CRT [33]. Univariate statistical analysis showed that the SUV after neoadjuvant therapy and percentage of change in SUV were prognostic factors for overall survival (OS). Contrarily, SUV before CRT did not have any prognostic significance. In their set of patients, they had no deaths in the group of patients with an SUV less than 3 after CRT. Analysis showed that only SUV after CRT (HR 1.401; 95% CI, 1.061–1.850) had prognostic significance for OS; conversely age, gender, surgical procedure, histology, tumor length and stage were not significant.
The findings that surgical resection does not improve survival was studied by Shridhar R et al, who reasoned that it is due to the biological character of the tumor in patients who do not have a complete response on PET [26]. These tumors are probably hypoxic and therefore have a higher radioresistance and remain PET negative because of their low metabolic activity or small size. This may explain why surgical resection in these patients did not affect OS, because these tumors may be in a dormant stage.
Several works are of the opinion that response to chemotherapy correlates with survival and increases the probability of an R0 resection. Results of PET/CT may therefore be used to monitor the effect of therapy [28,29,30,34]. These studies showed that the PET result had a predictive significance on survival if the SUV decreased by at least 36% (p=0.04). These data led to the creation of the MUNICON study [29]. This study included patients with carcinoma of the distal esophagus or gastroesophageal junction. PET was performed after the first cycle of induction chemotherapy. Patients with a positive response to therapy (responders), defined by a decrease in SUV by 35%, continued with chemotherapy prior to the resection and non-responders immediately underwent surgical resection. Median follow-up was 2.3 years, and during this period median survival was not reached by the responder group; non-responders had a median survival of 26 months (p=0.015). The subsequent study MUNICON II analyzed the role of salvage chemoradiotherapy in PET non-responders with gastroesophageal cancer [35]. Two-year overall survival in responders was 71% compared to 42% in non-responders (p=0.1).
All of these studies show the significance of PET and PET/CT in evaluating the effect of neoadjuvant therapy in esophageal cancer and in finding optimal indications for surgical resection. Only implementation of this examination method is able to accurately and objectively assess the obtained results and approach optimal determination of the best treatment, based on precise statistical analysis, which is in agreement with the requirements of evidence based medicine.
Although in previous years a number of interesting and well compiled studies have been published which favorably assess the significance of neoadjuvant therapy in esophageal cancer, further studies on larger patient groups with precise statistical evaluation are needed to definitely answer this question.
Our results also show interesting associations in the evaluation of survival between the groups as shown in table 6.
Assessment of the significance of neoadjuvant therapy was not the aim of this work; we only studied the significance and possibilities of PET/CT in this evaluation. However, this study is a foundation for the possibility of future reliable evaluation of the results of neoadjuvant therapy. We will continue to follow-up our present patients as well as recruit new ones, and we expect that with a larger set of patients and after evaluation of survival between the individual groups after a longer follow-up period we will be able to more accurately assess the significance of neoadjuvant therapy. The current results summarized in table 6 already indicate a significant difference (p=0.0004) in survival between groups with different responses to neoadjuvant therapy (CR (n=38), regression (n=89), stationary findings (n=10), progression (n=19)) favoring patients with a better response to neoadjuvant therapy, regardless of additional treatment after completion of neoadjuvant therapy. A significant difference in survival was also observed between the group of patients who completed neoadjuvant therapy and was radically operated (n=85) and the group that completed neoadjuvant therapy and did not undergo operation (n=59) in favor of the operated patients (p=0.003). The longest average survival, 38.6 months (median 29.0 months) was achieved by the group of operated patients who had a complete response on follow-up PET/CT. However, no significant difference (p=0.587) was seen between the results of the operated group versus the groups with varying results on follow-up PET/CT (regression, stationary findings). Interesting results were also seen when comparing grading at diagnosis and grading of the resected specimen after neoadjuvant therapy. The results in table 4 show that there was a significant decrease in grading in the individual groups in favor of lower grades. This result seems to demonstrate that neoadjuvant treatment leads to a decrease in grading in individual patients. These findings will be analyzed further in cooperation with pathologists.
Conclusion
Based on our results and findings from published studies, our work documented:
- The benefit of PET/CT in the initial staging of esophageal cancer and in the detection of metastatic disease - both lymph node involvement and distant metastases.
- The significance of PET/CT in evaluating the effect of neoadjuvant therapy and the presentation of initial results during the current evaluation.
This work was dedicated to the University Hospital Olomouc.
Conflict of Interests
The authors declare that they have not conflict of interest in connection with the emergence of and that the article was not published in any other journal.
MUDr. Katherine Vomáčková
I. chirurgická klinika LF UP a FN Olomouc
I. P. Pavlova 6 775 20 Olomouc
e-mail: katherineruzicka@hotmail.com
Zdroje
1. Dušek L, et al. Czech cancer care in number 2008−2009. Praha, Grada Publishing 2009.
2. Duda M, Adamčík L, Dušek L, Škrovina M, Jínek T. Zhoubné nádory jícnu v České republice. Rozhl Chir 2012;91 : 132−40.
3. Duda M, a kol. Jícen: pohled z mnoha úhlů v zrcadle zkušeností olomoucké jícnové školy. 2.vyd. Olomouc, Univerzita Palackého Olomouc 2012.
4. Holub E, Šimeček C. Pneumomediastinography in carcinoma of the oesophagus. Thorax 1968;23 : 77–82.
5. Duda M, Janda P, Häringová K, Roček V, Dlouhý M. Přínos výpočetní tomografie (CT) v předoperační rozvaze chirurga u karcinomu jícnu. Čs Gastroent Výž 1989;43 : 312–8.
6. Dlouhý M, Šimek I, Duda M, Janda P. Endokavitární sonografie jícnu. In: Válek V, editor. Zobrazovací a výpočetní technika v gastroenterologii. Učební texty. Brno, IDVPZ 1994 : 49–53.
7. Neoral C, Vomackova K, Vrba R, Aujesky R, Cincibuch J, et al. The benefit of PET-CT in the therapeutic algorithm of esophageal cancer. World J Surg 2009;33(Suppl.1):S121.
8. Vomáčková K, Neoral Č, Aujeský R, Vrba R, Kysučan J, et al. Využití PET-CT v plánování léčby karcinomu jícnu. Miniinvazna chirurgia a endoskopická chirurgia súčasnosti 2010;14 : 24–7.
9. Kato H, Miyazaki T, Nakajima M, Takita J, Kimura H, et al. The incremental effect of positron emission tomography on diagnostic accuracy in the initial staging of esophageal carcinoma. Cancer 2005;103 : 148–56.
10. Ott K, Weber WA, Finj U, et al. Fluorodeoxyglucose-positron emission tomography in the adenocarcinomas of the distal esophagus and cardia. World J Surg 2003;27 : 1035−9.
11. Van Westreenen HL, Westerterp M, Bossuyt PM, et al. Systematic review of the staging performance of 18F-fluorodeoxyglucose positron emission tomography in esophageal cancer. J Clin Oncol 2004;22 : 3805−12.
12. Kelly S, Harris KM, Berry E, et al. A systematic review of the staging performance of endoscopic ultrasound in gastro-oesophageal carcinoma. Gut 2001;49 : 534−9.
13. Flamen P, Lerut A, Van Cutsem E, Cambier JP, Maes A, et al. The utility of positron emission tomography for the diagnosis and staging of recurrent esophageal cancer. J Thorac Cardiovasc Surg 2000;120 : 1085–92.
14. Block MI, et al. Improvement in staging of esophageal cancer with the addition of positron emission tomography. Ann Thorac Surg 1997;64 : 770–6. Discussion776–7.
15. Flamen P, et al. Utility of positron emission tomography for the staging of patiens with potentially operable esophageal carcinoma. J Clin Oncol 2000;18 : 3202–10.
16. Flanagan FL, et al. Staging of esophageal cancer with 18Ffluorodeoxyglucose positron emission tomography. AJR Am J Roentgenol 1997;168 : 417–24.
17. Luketich JD, et al. Evaluation of distant metastases in esophageal cancer: 100 consecutive positron emission tomography scans. Ann Thorac Surg 999;68 : 1133–6. discussion 1136–7.
18. Meyers BF, et al. The utility of positron emission tomography in staging of potentially operable carcinoma of the thoracic esophagus: results of the American College of Surgeons Oncology Group Z0060 trial. J Thorac Cardiovasc Surg 2007;133 : 738–45.
19. Rasanen JV, et al. Prospective analysis of accuracy of positron emission tomography, computed tomography, and endoscopic ultrasonography in staging of adenocarcinoma of the esophagus and the esophagogastric junction. Ann Surg Oncol 2003;10 : 954–60.
20. van Vliet EP, Heijenbrok-Kal MH, Hunink MG, Kuipers EJ, Siersema PD. Staging investigations for oesophageal cancer: a meta-analysis. Br J Cancer 2008;98 : 547–57.
21. Weber WA, Ott K. Imaging of esophageal and gastric cancer. Semin Oncol. 2004;31 : 530−41.
22. Pan L, et al. Prognostic significance of SUV on PET/CT in patients with esophageal cancer: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol 2009;21 : 1008–15
23. Rizk NP, et al. Predictive value of initial PET-SUVmax in patients with locally advanced esophageal and gastroesophageal junction adenocarcinoma. J Thorac Oncol 2009;4 : 875–9.
24. Mysliveček M, Neoral Č, Vrba R, Vomáčková K, Cincibuch J, et al. The value of 18F-FDG PET/CT in assessement of metabolic response in esophageal cancer for prediction of histopathological response and survival after preoperative chemoradiotherapy. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2011;156 : 171−9.
25. van Hagen P, Hulshof MCCM, van Lanschot JJB, Steyerberg EW, van Berge Henegouwen MI, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366 : 2074−2084.
26. Shridhar R, Imani-Shikhabadi R, Davis B, Streeter OA, Thomas Jr CR. Curative treatment of esophageal cancer; An evidenced based review. J Gastrointest Canc 2013;44 : 375−84.
27. Bruzzi JF, Swisher SG, Truong MT, Munden RF, Hofstetter WL, et al. Detection of interval distant metastases: clinical utility of integrated CT-PET imaging in patients with esophageal carcinoma after neoadjuvant therapy. Cancer 2007;109 : 125−34.
28. Downey RJ, et al. Whole body 18FDG-PET and the response of esophageal cancer to induction therapy: results of a prospective trial. J Clin Oncol 2003;21 : 428–32.
29. Lordick F, et al. PET to assess early metabolic response and to guide treatment of adenocarcinoma of the oesophagogastric junction: the MUNICON phase II trial. Lancet Oncol 2007;8 : 797–805.
30. Wieder HA, et al. Time course of tumor metabolic activity during chemoradiotherapy of esophageal squamous cell carcinoma and response to treatment. J Clin Oncol 2004;22 : 900–8.
31. Monjazeb AM, et al. Outcomes of patients with esophageal cancer staged with [¹⁸F]fluorodeoxyglucose positron emission tomography (FDG-PET): can postchemoradiotherapy FDG-PET predict the utility of resection? J Clin Oncol 2010; 28 : 4714–21.
32. McLoughlin JM, et al. Are patients with esophageal cancer who become PET negative after neoadjuvant chemoradiation free of cancer? J Am Coll Surg 2008;206 : 879–86, discussion 886–7.
33. Lomas H, et al. Post chemoradiation PET SUV is highly predictive of overall survival in esophageal cancer. J Nucl Med Radiat Ther 2012;3 : 125.
34. Weber WA, et al. Prediction of response to preoperative chemotherapy in adenocarcinomas of the esophagogastric junction by metabolic imaging. J Clin Oncol 2001; 9 : 3058–65.
35. Zum Buschenfelde CM, et al. (18)F-FDG PET-guided salvage neoadjuvant radiochemotherapy of adenocarcinoma of the esophagogastric junction: the MUNICON II trial. J Nucl Med 2011;52 : 1189–96.
Štítky
Chirurgie všeobecná Ortopedie Urgentní medicína
Článek Phyllodes breast tumors
Článek vyšel v časopiseRozhledy v chirurgii
Nejčtenější tento týden
2015 Číslo 1- Metamizol jako analgetikum první volby: kdy, pro koho, jak a proč?
- Nejlepší kůže je zdravá kůže: 3 úrovně ochrany v moderní péči o stomii
- Stillova choroba: vzácné a závažné systémové onemocnění
- Metamizol v léčbě různých bolestivých stavů – kazuistiky
-
Všechny články tohoto čísla
- 100 let od narození prof. Josefa Nováka
- Strategies of treatment of chest wall tumors and our experience
- Atlas anatomie člověka I. Končetiny, stěna trupu
- Breast cancer at the 1st Surgical Department, University Hospital Olomouc − assessing the number and age of patients and benefit of breast screening
- K diskuzi o sekcích České chirurgické společnosti
- Intramural haematoma of the oesophagus – a case report and literature review
- Swimming pool suction injury: etiology, profylaxis and management
- Zápis z jednání schůze Redakční rady časopisu Rozhledy v chirurgii, konané dne 10. 12. 2014
- Phyllodes breast tumors
- The benefit of PET/CT in the diagnosis and treatment of esophageal cancer
- Rozhledy v chirurgii
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Phyllodes breast tumors
- Strategies of treatment of chest wall tumors and our experience
- Intramural haematoma of the oesophagus – a case report and literature review
- Swimming pool suction injury: etiology, profylaxis and management
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



