-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Prenatal Treatment for Serious Neurological Sequelae of Congenital Toxoplasmosis: An Observational Prospective Cohort Study
Background:
The effectiveness of prenatal treatment to prevent serious neurological sequelae (SNSD) of congenital toxoplasmosis is not known.Methods and Findings:
Congenital toxoplasmosis was prospectively identified by universal prenatal or neonatal screening in 14 European centres and children were followed for a median of 4 years. We evaluated determinants of postnatal death or SNSD defined by one or more of functional neurological abnormalities, severe bilateral visual impairment, or pregnancy termination for confirmed congenital toxoplasmosis. Two-thirds of the cohort received prenatal treatment (189/293; 65%). 23/293 (8%) fetuses developed SNSD of which nine were pregnancy terminations. Prenatal treatment reduced the risk of SNSD. The odds ratio for prenatal treatment, adjusted for gestational age at maternal seroconversion, was 0.24 (95% Bayesian credible intervals 0.07–0.71). This effect was robust to most sensitivity analyses. The number of infected fetuses needed to be treated to prevent one case of SNSD was three (95% Bayesian credible intervals 2–15) after maternal seroconversion at 10 weeks, and 18 (9–75) at 30 weeks of gestation. Pyrimethamine-sulphonamide treatment did not reduce SNSD compared with spiramycin alone (adjusted odds ratio 0.78, 0.21–2.95). The proportion of live-born infants with intracranial lesions detected postnatally who developed SNSD was 31.0% (17.0%–38.1%).Conclusion:
The finding that prenatal treatment reduced the risk of SNSD in infected fetuses should be interpreted with caution because of the low number of SNSD cases and uncertainty about the timing of maternal seroconversion. As these are observational data, policy decisions about screening require further evidence from a randomized trial of prenatal screening and from cost-effectiveness analyses that take into account the incidence and prevalence of maternal infection.
: Please see later in the article for the Editors' Summary
Published in the journal: . PLoS Med 7(10): e32767. doi:10.1371/journal.pmed.1000351
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1000351Summary
Background:
The effectiveness of prenatal treatment to prevent serious neurological sequelae (SNSD) of congenital toxoplasmosis is not known.Methods and Findings:
Congenital toxoplasmosis was prospectively identified by universal prenatal or neonatal screening in 14 European centres and children were followed for a median of 4 years. We evaluated determinants of postnatal death or SNSD defined by one or more of functional neurological abnormalities, severe bilateral visual impairment, or pregnancy termination for confirmed congenital toxoplasmosis. Two-thirds of the cohort received prenatal treatment (189/293; 65%). 23/293 (8%) fetuses developed SNSD of which nine were pregnancy terminations. Prenatal treatment reduced the risk of SNSD. The odds ratio for prenatal treatment, adjusted for gestational age at maternal seroconversion, was 0.24 (95% Bayesian credible intervals 0.07–0.71). This effect was robust to most sensitivity analyses. The number of infected fetuses needed to be treated to prevent one case of SNSD was three (95% Bayesian credible intervals 2–15) after maternal seroconversion at 10 weeks, and 18 (9–75) at 30 weeks of gestation. Pyrimethamine-sulphonamide treatment did not reduce SNSD compared with spiramycin alone (adjusted odds ratio 0.78, 0.21–2.95). The proportion of live-born infants with intracranial lesions detected postnatally who developed SNSD was 31.0% (17.0%–38.1%).Conclusion:
The finding that prenatal treatment reduced the risk of SNSD in infected fetuses should be interpreted with caution because of the low number of SNSD cases and uncertainty about the timing of maternal seroconversion. As these are observational data, policy decisions about screening require further evidence from a randomized trial of prenatal screening and from cost-effectiveness analyses that take into account the incidence and prevalence of maternal infection.
: Please see later in the article for the Editors' SummaryIntroduction
Congenital toxoplasmosis occurs when a woman first acquires Toxoplasma gondii infection during pregnancy. Infection can be acquired from oocysts ingested from contaminated soil or water, or tissue cysts from infected meat, but can only reliably be detected by seroconversion (change from negative to positive toxoplasma-specific antibodies) [1],[2]. Overall, the proportion of mothers who transmit infection to their fetus averages 25% but increases steeply with the gestational age at maternal seroconversion [3],[4]. Congenital toxoplasmosis leads to postnatal clinical manifestations of retinochoroiditis and/or intracranial lesions in one in six (17%) infected infants [3], and further eye lesions can appear at any age [5]. Less is known about the risk of neurological impairment, even though the main purpose of prenatal screening is to prevent serious neurological sequelae or death (SNSD) [3]. No prospective, comparative studies have evaluated the effectiveness of prenatal treatment for reducing SNSD.
We used variation in screening practices across Europe to determine the effect of prenatal treatment on SNSD. Our data derive from two prospective cohort studies in 14 centres in six European countries [6],.
Methods
Study Population
We studied infected fetuses that were prospectively identified by universal prenatal screening and newborn screening for congenital toxoplasmosis [4],[6]–[8]. Criteria for congenital toxoplasmosis are reported elsewhere [4],[6]. To avoid referral bias, we included only mothers or children in whom a positive screen test result preceded prenatal treatment or diagnostic investigations.
Follow-Up
Infected fetuses were enrolled into the study after confirmation of a positive prenatal screening test in France, Austria, and Italy, or neonatal screening test in Denmark, Sweden, or Poland. Screening and treatment schedules are summarized in Tables 1 and 2. Information on clinical and laboratory findings and treatment were collected at the end of pregnancy, at 1 mo postnatally, and at every pediatric and ophthalmic examination for toxoplasmosis at approximately 6 and 12 mo, and then annually until at least 4 y of age [5],[6].
Tab. 1. Characteristics of universal prenatal screening protocols and patients in study centres. 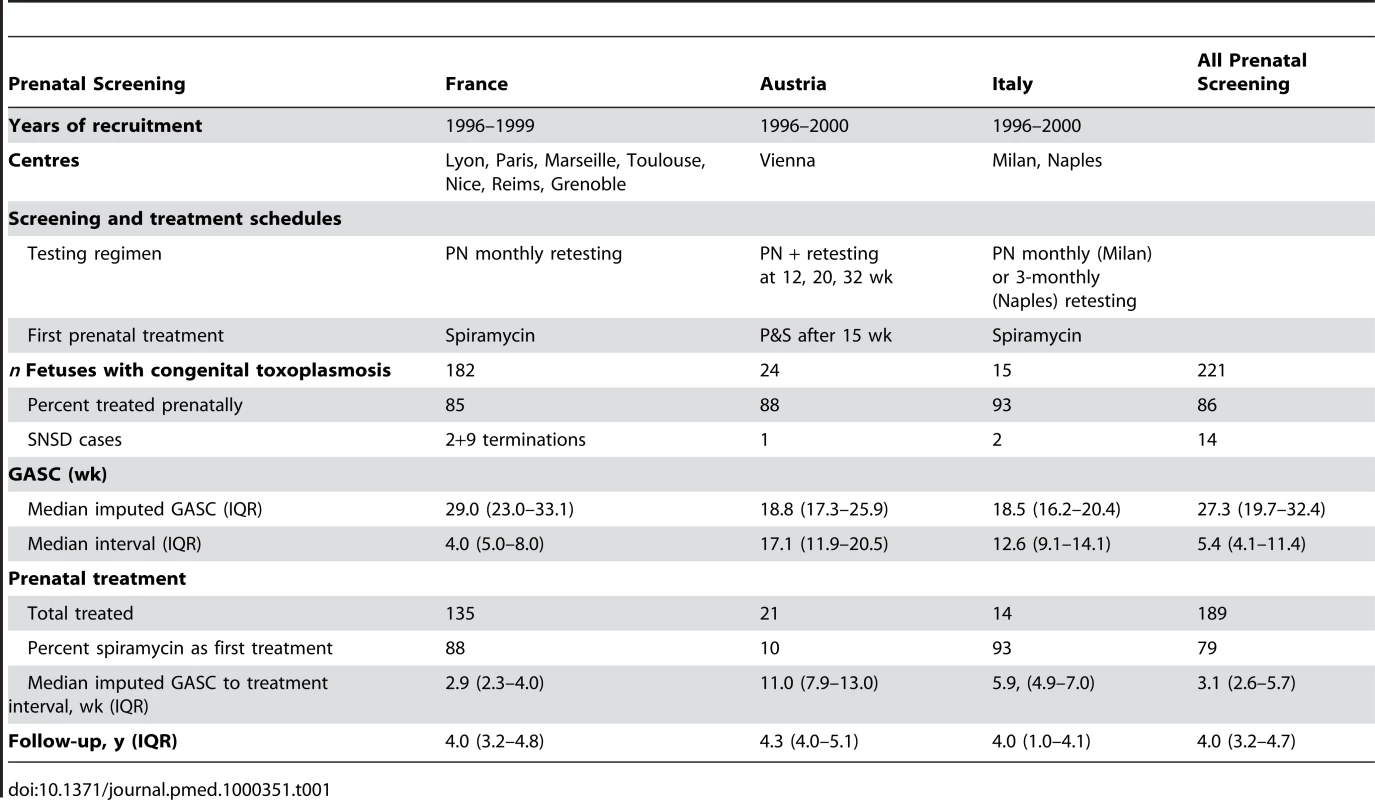
Tab. 2. Characteristics of neonatal screening protocols and patients in study centres. 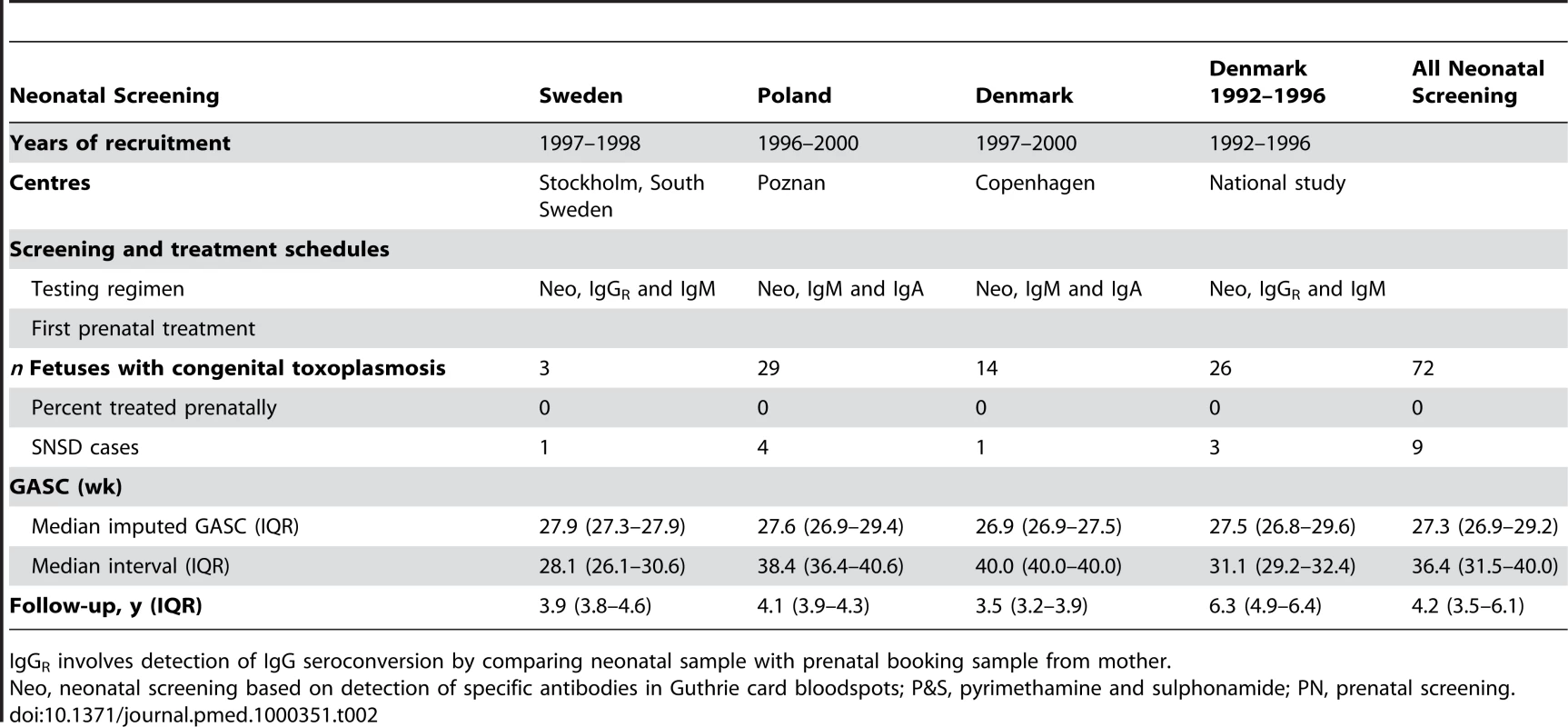
IgGR involves detection of IgG seroconversion by comparing neonatal sample with prenatal booking sample from mother. Details recorded during pregnancy included the date of the first abnormal or last normal fetal ultrasound scan, the results of PCR testing of amniotic fluid for toxoplasma DNA, autopsy findings for terminated fetuses, and the start and end dates of any prenatal treatment. Mothers of infants detected in neonatal screening centres were not treated. The variation between centres in the delay before starting prenatal treatment after maternal seroconversion and in the use of spiramycin or pyrimethamine-sulphonamide combinations as first-line treatment is shown in Table 1 for infected babies identified by universal prenatal screening and in Table 2 for babies identified by neonatal screening, and reported in detail elsewhere [6]. All centres treated infected babies postnatally, but there were minor differences in the use of pyrimethamine-sulphonamide alone or alternating with spiramycin [6].
Serious Neurological Sequelae
The primary outcome was serious neurological sequelae or death (SNSD), a composite outcome, comprising a pediatric report at any age of microcephaly, insertion of intraventricular shunt, an abnormal or suspicious neurodevelopmental examination that resulted in referral to a specialist, seizures during infancy or at an older age that required anticonvulsant treatment, severe bilateral visual impairment (visual acuity of Snellen 6/60 or less in both eyes assessed after 3 y), cerebral palsy, or death from any cause before 2 y of age including termination of pregnancy [9]. The consistency of SNSD findings was checked through multiple assessments. We did not require evidence that SNSD was attributable to congenital toxoplasmosis as this difficult clinical judgment could have biased inclusion of cases. Fetuses terminated for congenital toxoplasmosis were assumed to have SNSD, owing to long-standing policy discouraging termination unless there is evidence of intracranial lesions or other adverse sequelae affecting the fetus [10]–[12]. This assumption allows a conservative analysis of the effect of prenatal treatment as women were always treated before termination. Sensitivity analyses explored the increase in treatment effect after assuming no SNSD for terminated fetuses.
Gestational Age at Maternal Seroconversion
In order to avoid well-known biases introduced by imputing a covariate as an unadjusted midpoint for interval values of the gestational age at maternal seroconversion (GASC), we imputed values for GASC using all the serological information available to us [13]. For women screened prenatally, we imputed seroconversion at the midpoint between the last negative and first positive immunoglobulin M (IgM) tests, unless the woman was IgG negative at the first IgM positive test, when seroconversion was assumed to be 14 d beforehand. This method was derived from a previous analysis of a cohort from Lyon, France [14]–[16], and has been confirmed with subsequent cohorts [6].
For babies identified by neonatal screening, maternal seroconversion was based on an adjusted midpoint between conception, or in Sweden and Denmark 1992–1996, between the first prenatal booking sample and birth, using a previously reported log linear regression model [5]. The model was derived in the cohort of prenatal screened women and used ranked IgG titre and IgM status at the first neonatal test as predictors of GASC. These modifications shifted the imputed GASC to the right of the midpoint. This shift is consistent with evidence that the probability of a positive IgM result in an infected baby increases with GASC and that increased IgG titre is associated with recent infection [17],[18].
Analyses
All live births had at least one pediatric assessment during infancy (up till 12 mo old). Children with a normal pediatric examination during infancy who were subsequently lost to follow-up were assumed to have no SNSD [19].
We used WinBUGS version 1.4.3 to estimate odds ratios for the effect of prenatal exposures on SNSD. All models were adjusted for GASC [5]. The Bayesian framework was chosen because there were numerical problems, caused by the low event rate and the relatively small sample size, in the optimization algorithms required to find maximum likelihood estimates, which meant that the asymptotic normal approximation required to construct confidence intervals was unreliable. The Bayesian framework avoids relying on the large-sample normal approximation and has the advantage of evaluating the uncertainty from derived, nonlinear quantities without further using permutational or resampling methods. An example of this type of propagation of errors can be seen in the credible intervals presented in Figure 1. Our inferences were based on 95% Bayesian credible intervals (BCIs) referring to the posterior distributions of the models' parameters and derived quantities. All models were adjusted for gestational age at seroconversion, as previously reported [5].
Fig. 1. Probability of SNSD. 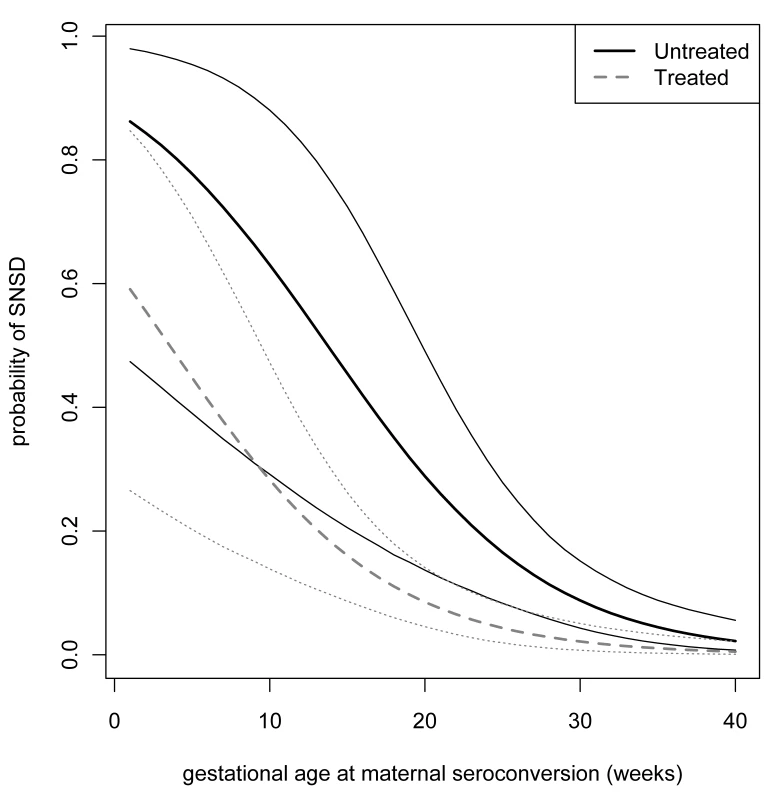
Probability of SNSD according to imputed gestational age at seroconversion and 95% Bayesian credible limits: dotted lines denote treated pregnancies; solid lines denote untreated pregnancies. The posterior probability distributions of SNSD for each level of exposure were calculated using Markov Chain Monte Carlo (MCMC) iterations. We used noninformative priors in two mixing Markov chains with different starting values and allowed 1,000 MCMC burn-in iterations; estimates of all posterior distributions were based on 10,000 realizations of each chain. A linear transformation was applied to the imputed values of gestational age at maternal seroconversion by centering around its mean; this improved the MCMC convergence, assessed by the method proposed by Brooks and Gelman [20], and made the autocorrelation functions close to zero. Inclusion of a quadratic term for GASC did not improve the goodness of fit according to the Deviance Information Criterion [21]. This method produced similar results for the effect of prenatal treatment to a previously used maximum likelihood estimation procedure [6].
Sensitivity analyses were used to determine the robustness of the findings to different assumptions. First we explored under-reporting of terminations by excluding data from Austria and Italy, as no terminations were reported for these centres. We also determined whether adding 18 unreported terminated fetuses to the analyses would alter the treatment effect. The 18 unreported fetuses had the same characteristics (GASC and prenatal treatment delay) as the nine terminated fetuses included in the study. Second, we estimated the effect of treatment under the extreme assumption that all terminated fetuses would have been born alive and would have developed normally. We revised this analysis assuming that the three fetuses with normal fetal ultrasound scans and no evidence of disseminated infection would have developed normally, even though two fetuses had their last scan before 21 wk of gestation. Third, we reclassified three live-born, untreated children with SNSD but without intracranial calcification as no SNSD. This reclassification restricted the outcome of SNSD to cases almost certainly attributable to congenital toxoplasmosis. Fourth, we explored potential error in the imputed gestational age at maternal seroconversion for children identified by neonatal screening by assuming seroconversion 1 mo earlier.
We calculated the number of women needed to be treated (NNT) to prevent one case of SNSD as a clinically meaningful measure for counseling women about the absolute difference in the probability of their child developing SNSD with and without treatment. We used the posterior probability distributions and 95% credible interval calculated from the regression model to estimate the NNT for women after a positive prenatal diagnosis (i.e., the number of women with an infected fetus who need to be treated). To estimate the NNT for women without prenatal diagnosis, we multiplied the difference in probability with the estimated risk of mother to child transmission of toxoplasmosis derived from a meta-analysis of all available cohort studies [3]. This simple estimation was based on the assumption that prenatal treatment has no effect on mother to child transmission. We did not take into account random error in the estimation of mother to child transmission according to GASC.
To inform prognostic counseling for parents of live-born babies, we determined the probability of SNSD according to an exclusive hierarchy of postnatal clinical manifestations (ventricular dilatation and/or intracranial calcification, lymphadenopathy or hepatosplenomegaly, and/or retinochoroiditis) that would be apparent after pediatric examination, ophthalmoscopy, and postnatal cranial ultrasound completed by 6 mo postnatal age [1]. These analyses excluded the Danish cohort of 1992 to 1996 (n = 26) owing to missing data for these early manifestations [7].
Research ethics approval was granted in countries where screening was offered as part of a research study [7],[22],[23], but was not required where screening was routine practice.
Results
Study Population and Neurological Sequelae
The combined cohorts comprised 293 infected fetuses, of whom 284 were born alive. 23 fetuses consisting of nine terminated pregnancies and 14 live-born children died or were classified as having serious neurological sequelae (Tables 1–4).The distribution of GASC and the intervals from which GASC was imputed are summarized in Tables 1 and 2, and Figures S1 and S2. All live-born children had at least one pediatric examination. The last examination was before 6 mo of age for 6/284 (2%) children and between 9 and 12 mo for a further six children. 238/284 (84%) children were followed beyond 2 y of age. Overall, the median duration of follow-up was 4.05 y (interquartile range [IQR] 3.21–4.88). Follow-up was longer for 26 children enrolled in the Danish cohort in 1992–1996 (6.3 y, IQR 4.9–6.4) (Table 2), but did not differ significantly between live-born children whose mothers were treated (4.05, IQR 3.25–4.76) compared with not treated (4.13, IQR 3.17–5.19, Wilcoxon-Mann-Whitney test p = 0.39).
Tab. 3. Characteristics of live births with congenital toxoplasmosis and SNSD. 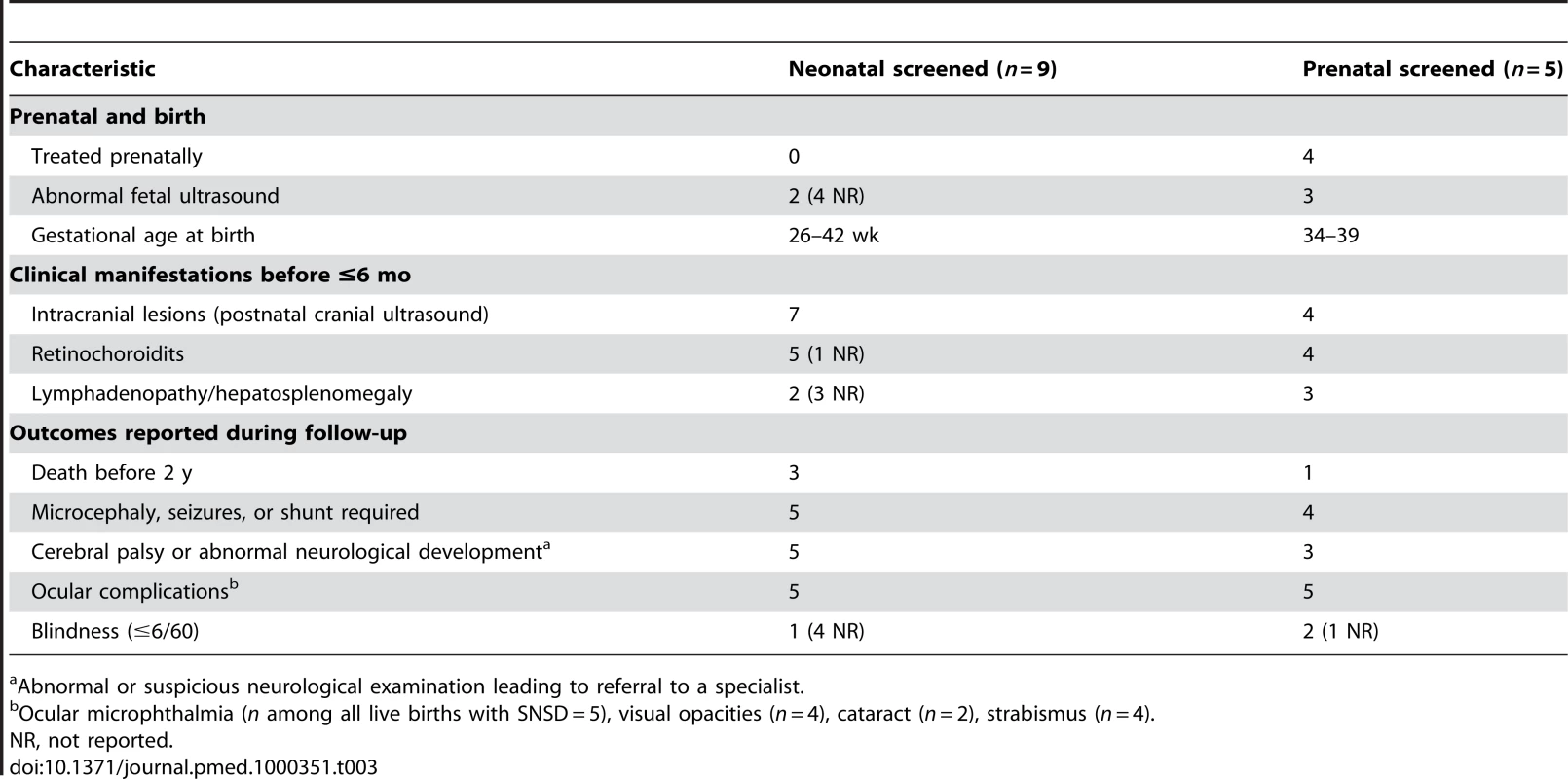
Abnormal or suspicious neurological examination leading to referral to a specialist. Tab. 4. Characteristics of fetuses terminated with congenital toxoplasmosis. 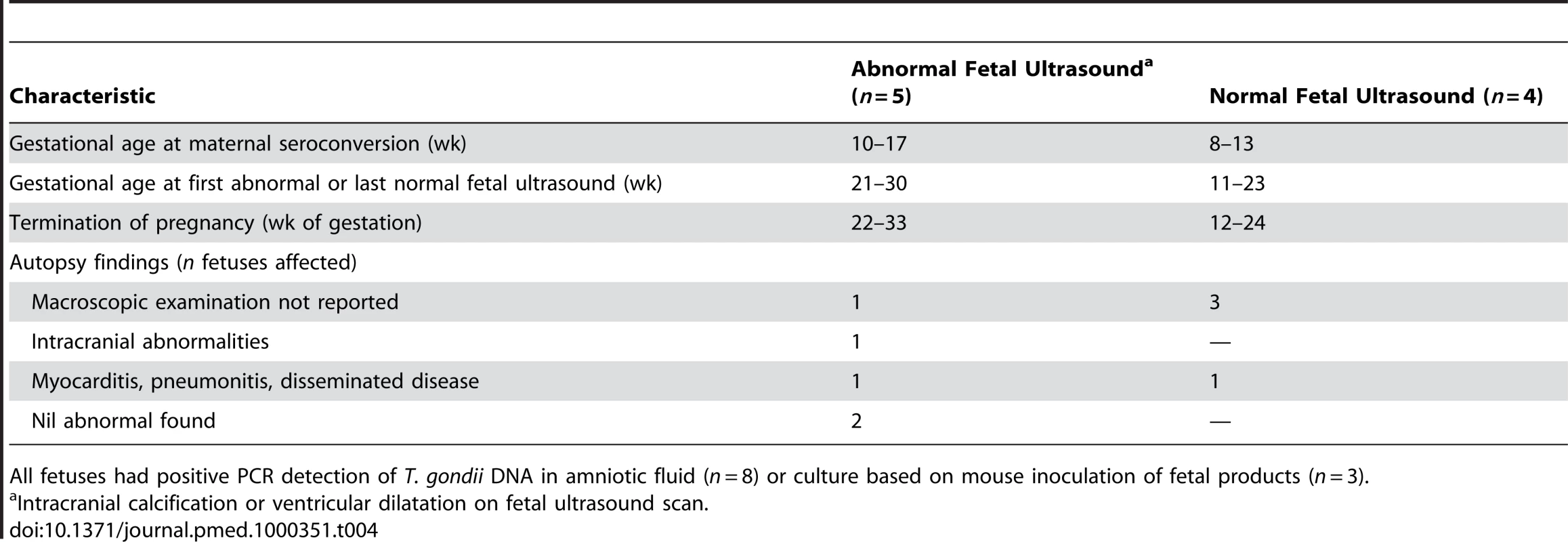
All fetuses had positive PCR detection of T. gondii DNA in amniotic fluid (n = 8) or culture based on mouse inoculation of fetal products (n = 3). Of the 221 women identified by prenatal screening, 86% (189/221) received prenatal treatment, most (71%, 134/189) within 5 wk of the imputed date of maternal seroconversion (Table 4). Untreated women (n = 32) identified by prenatal screening had their first positive serological test at a median gestational age of 38.6 wk (IQR 37.4–40.0 wk). None of the 72 women with infected live-born infants identified by neonatal screening received prenatal treatment (Table 2).
The characteristics of the 23 fetuses with SNSD are summarized in Tables 3 and 4. Among the 14 live births, most (11/14) had SNSD first detected in the first 6 mo of life. Three untreated, live-born children had no intracranial lesions detected postnatally. Two of the three were identified by neonatal screening. One had no ocular manifestation but was classified with SNSD because the baby died at 3 mo from staphylococcal septicemia. The other two had ocular signs: one had retinochoroiditis and strabismus and the other had a cataract. Less information was available for the nine terminated fetuses (Table 4): six had intracranial abnormalities on fetal ultrasound and/or macroscopic abnormalities at autopsy.
Characteristics Associated with Serious Neurological Sequelae
Three prenatal factors strongly predicted serious neurological sequelae: the gestational age at maternal seroconversion, prenatal treatment, and an abnormal fetal ultrasound of the brain (Table 4). The odds of SNSD, adjusted for prenatal treatment, decreased by 13% (95% BCI 7%–19%) for every week increase in gestational age at maternal seroconversion (odds ratio 0.866, 95% BCI 0.806–0.925) (Table 5).
Tab. 5. Prenatal characteristics associated with SNSD. 
Adjusted for gestational age at maternal seroconversion. Prenatal treatment substantially reduced the risk of serious neurological sequelae. The adjusted odds ratio for any prenatal treatment compared with no treatment was 0.236 (95% BCI 0.071–0.708), and the average risk difference between treated and untreated mothers was 8.7% (2.0%–18.1%) (Table 5). In infected fetuses, the absolute risk difference between SNSD in treated and untreated pregnancies declined steeply with the gestational age at maternal seroconversion (Figure 1). After maternal seroconversion at 10 wk of gestation, the estimated risk of SNSD in fetuses of treated women was 25.7% (12.9%–43.0%) and in untreated women 60.0% (27.6%–85.9%). The risk difference was 33.3% (6.9%–56.1%), and the NNT of mothers with infected fetuses to prevent one case of SNSD was 3 (2–15). After seroconversion at 20 wk, the risk difference was 18.5 (3.6–38.3, NNT 6 [3]–[28]), and after seroconversion at 30 wk, the risk difference was 5.7 (1.3–11.5, NNT 18 [9–75]). The NNT to prevent one case of SNSD for women who do not know whether their fetus is infected or not was estimated to be 28 (17–132) after seroconversion at 10 wk of gestation; 20 (10–99) at 20 wk, the lowest point; 32 (16–137) at 30 wk; and 51 (23–201) after seroconversion at 35 wk of gestation. These estimates apply only to women with confirmed seroconversion.
Table 6 shows that sensitivity analyses did not alter the finding of a significant treatment effect at the 5% level, except when three untreated live-born children with SNSD but no intracranial lesions were reclassified without SNSD. The adjusted odds ratio for any prenatal treatment compared with none was reduced in analyses that excluded Austria and Italy and when terminated fetuses were reclassified without SNSD (Table 6). The addition of a further 18 fictitious, unreported pregnancy terminations with SNSD marginally reduced the treatment effect. Assuming maternal seroconversion 1 mo earlier in cases identified by neonatal screening also increased the odds ratio, but the upper 95% credible interval still excluded 1.0 (Table 6).
Tab. 6. Sensitivity analyses (odds ratios for SNSD and 95% BCIs). 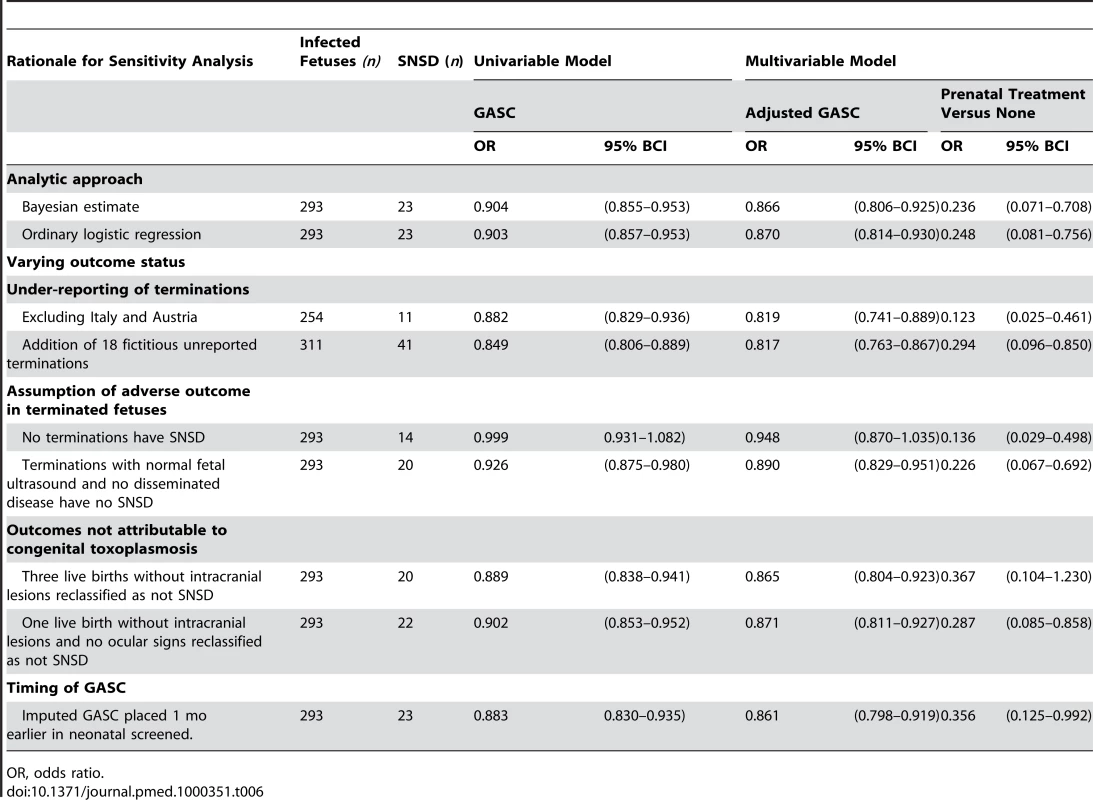
OR, odds ratio. The nine terminated pregnancies in France made up most of the SNSD cases that seroconverted in the first trimester (Figures S1 and S2). As a result, the effect of GASC on SNSD diminished and was no longer significant at the 5% level when all terminations were reclassified without SNSD (Table 6).
Among treated women, we found no evidence that delayed timing of treatment increased the proportion of fetuses with SNSD. However, the power to detect such an effect was limited. Moreover, most of the early treated fetuses with SNSD were pregnancy terminations (Table 4). There was no evidence that pyrimethamine-sulphonamide treatment was clinically or statistically more beneficial than spiramycin alone, although there was limited power to detect an effect at the 5% level (Table 5).
Fetal ultrasound abnormality was associated with SNSD (adjusted odds ratio 120, 7.04–6400), partly because this was a criterion for pregnancy termination, and hence classification with SNSD. The earliest gestational age at detection of intracranial abnormality on fetal ultrasound was 21 wk in five terminated fetuses and 26 wk in five live-born fetuses (three with SNSD). All five live-born fetuses with intracranial abnormality on fetal ultrasound had intracranial calcification and/or ventricular dilatation on postnatal cranial ultrasound (specificity 178/178, 100%), but few babies with abnormal postnatal scans had intracranial abnormalities reported on fetal ultrasound (5/18, sensitivity 28%).
Table 7 shows the distribution of serious neurological sequelae according to postnatal clinical manifestations detected in early infancy in live-born children with congenital toxoplasmosis. The proportion of children with serious neurological sequelae was higher among those with intracranial lesions detected by postnatal cranial ultrasound scan (30.2%, 95% BCI 13.7%–46.6%) than in those with no intracranial lesions (1.0%, 0.0%–2.3%).
Tab. 7. Postnatal clinical manifestations detected in early infancy and probability (%) of SNSD. 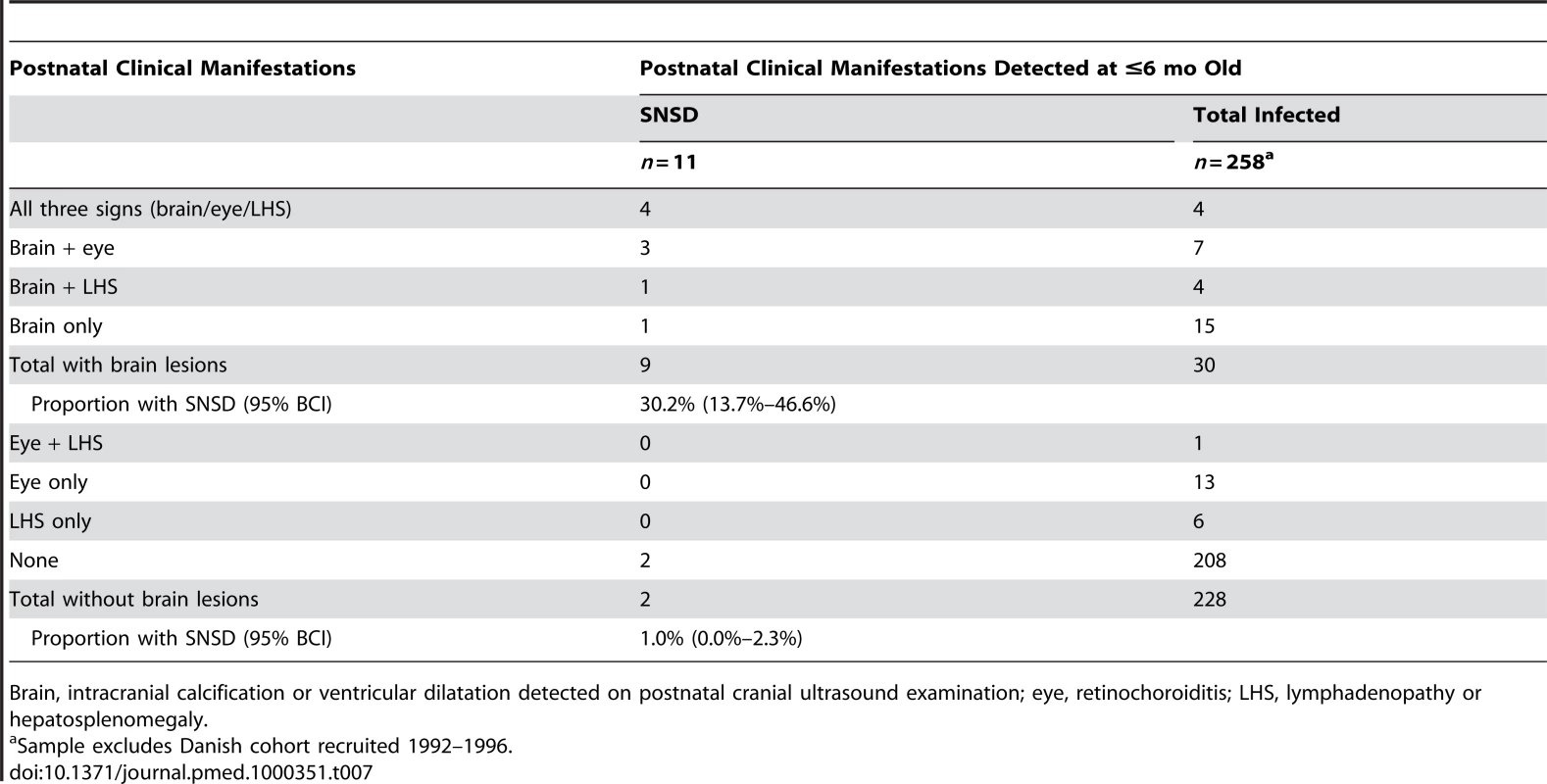
Brain, intracranial calcification or ventricular dilatation detected on postnatal cranial ultrasound examination; eye, retinochoroiditis; LHS, lymphadenopathy or hepatosplenomegaly. Discussion
Prenatal treatment substantially reduced the proportion of infected fetuses who developed SNSD. We found no evidence that a pyrimethamine-sulphonamide combination was more effective than spiramycin, which is less toxic [24]. Among infected fetuses, the difference in the proportion of treated and untreated fetuses with SNSD was highest when maternal infection was acquired during the first trimester. These findings should be interpreted with caution because of the low number of the SNSD cases and the uncertainty about the timing of maternal seroconversion. Abnormalities on fetal ultrasound, and intracranial abnormalities detected by cranial ultrasound after birth, were important prognostic markers for SNSD.
This is the first prospective cohort study, to our knowledge, to report the effect of prenatal treatment on serious neurological sequelae in fetuses with congenital toxoplasmosis. We minimized selective inclusion of pregnancies with complications ensuring that universal screening tests preceded prenatal treatment or diagnostic investigations for fetal infection status or abnormalities. Long-term follow-up and repeated pediatric assessments made it possible to ensure that, for the live births at least, SNSD were confirmed. In a retrospective study, Foulon et al. reported a similarly large effect of prenatal treatment on intracranial lesions and/or neurological sequelae detected up to 12 mo of age, but these results could have been explained by referral of untreated women with pregnancy complications to fetal medicine centres [25],[26].
Weaknesses of the study relate to selection biases inherent in observational studies. First, pregnancies terminated for fetal infection may not have been reported to the study [27], but sensitivity analyses that assumed twice as many treated women had “unreported” terminations did not nullify the treatment effect. Second, we did not include stillbirths in the analysis as these rarely have a definitive diagnosis of congenital toxoplasmosis [28], which could have underestimated the benefits of prenatal treatment if treatment led to the survival of fetuses with SNSD who would have miscarried or been stillborn in untreated women. Third, our method for estimating the gestational age at maternal seroconversion may have overestimated precision and could have introduced bias [13]. Fourth, we assumed that all SNSD outcomes were attributable to congenital toxoplasmosis. Reclassification of three children with SNSD but without intracranial lesions to non-SNSD rendered the effect of prenatal treatment nonsignificant at the 5% level. Fifth, adverse outcomes were limited to serious manifestations that were evident on pediatric examination in the early childhood years. Overall, these potential biases act in different directions and are likely to only partly account for the strong treatment effect observed. However, they may alter the magnitude of the effect, which can only be reliably determined by a large randomized controlled trial.
Implications for Practice
The benefits of prenatal treatment are high for women with a positive prenatal diagnosis for congenital toxoplasmosis. The NNT to prevent one case of SNSD varies from three to 18 depending on the gestational age at maternal seroconversion. As fetal diagnosis carries a risk of fetal loss due to amniocentesis, some women may prefer to trade a lower chance of benefit in order to avoid amniocentesis, provided a treatment such as spiramycin is used, which has no serious side effects [24]. The lowest estimate of the number of women with no prenatal diagnosis and unknown congenital infection status of their fetus who need to be treated to prevent one case of SNSD was 20 (ten to 99) after maternal seroconversion at 20 wk of pregnancy, rising to 28 (17 to 132) after seroconversion at 10 wk and 51 (23, 201) at 35 wk of gestation. These estimates apply only to women with confirmed seroconversion. The NNT would be much higher for women whose infection is diagnosed by tests for recent infection such as a rising titre, or low IgG avidity, as most of these women would have acquired infection before conception [4],[29].
Termination of pregnancy should be limited to fetuses with abnormal intracranial ultrasound findings, otherwise the vast majority of terminations would involve unaffected fetuses; this means deferring termination until 22 wk or later as ultrasound abnormalities do not develop until 21 wk of gestation at the earliest [30]. Termination at this late gestation involves feticide and is unlikely to be acceptable to many women or clinicians.
In terms of the type of treatment, our findings add to previous comparative studies, which have consistently found no evidence that pyrimethamine-sulphonamide combinations are more effective than the less toxic alternative of spiramycin [3],[4],[6],[15],[16],[25]. However, our study lacked power to detect an effect on SNSD.
Whether the benefits of prenatal treatment translate into an effective prenatal screening program remains to be determined by a randomised controlled trial of prenatal screening. In the meantime, cost-effectiveness analyses that take into account regional variation in the prevalence of susceptible women, the incidence of maternal infection, the timing, uptake, and accuracy of repeated screening tests to detect maternal seroconversion, and the timing of prenatal treatment, could provide valuable information for policy makers and for research funders contemplating investment in a large trial.
Finally, our results relate to the relatively benign type II strain of T. gondii, which predominates in Europe and North America. Trials are urgently needed to determine the most effective timing and type of prenatal treatment for the more virulent parasite strains that predominate in South America [31].
Supporting Information
Zdroje
1. RemingtonJS
McLeodR
ThulliezP
DesmontsG
2006 Toxoplasmosis.
RemingtonJS
KleinJ
WilsonCB
BakerCJ
Infectious disease of the fetus and newborn infant Philadelphia Elsevier Saunders 947 1092
2. Ferreira-da-SilvaMF
TakacsAC
BarbosaHS
GrossU
LuderCG
2009 Primary skeletal muscle cells trigger spontaneous Toxoplasma gondii tachyzoite-to-bradyzoite conversion at higher rates than fibroblasts. Int J Med Microbiol 299 381 388
3. ThiebautR
LeproustS
CheneG
GilbertRE
The SYROCOT Study Group 2007 Effectiveness of prenatal treatment for congenital toxoplasmosis: a meta-analysis of individual patients' data. Lancet 369 115 122
4. GilbertR
GrasL
2003 Effect of timing and type of treatment on the risk of mother to child transmission of Toxoplasma gondii. BJOG 110 112 120
5. FreemanK
TanHK
PrusaA
PetersenE
BuffolanoW
2008 Predictors of retinochoroiditis in children with congenital toxoplasmosis: European, prospective cohort study. Pediatrics 121 e1215 e1222
6. GrasL
WallonM
PollakA
Cortina-BorjaM
EvengardB
2005 Association between prenatal treatment and clinical manifestations of congenital toxoplasmosis in infancy: a cohort study in 13 European centers. Acta Paediatrica Scandinavia 94 1721 1731
7. LebechM
AndersenO
ChristensenNC
HertelJ
NielsenHE
1999 Feasibility of neonatal screening for toxoplasma infection in the absence of prenatal treatment. Lancet 353 1834 1837
8. PaulM
PetersenE
SzczapaJ
2001 Prevalence of congenital Toxoplasma gondii infection among newborns from the Poznan region of Poland: validation of a new combined enzyme immunoassay for Toxoplasma gondii-specific immunoglobulin A and immunoglobulin M antibodies. J Clin Microbiol 39 1912 1916
9. TanHK
SchmidtD
StanfordM
Tear-FahnehjelmK
FerretN
2007 Risk of visual impairment in children with congenital toxoplasmic retinochoroiditis. Am J Ophthalmol 144 648 653
10. BerrebiA
KobuchWE
BessieresMH
BloomMC
RollandM
1994 Termination of pregnancy for maternal toxoplasmosis. Lancet 344 36 39
11. Gay-AndrieuF
MartyP
PialatJ
SourniesG
DrierdL
2003 Fetal toxoplasmosis and negative amniocentesis: necessity of an ultrasound follow-up. Prenat Diagn 23 558 560
12. BerrebiA
BardouM
BessieresMH
NowakowskaD
CastagnoR
2007 Outcome for children infected with congenital toxoplasmosis in the first trimester and with normal ultrasound findings: a study of 36 cases. Eur J Obstet Gynecol Reprod Biol 135 53 57
13. GomezG
EspinalA
LagakosW
2003 Inference for a linear regression model with an interval-censored covariate. Stat Med 22 409 425
14. DunnD
WallonM
PeyronF
PetersenE
PeckhamCS
1999 Mother to child transmission of toxoplasmosis: risk estimates for clinical counselling. Lancet 353 1829 1833
15. GilbertRE
GrasL
WallonM
PeyronF
AdesAE
2001 Effect of prenatal treatment on mother to child transmission of Toxoplasma gondii: a cohort study of 554 mother-child pairs in Lyon, France. Int J Epidemiol 30 1303 1308
16. GrasL
GilbertRE
AdesAE
DunnDT
2001 Effect of prenatal treatment on the risk of intracranial and ocular lesions in children with congenital toxoplasmosis. Int J Epidemiol 30 1309 1330
17. WallonM
DunnD
SlimaniD
GiraultV
Gay-AndrieuF
1999 Diagnosis of congenital toxoplasmosis at birth: what is the value of testing for IgM and IgA? Eur J Pediatr 158 645 649
18. GilbertRE
ThalibL
TanHK
PaulM
WallonM
2007 Screening for congenital toxoplasmosis: accuracy of immunoglobulin M and immunoglobulin A tests after birth. J Med Screen 14 8 13
19. HeinemanKR
Hadders-AlgraM
2008 Evaluation of neuromotor function in infancy-A systematic review of available methods. J Dev Behav Pediatr 29 315 323
20. BrooksS
GelmanA
1998 General methods for monitoring convergence of iterative simulations. J Comput Graph Stat 7 434 455
21. SpiegelhalterD
BestN
CarlinB
van der LindeA
2002 Bayesian measures of complexity and fit. J R Stat Soc Series B Stat Methodol 583 639
22. PaulM
PetersenE
PawlowskiZS
SzczapaJ
2000 Neonatal screening for congenital toxoplasmosis in the Poznan region of Poland by analysis of Toxoplasma gondii-specific IgM antibodies eluted from filter paper blood spots. Pediatr Infect Dis J 19 30 36
23. EvengardB
PettersonK
EngmanM-L
WiklundS
IvarssonSA
2001 Low incidence of toxoplasma infection during pregnancy and in newborns in Sweden. Epidemiol Infect 127 121 127
24. DaveluyA
HaramburuF
BricoutH
CostanzoMC
FourrierA
2005 Review of data related to side effects of drugs used in congenital toxoplasmosis. Panel 2. [Unpublished report]. Bordeaux (France): The Eurotoxo Group. Available at http://eurotoxo.isped.u-bordeaux2.fr/WWW_PUBLIC/DOC/Side_effects_main_drugs_v3.pdf. Accessed 10 September 2010
25. FoulonW
VillenaI
Stray-PedersenB
DecosterA
LappalainenM
1999 Treatment of toxoplasmosis during pregnancy: a multicentre study of impact on fetal transmission and children's sequelae at age 1 year. Am J Obstet Gynecol 180 410 415
26. GilbertRE
PeckhamCS
2002 Congenital toxoplasmosis in the United Kingdom: to screen or not to screen? J Med Screen 9 135 141
27. HernanMA
Hernandez-DiazS
WerlerMM
MitchellAA
2002 Causal knowledge as a prerequisite for confounding evaluation: an application to birth defects epidemiology. Am J Epidemiol 155 176 184
28. FreemanK
OakleyL
PollakA
BuffolanoW
PetersenE
2004 Congenital toxoplasmosis and preterm birth, low birth weight, and small for gestational age birth. BJOG 112 31 37
29. GrasL
GilbertRE
WallonM
PeyronF
Cortina-BorjaM
2004 Duration of the IgM response in women acquiring Toxoplasma gondii during pregnancy: implications for clinical practice and cross-sectional incidence studies. Epidemiol Infect 132 541 548
30. LevineD
FeldmanHA
TannusJF
EstroffJA
MagninoM
2008 Frequency and cause of disagreements in diagnoses for fetuses referred for ventriculomegaly. Radiology 247 516 527
31. GilbertRE
FreemanK
LagoEG
Bahia-OliveiraLM
TanHK
2008 Ocular sequelae of congenital toxoplasmosis in Brazil compared with Europe. PLoS Negl Trop Dis 2 e277 doi:10.1371/journal.pntd.0000277
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2010 Číslo 10- Není statin jako statin aneb praktický přehled rozdílů jednotlivých molekul
- Magnosolv a jeho využití v neurologii
- Moje zkušenosti s Magnosolvem podávaným pacientům jako profylaxe migrény a u pacientů s diagnostikovanou spazmofilní tetanií i při normomagnezémii - MUDr. Dana Pecharová, neurolog
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
- S prof. Vladimírem Paličkou o racionální suplementaci kalcia a vitaminu D v každodenní praxi
-
Všechny články tohoto čísla
- Systematic Evaluation of Serotypes Causing Invasive Pneumococcal Disease among Children Under Five: The Pneumococcal Global Serotype Project
- The Persisting Burden of Intracerebral Haemorrhage: Can Effective Treatments Be Found?
- Conflicts of Interest at Medical Journals: The Influence of Industry-Supported Randomised Trials on Journal Impact Factors and Revenue – Cohort Study
- An Urgent Need to Restrict Access to Pesticides Based on Human Lethality
- A Field Training Guide for Human Subjects Research Ethics
- Oral Ondansetron Administration in Emergency Departments to Children with Gastroenteritis: An Economic Analysis
- Being More Realistic about the Public Health Impact of Genomic Medicine
- Epigenetic Epidemiology of Common Complex Disease: Prospects for Prediction, Prevention, and Treatment
- Acute Human Lethal Toxicity of Agricultural Pesticides: A Prospective Cohort Study
- Information from Pharmaceutical Companies and the Quality, Quantity, and Cost of Physicians' Prescribing: A Systematic Review
- Increased Responsibility and Transparency in an Era of Increased Visibility
- Editors, Publishers, Impact Factors, and Reprint Income
- Reflections on Pandemic (H1N1) 2009 and the International Response
- Prenatal Treatment for Serious Neurological Sequelae of Congenital Toxoplasmosis: An Observational Prospective Cohort Study
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Epigenetic Epidemiology of Common Complex Disease: Prospects for Prediction, Prevention, and Treatment
- Editors, Publishers, Impact Factors, and Reprint Income
- Oral Ondansetron Administration in Emergency Departments to Children with Gastroenteritis: An Economic Analysis
- Systematic Evaluation of Serotypes Causing Invasive Pneumococcal Disease among Children Under Five: The Pneumococcal Global Serotype Project
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



