-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
NEONATAL SCREENING, THE EUROPEAN PERSPECTIVE Preamble of President, International Society for Neonatal Screening (ISNS)
Autoři: J. Gerard Loeber
Působiště autorů: National Institute for Public Health (RIVM), Bilthoven, The Netherlands
Vyšlo v časopise: Čes-slov Pediat 2009; 64 (4): 159-161.
Kategorie: Editorial
Neonatal screening originated in the USA around 1960 and found its way over the globe in the ensuing years, first in New Zealand, Australia, Japan and in western Europe, and followed between 1970 and 1980 by other European countries and some in Latin America. After 1980 pilot programmes were started in a large number of countries in Asia and the rest of Latin America. At present, screening is available in North America, Australia/New Zealand and Europe, but still in its early phases in a number of countries in Latin America and Asia, and hardly at all in Africa. On average only around 25 % of the worlds newborn infants is screened at all. What about the present situation in Europe?
Geographical Europe is bordered by the Atlantic Ocean in the West, the Mediterranean Sea in the South and the Ural Mountains and Caspian See in the East, but political Europe, i.e. the member states of the Council of Europe, includes Turkey, Armenia and Azerbaijan and of course the Asian part of Russia, in total 46 countries.
With respect to neonatal screening coverage Europe is doing reasonably well, with a coverage of around 80%. In a number of Eastern European countries the coverage is lagging behind, e.g. in Romania but especially in Turkey, which has one of the largest populations.
Besides coverage another interesting marker is the scope of each screening programme. In almost all countries screening started with phenylketonuria [1], because the methodology was relatively cheap, closely followed by the screening for congenital hypothyroidism [2]. The way in which other conditions have been added and the reasoning behind it differs very much from country to country [3, 4]. Factors as prevalence and available methodology played a major role, but quite often also the personal interest of leading professionals for a certain condition or group of conditions seemed to be involved.
Table 1 shows per country the starting year for screening for certain conditions. However, not all infants in a country are screened for all conditions in the table. This is caused by the fact that in many countries there is no, or only a partly, defined national programme, but instead individual screening centres determine what list of conditions they elect to screen for.
Tab. 1. Conditions screened for in the European countries and starting year. 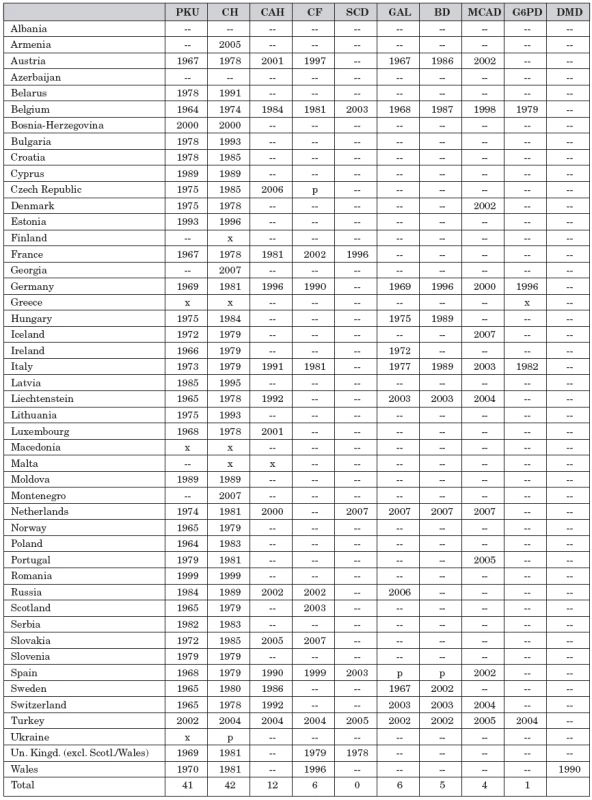
PKU = phenylketonuria, CH = congenital hypothyroidism, CAH = congenital adrenal hyperplasia, CF = cystic fibrosis, SCD = sickle cell disease, GAL = galactosemia, BD = biotinidase deficiency, MCAD = medium chain acyl-CoA dehydrogenase deficiency, G6PD = glucose-6-phosphate dehydrogenase deficiency, DMD = Duchenne muscular ystrophy -- = no data available, x = nation wide program, p = pilot program or in part of the laboratories An important determinant is the availability of funding. In the last decade the introduction of tandem mass spectrometry has made the expansion of the scope of the programmes rapidly possible. However, this technique is expensive and not all countries can afford it. On the other hand, in some countries there is pressure from the population to have the screening programme started or expanded irrespective of cost and the acquisition of perhaps unwanted health information.
At present, the European screening map looks like a random patchwork.
Not so long ago, this was also the case for the USA. Some years back, the American College of Medical Genetics undertook a study how to harmonise the state’s screening programmes and developed a questionnaire in which around 80 known conditions were discussed and scored. The result was a list of 29 conditions that ACMG recommended to all states to screen for [5]. It seems logical to use a similar approach in Europe based on the ACMG model. In view of the large differences concerning language, culture, ethics, history and health care structures this will be a time-consuming and complicated enterprise, but very worth the effort!
The International Society for Neonatal Screening (ISNS) may be instrumental in bringing together experts to exchange views and opinions, aided by the development of global information databases. During the last decade ISNS started organising scientific meetings in Europe, first as small scale workshops by invitation (Königstein, Germany, 2001; Pultusk, Poland, 2003; Sevilla, Spain, 2004), followed by open conferences (Paris, France, 2005; Reykjavik, Iceland, 2007), culminating in the present meeting in Prague, Czech Republic, 2009. Apart from condition-related reports, a major part of the meeting will concern the discussion on how to harmonise the European screening programmes, based on the experience of colleagues from the US. The role of parents and advocacy groups in this process is of great importance.
Zdroje
1. Guthrie R, Susi A. A simple phenylalanine method for detecting phenylketonuria in large populations of newborn infants. Pediatrics 1963;32 : 338–343.
2. Dussault JH, Coulombe P, Laberge C, Letarte J, Guyda H, Khoury K. Preliminary report on a mass screening program for neonatal hypothyroidism. J. Pediatr. 1975;86 : 670–674.
3. Loeber JG. Neonatal screening in Europe; the situation in 2004. J. Inherit. Metab. Dis. 2007;30 : 430–438.
4. Bodamer OA, Hoffmann GF, Lindner M. Expanded newborn screening in Europe 2007. J. Inherit. Metab. Dis. 2007;30 : 439–444.
5. Watson M, Mann MY, Lloyd-Puryear MA, et al. Newborn screening: toward a uniform screening panel and system – executive summary. Pediatrics 2006;117: S296–S307.
Štítky
Neonatologie Pediatrie Praktické lékařství pro děti a dorost
Článek AbstraktaČlánek Abstrakta - pokračováníČlánek Abstrakta - pokračováníČlánek Abstrakta - pokračováníČlánek Abstrakta - pokračování
Článek vyšel v časopiseČesko-slovenská pediatrie
Nejčtenější tento týden
2009 Číslo 4- Horní limit denní dávky vitaminu D: Jaké množství je ještě bezpečné?
- Isoprinosin je bezpečný a účinný v léčbě pacientů s akutní respirační virovou infekcí
- Syndrom Noonanové: etiologie, diagnostika a terapie
-
Všechny články tohoto čísla
- The 6th ISNS European Regional Meeting in Neonatal Screening
- NEONATAL SCREENING, THE EUROPEAN PERSPECTIVE Preamble of President, International Society for Neonatal Screening (ISNS)
- LIST OF ABSTRACTS AND PRESENTING AUTHORS
- Abstrakta
- Abstrakta - pokračování
- Abstrakta - pokračování
- Abstrakta - pokračování
- Abstrakta - pokračování
- Česko-slovenská pediatrie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



