-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
FEASIBILITY OF RADIOIODINE DOSIMETRY USING A SMALL FIELD OF VIEW GAMMACAMERA; PILOT STUDY
Nowadays, it is crucial to deal with the dosimetry issues in the light of 2013/59 EURATOM recommendation, where it is clearly defined that dosimetry will be required even for the targeted radionuclide therapy (TRT). The aim of this pilot study was to investigate and to verify the possibilities of dosimetry for patients undergoing first Radioiodine therapy (RAIT) using a small single head mobile gamma camera.
Camera Solo mobile was used for quantitative imaging of 131I accumulation in remnants of patients' thyroids and 131I accumulating nodes (neck region). Vials with a known activity of 131I were used to calibrate the system. The patient-volunteers were around 3 months after thyreoablation due to thyroid carcinoma. The weight of the accumulating remnants or nodes was established using ultrasound or roughly estimated using phantom measurements.
The absorbed doses within remnants or nodes vary from 40 Gy up to 780 Gy with an uncertainty from 25% up to 100% depending mainly on the mass of the remnants estimation. A consequent follow-up is being done. The dose assessment could be done using cheaper small single-head gammacamera what would safe time of the standard ones. Uncertainty is supposed to be significantly reduced by further processes optimization.Keywords:
Radioiodine, therapy, dosimetry, quantitative imaging
Authors: Pavel Solný 1,2; Petr Vlček 2; Lenka Jonášová 2; Jaroslav Zimák 2
Authors place of work: Department of Dosimetry and Application of Ionizing Radiation Faculty of Nuclear Sciences and Physical Engineering, Czech Technical University in Prague, Czech Republic (DDAIR) 1; Department of Nuclear Medicine and Endocrinology 2nd Faculty of Medicine, Charles University in Prague and Motol University, Prague, Czech Republic (DNME) 2
Published in the journal: Lékař a technika - Clinician and Technology No. 3, 2015, 45, 82-87
Category: Původní práce
Summary
Nowadays, it is crucial to deal with the dosimetry issues in the light of 2013/59 EURATOM recommendation, where it is clearly defined that dosimetry will be required even for the targeted radionuclide therapy (TRT). The aim of this pilot study was to investigate and to verify the possibilities of dosimetry for patients undergoing first Radioiodine therapy (RAIT) using a small single head mobile gamma camera.
Camera Solo mobile was used for quantitative imaging of 131I accumulation in remnants of patients' thyroids and 131I accumulating nodes (neck region). Vials with a known activity of 131I were used to calibrate the system. The patient-volunteers were around 3 months after thyreoablation due to thyroid carcinoma. The weight of the accumulating remnants or nodes was established using ultrasound or roughly estimated using phantom measurements.
The absorbed doses within remnants or nodes vary from 40 Gy up to 780 Gy with an uncertainty from 25% up to 100% depending mainly on the mass of the remnants estimation. A consequent follow-up is being done. The dose assessment could be done using cheaper small single-head gammacamera what would safe time of the standard ones. Uncertainty is supposed to be significantly reduced by further processes optimization.Keywords:
Radioiodine, therapy, dosimetry, quantitative imaging1 Introduction
Currently, databases of KNME show high success rate in the first year after the radioiodine therapy (up to 97% of patients treated for the first time at KNME is during the first year in remission). However, the data indicate increasing number of repeated treatments in the range of 2 to 5 years (7-15% of the all patients). Percentage of patients undergoing repeated treatment reach 20% in the time scale from 5 to 10 years after primal therapy.
Such high number of patients treated more than once may be attributed to not completely individualized patient therapy planning. It leads to hypothesis that remains of the thyroid gland with tumor or lymph nodes do not receive a sufficient therapeutic dose. Absorbed dose satisfying therapeutic purposes should be greater than 300 Gy for thyroid residues and no less than 80 Gy for lymph nodes [1], [3], [9]. The absorbed dose to the target volume cannot be traced retrospect-tively and thus in-vivo dosimetric measurements are needed for assessment. The absorbed dose is only qualitative marker, which has the potential to predict subsequent development of disease and that can be related to the outcome of therapy in terms of the destruction of the target tissue. The absorbed dose is a key factor for a design and evaluation of any TRT (targeted radionuclide therapy) and this fact is reflected in the Council Directive 2013/59 /Euratom issued on 5th December 2013. In the document it is made clear that any therapy should be subjected to planning and verification. In other words, it means that therapy planning have to be based on dosimetric measurements. The content of this directive will be propagated in the national legislation no later than February 2018. Based on this requirement, it is necessary to study given issue in order to determine benefits of planning and verification of the therapy.
1.1 Differentiated thyroid cancer treatment and current state-of-the-art
Differentiated thyroid cancer (DTC) is defined as a carcinoma deriving from the follicular epithelium and retaining basic biological characteristics of healthy thyroid tissue (specific iodine uptake preserved). DTC incidence generally shows a noticeable increase [2]. Consecutive autopsy studies together with local experience at Department of Nuclear Medicine and Endocrinology, 2nd Faculty of Medicine, Motol University Hospital, Prague (DNME) have shown that papillary microcarcinoma is frequent in the general population. Improved detection of some of these subclinical tumors may be partially responsible for the increase in DTC incidence.
The standard treatment procedure is as follows: total or near-total thyroidectomy, respectively, (six level lymph nodes dissection and dissection of the lateral-cervical lymph nodes of the same side as the primary lesion). After thyroidectomy, it is now generally considered to be contributive to ablate the thyroid remnant with 131-radioiodine therapy (RaIT). Ablation of thyroid remnant (TRA) allows better management of the follow-up of DTC patients. The Thyroglobulin serum (hTg) levels should be undetectable or very close to zero in case of successful treatment. Potential enhancement of hTg serum can be considered as a relapse of disease. RAIT can be carried out in hypothyroidism state (Thyroid-stimulating hormone (TSH) > 30 or after external TSH stimulation). Post-therapeutic whole body scan and static images of the head, neck and thorax, optionally, are acquired 4-8 days after RAIT. Post-therapeutic examination allows us to identify the thyroid remnant and metastases. [1], [8], [10]
Nowadays, RAIT activities are generally empirically determined and fixed for each institution. Therefore treatment activity is “particularly patient specific” (PPS). PPS treatment is usually based on several criteria such as disease characteristics and progress, patient age, thyroglobulin levels and others. Administered activity for radioiodine ablation of post-surgical thyroid residues vary from 1.11 up to 5.0 GBq [1], [8].
Currently, at the KNME, patients are administered with fixed particularly patient specific treatment activity (PPSTA) of 3.0; 3.7; 4.4; and 5.5 GBq. PPSTA depends on the tumor histology, accumulation of 131I within the remnants or nodes, thyreoglobulin concentrations, staging of the disease and consideration of other medical risk factors. Although, PPSTA is obviously popular at some departments conducting RAIT and is believed to be optimized and successful, it has to be stated that dosimetry is conducted only in few cases in different ways making the among-study comparison inaccurate.
The prognosis of the disease is generally excellent mainly due to quite time-tested treatment procedures. Based on literature research from EANM guidelines the 10-year survival rate in cases of distant metastasis is approximately 25–40%, the 10-year overall cause-specific survival for DTC patients as a whole is estimated at approximately 85%. Even the lifetime recurrence rate is high, reaching 10–30%. Lifelong follow-up is proposed for all DTC survivors and subsequent therapy in an appreciable number of patients should be done.
As EANM guidelines state, the relatively low prevalence of the malignancy and the lengthy overall survival of most patients create the need for large sample sizes and very long follow-ups. So that prospective studies are complicated, especially on new therapies. The entire situation flows up into recommendation based on clinical experience together with literature and limited studies based on observation and critical review. EANM guidelines provide citations to key studies underlying their recommendations rather than formally classifying strength of evidence for proposed treatment strategies. [1], [6].
2 Materials and methods
The idea of this study was to use appropriate imaging device which enable quantitative analysis of the acquired image even shortly after administration of 131I (high photon fluxes). Basically, it is possible to use standard gamma camera [7]. However, the camera time is quite expensive and hardly reachable in case of necessity of extra examination. Moreover, bigger field of view detects more photons and it can be overloaded in case of high activities. Therefore, extra device was required. Considering relatively small region of interest (ROI) – chin, neck, the very upper part of thorax, operating small field of view (FOV) gamma camera seemed to be more profitable for most of the patients.
Search for appropriate device was done and DDD Company which presented their products at 40th Days of Nuclear Medicine (Příbram) 2013 was asked for cooperation.
Demo of Single head gama camera Solo mobile was lent to DNME for testing. During the testing, it was confirmed that this camera is suitable also for the dosimetry measurements of the thyroid remnants.
Consequently, the pilot study was proposed and realized.
2.1 Experiment outline
As most of the patients treated at DNME undergo their first RAIT (80%), the dosimetry measurements were preliminary planned to be set for them. Standard schedule of the hospitalization at DNME is as follows:
- diagnostic administration (DA): 2nd day morning,
- administered activity: 110 MBq,
- 131I retention measurements 1 h, 24 h, 48 h after DA,
- accumulation test from 24 h up to 25 h after DA,
- therapeutic administration (TA) 50 h after DA,
- dose rate measurement 96 h after TA (radio-hygienic purpose),
- WB scintigraphy from 96 h up to 120 h after TA.
The hospitalization schedule had to be untouched by any extra measurements. Regarding this requirement first measurement were set just to test the schedule of dosimetry measurements in clinical operation.
Dosimetry schedule was than proposed as follows:
- measurements of 131I activity in the thyroid remnants 1 h, 3 h, 7 h, 22 h, 26 h, 48 h after DA (dose rate 1 m from the patient was measured at the same time),
- therapeutic administration (TA) 50 h after DA,
- measurements of 131I activity in the thyroid remnants 5-7 h, 22 h, 30 h, 46 h 55 h 74 h, 94 h and 124 h after TA (dose rate 1 m from the patient was measured at the same time).
Proposed dosimetry schedule was usually kept up to one or two measurements which had to be canceled or delayed.
Participating patient-volunteers were around 3 months after thyreoablation due to thyroid carcinoma. They were asked to participate and all the measurements were explained and detailed to them.
Examination was usually done in separate room during diagnostic measurements. The post-therapeutic measurement was done in corridor of bed ward as it was closer to the patient's rooms. Examination was done in the closest patient-to-detector geometry (see Fig. 1). Patients' neck distance from the detector was measured so distance correction could be done.
Fig. 1: Patient position in front of the camera. 
2.2 Imaging and activity determination
Measurements of 131I accumulated in the remnants of patients' thyroids and 131I accumulating nodes (neck region) were done by the Solo Mobile camera.
Two types of collimators - MEGP (medium energy general purpose) and HEGP (high energy general purpose) were tested. This was done due to quite problematic operation with the HEGP collimators. The first group (six patients) was examined using the MEGP collimator (one patient was excluded because of repeated therapy); HEGP was used for the other group (five patients). The first group of patients underwent more than 5 measurements after diagnostic adminis-tration. Consequently, more than five measurements were done after therapeutic administration. For the second group six measurements after diagnostic and seven measurements after therapy were performed.
Vials with a known activity of 131I were used to do a basic calibration of the camera. Detected counts dependence on activity in various source-to-camera within and without PMMA neck phantom. Calibration then served to determine the activity of 131I within lesions from the static scintigraphy image. During the first 72 hours of measurement standardized activity was present within the FOV together with patient (see Fig. 2). This enabled more direct calculation of the lesions activity without the need of dead time correction and demands for detailed calibration measurements. Activity in lesion Al for each measurement was then determined as in equation (1).
Here AS is the standard activity, Cl is counts within the ROI in lesion, CS is counts in the ROI of standard, Bgl, and BgS are pixel-normalized background counts (see Fig. 2). Further, d is distance correction. Depth correction of the lesion was neglected.
Fig. 2: Standard position within the FOV. 
2.3 Mass of the lesion
The weight of the accumulating remnants or nodes was established using volume measured by ultrasound. For simplification, lesions and nodes are considered to be ellipsoids, thus the volume is equal to (4/3)πabc in the first approximation and the density was set to 1 g per ccm. In case that the scintigraphy image didn’t correlate with sonography, volume of the accumulating lesions was roughly estimated using phantom measurements or known volume of similarly accumulating lesions of other patients. Mass of the lesions estimation error was proposed to be from 30% up to 50% based on the physicians’ experiences.
2.4 Dose determination
Using quantitative imaging for activity determination within the remnants of thyroid and nodes cumulated activity was calculated as an integral of the curve fitting the data (example Fig. 3). Calculation of cumulated activity was usually done for the curve from 8 hours up to 200 hours (extrapolation) after treatment administration. First 8 hours of cumulated activity (uptake) was approximate to be 2/3 of the activity after 8 hours.
Fig. 3: Example of fitting measured data for multiple accumulating lesions. 
Absorbed dose from emitted electrons was determined and was proposed as the therapeutic dose from radioiodine therapy according the equation (2).
Absorbed dose due to photons was neglected as for such small lesions (less than 1 ccm) is insignificant.
3 Results
Absorbed doses were determined for all the participating patients. Results are in Tab. 1 and Tab. 2. Red-dyed cells correspond to lesions where either lesions mass was estimated or no scintigraphy correlation with sonographic examination was observed.
Tab. 1. Dosimetry results for the MEGP measurements. 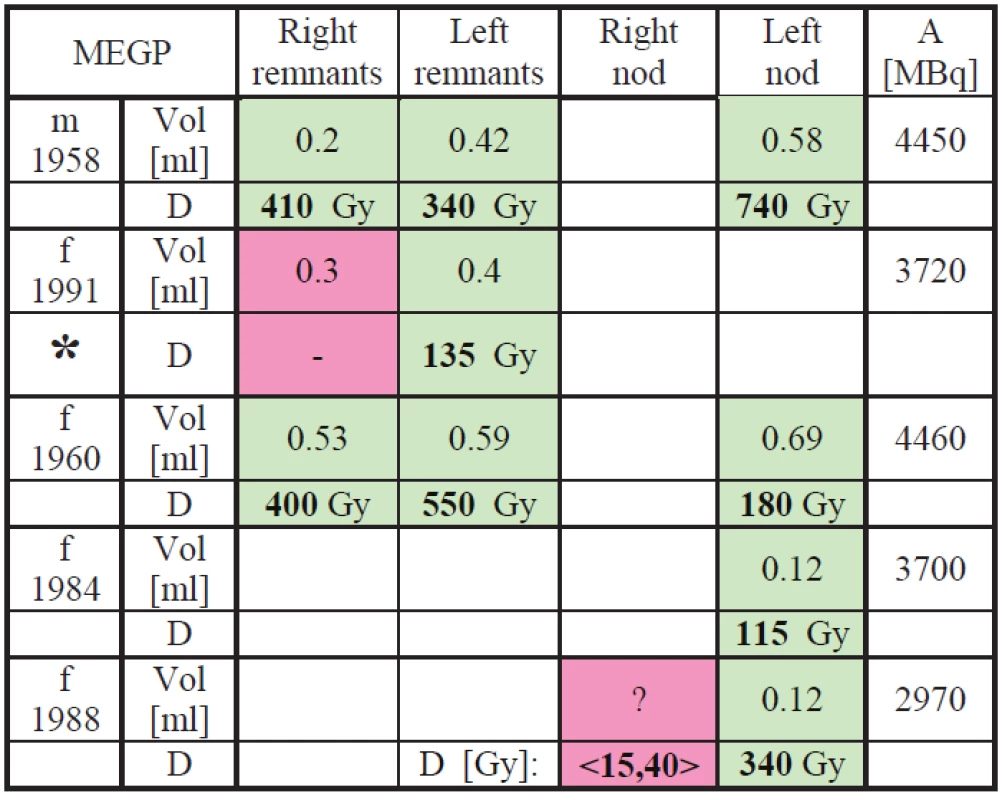
Tab. 2. Dosimetry results for the HEGP measurements. 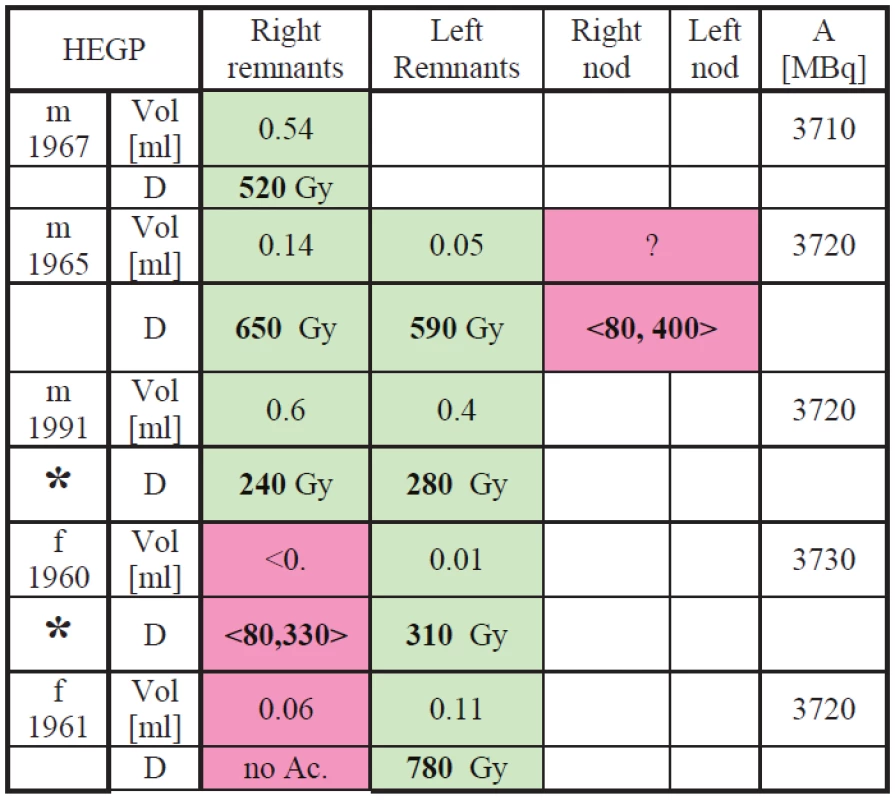
In the tables results in <,> brackets correspond to the lowest and the highest dose estimation using the highest expectable lesion volume and the lowest expectable lesion volume based on estimation from patient similar medical finding. Patients marked with * are supposed to come for repeated treatment because the treatment dose calculated from our measurements is lower than the generally recognized limit 300 Gy for thyroid tissue and 80 Gy for nod (see [1]).
4 Discussion
As this work was mainly intended to be a pilot study most of the errors are only an estimation based on limited number of measurements.
The smallest error (10%) is supposed to be in the activity determination because standard with known activity was used in the FOV and neck-to-camera distance correction was applied. Lesions volume determination using sonography is also quite small (from 5% to 15%) influenced mainly by the physician. Uncertainty from data fitting and cumulative activity determination is supposed to be lower than 15%. Combined error resulting from limited time for calibration and first 24 h after TA administration measurements is supposed to be less than 20%. So that the pilot-study-lowest combined error was estimated to be 25% for patients where the sonography is correlated with scintigraphy image. However in cases, where the sonography is not correlated with the scintigraphy (the nod or remnant can’t be measured than), it is hard to do any dose calculation because the mass of the lesion is only estimate. Nevertheless, it is still possible to presume that the accumulating lesion has a limited mass due to physicians’ long-professional-life experiences. Based on that mass was estimated conservatively (the lowest dose) as a ten times larger remnant than the correlated one and optimistic estimation was only two or three times larger remnant than the correlated one. This “estimation” could vary the dose calculation than for more than +/ - 100% so that 100% error is to be considered than.
This estimation is still very problematic and the error is large so that interval of dose is putted down and marked by pink color.
5 Conclusions
Results show that most of the patients are administered with sufficient therapeutic activity. Nevertheless; three out of ten patients are supposed to come back for repeated treatment (administered activity was unable to deliver sufficient treatment dose). Quite big dispersion through the studied groups was observed and it is in an agreement with recent findings.
Acknowledgements
Here it is necessary to heartily thanks to patient volunteers, who participate in this research and wish them all the best and get well soon.
We also have to frankly thanks to DDD Diagnostic and Medconsult s.r.o. for lending the camera and their technical support during testing.
Ing. Pavel Solný
Department of Dosimetry and Application of Ionizing Radiation Faculty of Nuclear Sciences and Physical Engineering, Czech Technical University in Prague
Department of Nuclear Medicine and Endocrinology 2nd Faculty of Medicine, Charles University in Prague and Motol University, Prague, Czech Republic
E-mail: pavel.solny@fnmotol.cz
Phone: +420 224 734 612
Zdroje
[1] Maxon, H. R., Englaro, E. E., Thomas, S. R., Hertzberg, V. S., Hinnefeld, J. D., Chen, L. S., Aden, M. D. (1992). Radioiodine-131 therapy for well-differentiated thyroid cancer--a quantitative radiation dosimetric approach: outcome and validation in 85 patients. Journal of Nuclear Medicine : Official Publication, Society of Nuclear Medicine, 33(6), 1132–6. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/1597728
[2] Luster, M., Clarke, S. E., Dietlein, M., Lassmann, M., Lind, P., Oyen, W. J. G., Bombardieri, E. (2008). Guidelines for radioiodine therapy of differentiated thyroid cancer. European Journal of Nuclear Medicine and Molecular Imaging, 35(10), 1941–59. doi:10.1007/s00259-008-0883-1.
[3] Lassmann, M., Hänscheid, H., Chiesa, C., Hindorf, C., & Flux, G. (2008). EANM Dosimetry Committee series on standard operational procedures for pre-therapeutic dosimetry I : blood and bone marrow dosimetry in differentiated thyroid cancer therapy, 1405–1412. doi: 10.1007/s00259-008-0761-x.
[4] Flux, G. D., Haq M., Chittenden, S. J., et al. (2009). A dose-effect correlation for radioiodine ablation in differentiated thyroid cancer. European Journal of Nuclear Medicine and Molecular Imaging, 37; 270–275.
[5] Lassmann, M., Chiesa, C., Flux, G., & Bardiès, M. (2010). EANM Dosimetry Committee guidance document : good practice of clinical dosimetry reporting. doi: 10.1007/s00259-010-1549-3.
[6] Hänscheid, H., Canzi, C., Eschner, W., Flux, G., Luster, M., Strigari, L., & Lassmann, M. (2013). EANM Dosimetry Committee series on standard operational procedures for pre-therapeutic dosimetry II. Dosimetry prior to radioiodine therapy of benign thyroid diseases. European Journal of Nuclear Medicine and Molecular Imaging, 40(7), 1126–34. doi: 10.1007/s00259-013-2387-x.
[7] Bardiès, M., Flux, G., Lassmann, M., Monsieurs, M., Savolainen, S., & Strand, S.-E. (2006). Quantitative imaging for clinical dosimetry. Nuclear Instruments and Methods in Physics Research Section A: Accelerators, Spectrometers, Detectors and Associated Equipment, 569(2), 467–471. doi:10.1016/j.nima.2006.08.068.
[8] Flux, G. D., Guy, M. J., Beddows, R., Pryor, M., & Flower, M. a. (2002). Estimation and implications of random errors in whole-body dosimetry for targeted radionuclide therapy. Physics in Medicine and Biology, 47(17), 3211–23. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12361219.
[9] Hackshaw A, et al. 131I activity for remnant ablation in patients with differentiated thyroid cancer: a systematic review. J Clin Endocrinol Metab. 2007; 92(1):28–38.
[10] Nikzad, S. (2011). Determination of organ doses in radioiodine therapy using medical internal radiation dosimetry (MIRD) method, 8(4), 249–252.
[11] Gholamrezanezhad A, 12 Chapters on nuclear medicine, Chapt. 2 Internal radiation models and applications; Amato E. et al. InTech, Janeza Trdine 9, 51000 Rijeka, Croatia; ISBN 978-953-307-802-1, 26–50.
[12] Snyder, W. et al. (1975). “S” absorbed dose per unit cumulated activity for selected radionuclides and organs MIRD Pamphlet No. 11. New York (NY): Society of Nuclear Medicine.
[13] Eschmann, S. M., Reischl, G., Bilger, K., Kupferschläger, J., Thelen, M. H., Dohmen, B. M., … Bares, R. (2002). Evaluation of dosimetry of radioiodine therapy in benign and malignant thyroid disorders by means of iodine-124 and PET. European Journal of Nuclear Medicine and Molecular Imaging, 29(6), 760–7. doi: 10.1007/s00259-002-0775-8.
[14] Sgouros, G., Kolbert, K. S., Sheikh, A., Pentlow, K. S., Mun, E. F., Barth, A., … Larson, S. M. (2004). Patient-Specific Dosimetry for 131 I Thyroid Cancer Therapy Using 124 I PET and.
Lassmann, M., Reiners, C., & Luster, M. (2010). Dosimetry and thyroid cancer: the individual dosage of radioiodine. Endocrine-Related Cancer, 17(3), R161–72. doi: 10.1677/ERC-10-0071.
[15] Dorn, R., Kopp, J., Vogt, H., Heidenreich, P., Carroll, R. G., & Gulec, S. a. (2003). Dosimetry-guided radioactive iodine treatment in patients with metastatic differentiated thyroid cancer: largest safe dose using a risk-adapted approach. Journal of Nuclear Medicine : Official Publication, Society of Nuclear Medicine, 44(3), 451–6. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12621014
[16] Furhang, E. E., Larson, S. M., Buranapong, P., & Humm, J. L. (1999). Thyroid cancer dosimetry using clearance fitting. Journal of Nuclear Medicine : Official Publication, Society of Nuclear Medicine, 40(1), 131–6. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9935068
[17] IAEA-TECDOC-1608. (2009), (March).
[18] Sisson, J. C. (2002). Practical Dosimetry of Carcinoma 131 I in Patients with Thyroid, 17(1), 101–106.
Štítky
Biomedicína
Článek vyšel v časopiseLékař a technika

2015 Číslo 3-
Všechny články tohoto čísla
- MONITORING VÝSKYTU PORÚCH OSOVÉHO ORGÁNU U ŠTUDENTOV DENTÁLNEJ HYGIENY
- COMPARISON OF DOSE CALCULATION ALGORITHMS FOR LEKSELL GAMMA KNIFE PERFEXION USING MONTE CARLO VOXEL PHANTOMS
- FEASIBILITY OF RADIOIODINE DOSIMETRY USING A SMALL FIELD OF VIEW GAMMACAMERA; PILOT STUDY
- DYNAMICAL CHARACTERISTICS OF SPEECH APPARATUS IN HUNTINGTON’S DISEASE
- RELIABILITA MERANÍ ZAŤAŽENIA NOHY PRI CHÔDZI
- Lékař a technika
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- RELIABILITA MERANÍ ZAŤAŽENIA NOHY PRI CHÔDZI
- MONITORING VÝSKYTU PORÚCH OSOVÉHO ORGÁNU U ŠTUDENTOV DENTÁLNEJ HYGIENY
- COMPARISON OF DOSE CALCULATION ALGORITHMS FOR LEKSELL GAMMA KNIFE PERFEXION USING MONTE CARLO VOXEL PHANTOMS
- FEASIBILITY OF RADIOIODINE DOSIMETRY USING A SMALL FIELD OF VIEW GAMMACAMERA; PILOT STUDY
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání





