-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaAssessment of the efficacy of photodynamic therapy in patients with chronic central serous chorioretinopathy
Authors: K. Manethová 1,2; J. Ernest 1; M. Hrevuš 1; N. Jirásková 3
Authors place of work: Oční klinika, Ústřední vojenská nemocnice – všeobecná fakultní nemocnice v Praze, U Vojenské nemocnice 1200, Praha 6, Přednosta: doc. MUDr. Martin Šín, Ph. D., FEBO 1; Oční oddělení, Thomayerova nemocnice, Vídeňská 800, Praha 4 Primářka: MUDr. Kateřina Manethová, FEBO 2; Oční klinika, Fakultní nemocnice Hradec Králové, Sokolská 581, Hradec Králové, Přednostka: prof. MUDr. Naďa Jirásková, Ph. D., FEBO 3
Published in the journal: Čes. a slov. Oftal., 75, 2019, No. 6, p. 298-308
Category: Původní práce
doi: https://doi.org/10.31348/2019/6/2Summary
Purpose: The aim of this prospective clinical study was to evaluate the anatomical and functional results of the treatment of 54 eyes with chronic form of central serous chorioretinopathy (CSC) using photodynamic therapy in a reduced (half) verteporfin (HD-PDT) dosing regimen.
Materials and Methods: Our prospective study included 54 eyes of 52 patients (40 males, 12 females) at an average age of 50.1 years (median 49.5, range 30–75 years) treated at the Ophthalmology Clinic of the First Faculty of Medicine and Military University Hospital in Prague from January 2012 to January 2018 for chronic form of CSC with a minimum disease duration of 3 months. In our study, we evaluated the improvement of the best corrected visual acuity (BCVA) and central retinal thickness (CRT) before treatment and at 1, 3, 6 and 12 months after HD-PDT.
Results: The mean baseline BCVA was 68.91 ± 10.5 ETDRS letters (median 71; range 35–85) and the mean baseline CRT was 385.6 ± 118.5 µm (median 367, 5 µm; range 245–1000 µm). At the end of the follow-up period, the average BCVA was 79 ± 11 ETDRS letters (median 82; range 38–93). The improvement in BCVA before and after treatment was statistically significant in all measurements (p < 0.0001). The mean CRT at the end of the follow-up period was 263.5 ± 52 µm (median 258.5 µm; range 162–404 µm). The decrease in CRT at all timepoints was statistically significant compared to baseline (p < 0.0001). In our set of patients, at the end of the follow-up period, the retinal finding was improved or stabilized in 50 eyes (92.6 %). In this study, we observed in 2 cases the development of secondary choroidal neovascularization (CNV).
Conclusion: HD-PDT is a long-term safe and effective method of treating chronic forms of CSC. However, despite a reduced dose of verteporfin, complications may occur.
Keywords:
chronic central serous chorioretinopathy – half-dose photodynamic therapy – secondary choroidal neovascularization
INTRODUCTION
Central serous chorioretinopathy is a pathology characterised by serous detachment of the neuroretina, especially in the posterior pole of the eye [13]. CSC is frequently accompanied by serous expulsion of the retinal pigment epithelium (RPE) and associated with the seepage of fluid into the subretinal space through the defective RPE. CSC most commonly affects men of productive age [15]. The precise pathophysiology of the disease is not entirely known. On the basis of an examination of angiography with indocyanine green (ICG), which detected increased permeability of the choroidal capillaries [47], and optical coherence tomography (OCT), demonstrating increased thickness of the choroidea [19], choroidal vasculopathy is presumed to be the primary cause of CSC. In most cases CSC has a good prognosis, with spontaneous resorption of subretinal fluid (SRF) and adjustment of visual functions. However, in a small percentage of patients the disease progresses to a chronic or recurrent course, and may lead to irreversible functional and anatomical changes of the retina, with a resulting final clinical picture of diffuse retinal pigment epitheliopathy (DRPE) [47].
The optimal therapeutic approach to patients with CSC remains controversial. In recent decades, countless different therapeutic procedures have been used in the treatment of chronic forms of CSC (cCSC); these have included for example laser photocoagulation [25], medicamentous treatment [14,41] standard PDT [9] or agents acting against vascular endothelial growth factor (anti-VEGF) [16]. In recent years experts in the treatment of cCSC have inclined towards less destructive methods such as photodynamic therapy in reduced-dose regimens (rPDT), either with a reduced dose of Visudyn (“reduced-dose”) or of the used laser beam energy (“reduced-fluence”) [21,22,43]. Chan, Uetani and Alkin demonstrated comparable efficacy and safety in the use of reduced regimens of PDT (reduced-dose or reduced-fluence PDT) in patients with cCSC, in whom an improvement of best corrected visual acuity (BCVA) and a reduction of subretinal fluid was achieved, wherein no complications were recorded in the observed cohort [1,7,49].
In the Czech Republic no standards for treatment of this clinical pathology exist to date. Because the disease typically afflicts younger patients of productive age with high demands for quality of vision, correct timing and determination of treatment of acute and especially chronic form of CSC remains a challenge. Whereas previously the treatment relied exclusively upon laser treatment of the retina [29], in recent years there has been an inclination towards more conservative methods such as photodynamic therapy or intravitreal application of antiangiogenic substances (anti-VEGF) [26,28].
AIM OF STUDY
The main aim of this prospective, non-randomised clinical trial was to evaluate the anatomical and functional results of the treatment of 52 patients (54 eyes) treated at the Department of Ophthalmology at the 1st Faculty of Medicine and Central Military Hospital-General University Hospital in Prague in the period of 2014-2018 for cCSC, with the aid of photodynamic therapy in a reduced-dose regimen (HD-PDT, half-dose verteporfin). HD-PDT was indicated as the primary intervention in the case of symptomatic chronic forms persisting for longer than 3 months, in which no resorption of subretinal fluid (SRF) took place spontaneously, or following insufficient response to conservative therapy.
Target of clinical evaluation:
- change of best corrected visual acuity (BCVA) before HD-PDT and in 1st, 3rd, 6th and 12th month after treatment.
- change of central retinal thickness (CRT) before HD-PDT and in 1st, 3rd, 6th and 12th month after treatment.
METHOD
The main entrance criteria included: patients older than 18 years, on OCT evident SRF and/or ablation of RPE persisting for a period of at least 3 months, presence of active hyperfluorescence on fluorescence angiography (FAG) typical of CSC, presence of choroidal vascular permeability and abnormally dilated choroidal capillaries on ICG.
The main exclusion criteria covered: symptoms of another macular pathology (choroidal neovascularisation – CNV, age-related macular degeneration, angioid streaks, polypoidal choroidal vasculopathy, pathological myopia, tilted disk etc.), laser treatment or intravitreal application of anti-VEGF within a period of 3 months before inclusion in the study, previous performance of PDT, non-transparent optical media preventing examination and actual performance of PDT, known hypersensitivity to any form of preparation for FAG, ICG or PDT.
In all the patients upon entrance we performed a complete ophthalmological examination, including: examination of BCVA (EDTRS optotypes) and intraocular pressure by non-contact tonometry (NCT), biomicroscopy of the anterior and posterior segment of the eye in artificial mydriasis, photo documentation according to protocol – OCT (Heidelberg Spectralis, Heidelberg Engineering, Germany) – high resolution linear horizontal macular scans, map of central retinal thickness with determination of CRT value, enhanced deep imaging (EDI), autofluorescence for determining the extent of damage to the RPE (Heidelberg Spectralis, Heidelberg Engineering, Germany), angiographic FAG/ICG examination (separate or simultaneous on instrument Heidelberg Spectralis, Heidelberg Engineering, Germany).
On the basis of the baseline examination, confirmed cCSC was defined as a diffuse area of hyperfluoresence in the macular landscape with serous elevation of the RPE and/or neuroepithelium, or as a deposit of seepage localised in an area of up to 500 µm from the centre of the fovea, contraindicated for laser photocoagulation, in which no spontaneous resorption of SRF took place. Patients were indicated for the performance of HD-PDT, which we performed at the latest within 30 days of the baseline examinations. The patients were then observed and examined 1, 3, 6 and 12 months after the performance of HD-PDT. A follow-up examination was always conducted for BCVA, IOP, anterior and posterior segment of the eye (biomicroscopically). We always produced control OCT images in a “follow-up” format, which thanks to the function of “eye-tracking” enabled an entirely precise comparison of images in individual measurements. In indicated cases we repeated FAG/ICG.
We considered stabilisation of the finding to mean absence of SRF, normalisation of CRT, and improvement of BCVA by 5 or more letters of ETDRS optotypes. We considered an active finding (persistence of SRF or recurrence) to cover presence of SRF or ablation of the RPE on OCT, activity on FAG/ICG, and decrease of BCVA by 5 or more ETDRS letters in combination with one of the previous two factors. In the case of persistent activity of the pathology, insufficient effect of HD-PDT or relapse of the pathology, we selected additive therapy with the aid of focal laser treatment, application of anti-VEGF preparation intravitreally, or we repeated HD-PDT. We repeated control FAG/ICG during the course of the observation period if we had not recorded an effect of HD-PDT, thus if persistence of fluid was evident on OCT, if recurrence of the pathology occurred, or if signs appeared of CNV complicating the course of the pathology.
We performed additive laser photocoagulation in the case of demonstration of an active deposit source of seepage at a distance of at least 500 µm from the centre of the fovea. We repeated HD-PDT in the case of demonstration of persistent activity or a finding of new active deposits displayed on a control angiogram. We applied anti-VEGF preparations (bevacizumap, aflibercept) into the intravitreal space if we diagnosed complicating CNV following performed HD-PDT, or if upon persistent exudation the RPE was so damaged that a further session of HD-PDT could have further extended or deepened the deposit of atrophy of the RPE.
General characteristics of cohort
Our prospective trial included 54 eyes of 52 patients (40 men, 12 women) with a mean age of 50.1 years (median 49.5, range 30-75 years) treated at the Department of Ophthalmology at the 1st Faculty of Medicine of Charles University and the Central Military Hospital-General University Hospital in Prague from January 2012 to January 2018 for chronic form of CSC with a minimum duration of the pathology lasting 3 months. The patients were indicated for HD-PDT therapy on the basis of the baseline examinations, at which a diagnosis of the basic pathology was determined – cCSC. The average length of duration of the pathology at the time of commencement of the treatment was approximately 11.5 months (range 3-120 months). In our cohort we had 5 patients with a medical history of general corticoid therapy (9.4%), 13 patients were being treated for hypertension (25%), 2 patients for depression (3.8%), multiple sclerosis (MS) had been diagnosed in 3 patients (5.8%), of whom in 1 patient the diagnosis of cCSC was preceded by the diagnosis of MS. On the basis of the baseline OCT/FAG/ICG examination, a point source of seepage was determined in 6 eyes (11.1%), and diffuse deposits of hyperfluorescence in 48 eyes (88.9%). We recorded signs of bilateral cCSC in 23 eyes (42.6%). The mean value of baseline BCVA before treatment was 68.91 ± 10.5 letters of ETDRS optotypes (median 71; in an interval from 35 to 85 ETDRS letters), and the mean value of baseline CRT was 385.6 ± 118.5 µm (median 367.5 µm; within a range from 245 µm to 1000 µm) (Table 1).
Tab. 1. Basic characteristics of cohort and baseline values of central retinal thickness and best corrected visual acuity 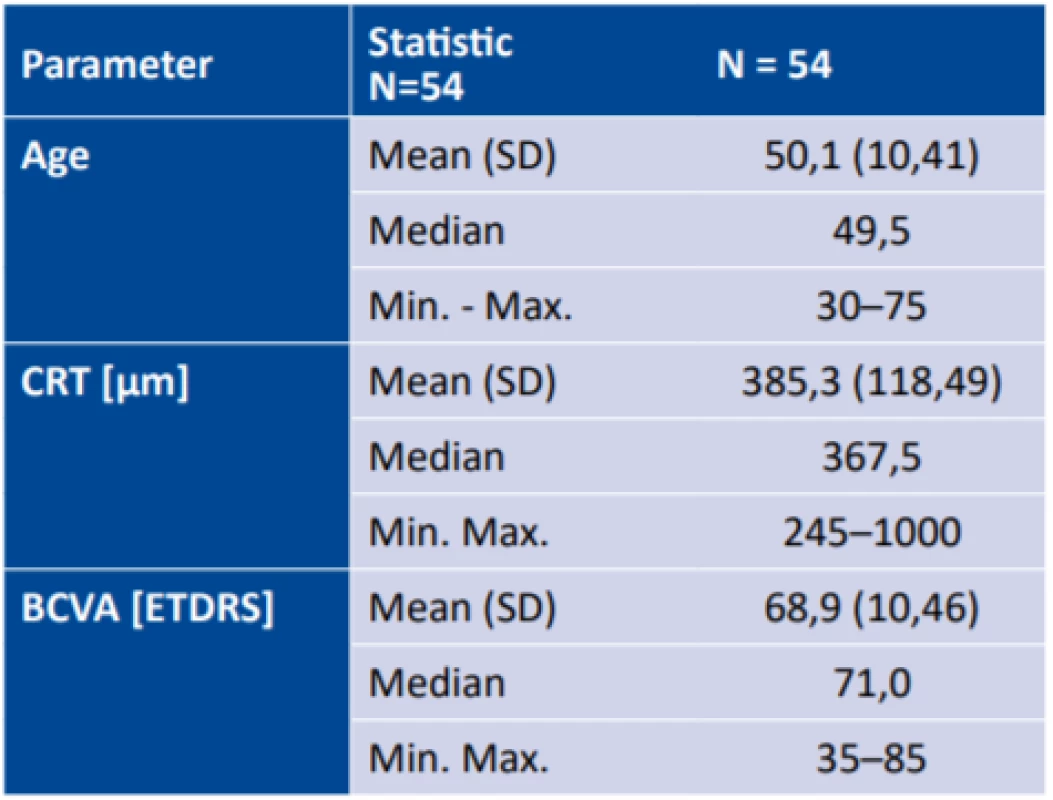
CRT – central retinal thickness, BCVA – best corrected visual acuity, SD – standard deviation Although the presence of cystoid macular degeneration (CMD) as a complication of long-term cCSC is considered a negative prognostic factor for treatment [18,38], patients with CMD were not excluded from our cohort. During the course of the observation period we excluded one patient, in whom uveal effusion syndrome later developed, and the initial manifestations characteristic of CSC were a masking syndrome of this rare pathology.
Statistical analysis of data
The values of CRT and BCVA at the times of 1M, 3M, 6M and 12M were statistically compared with the baseline value (0M) with the aid of a non-parametric Wilcoxon paired t-test, for the purpose of addressing the set target (Table 2). For statistical testing, a bilateral alternative hypothesis was selected, with a 5% level of significance. The stated P values were adjusted by Benjamini-Hochberg correction for multiple testing.
Tab. 2. Descriptive statistics of age, central retinal thickness and best corrected visual acuity, and statistical comparison of changes of observed parameter against baseline value (0M) 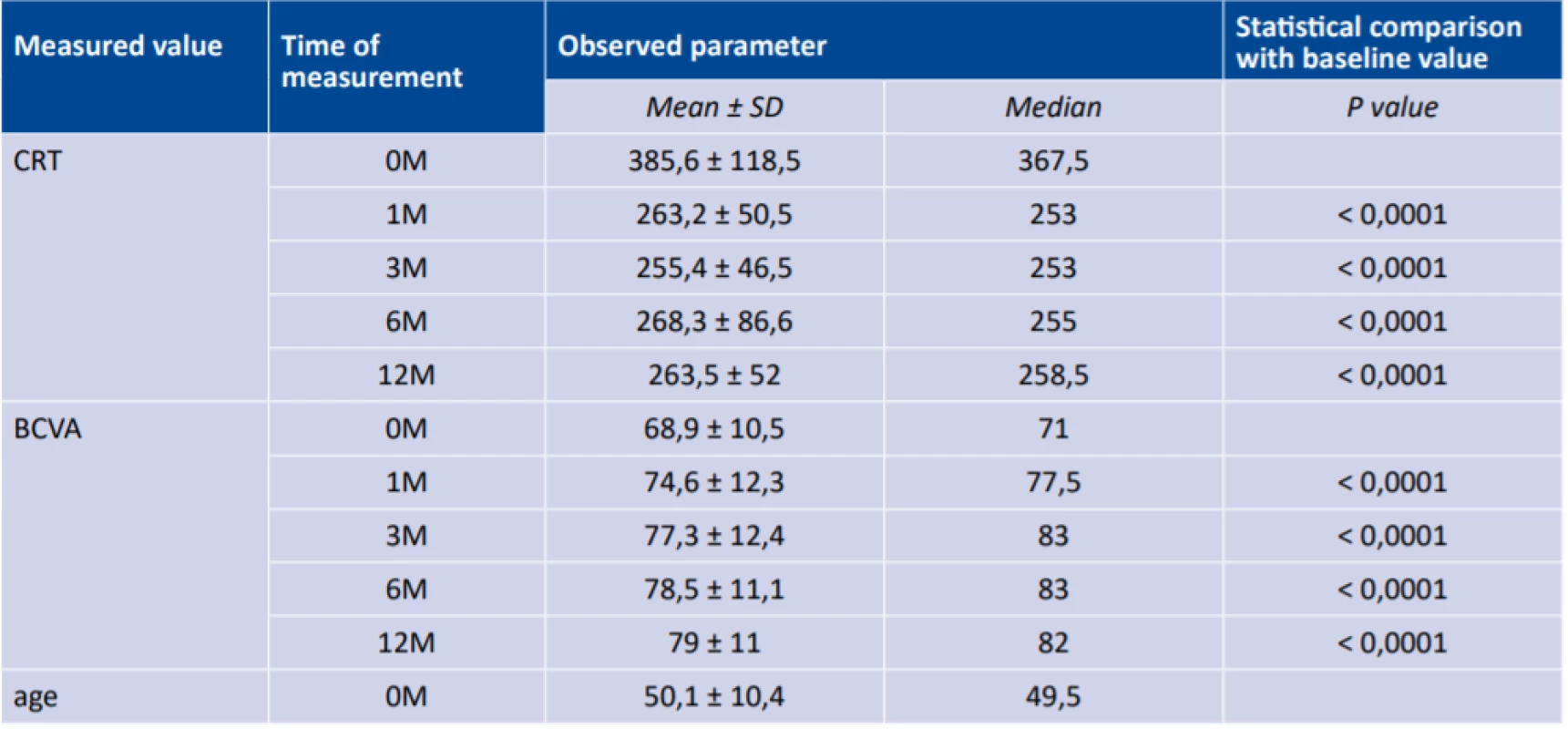
CRT – central retinal thickness, BCVA – best corrected visual acuity, SD – standard deviation Safety and complications
During the course of the observation period, no systemic complications appeared in direct correlation with the performed photodynamic therapy or with intravitreal application of an anti-VEGF agent.
From our cohort of 54 eyes with cCSC treated with the aid of HD-PDT, in two cases we detected the occurrence of complicating CNV 1 month after the performance of the procedure in the place of radiation of the angiographically demonstrated active lesion, and we evaluate these two cases as a complication of treatment. In both cases this concerned subfoveal, or more precisely juxtafoveal minor (though highly active) CNV of the classic type (Fig. 1, 2). Patients with advanced secondary CNV were treated with anti-VEGF therapy (aflibercept); in one case a minor pacific fibrovascular juxtafoveal scar resulted, in the second case anatomical adjustment occurred practically ad integrum, with regression of the entire classic portion of the neovascular complex (Fig. 3).
Fig. 1. (A) Simultaneous fluorescence and indocyanine angiography in prearterial and (B) late venous phase of angiogram displays rounded subfoveal deposit of gradual hyperfluorescence with demarcated width of beam for photodynamic therapy (yellow outlining), on angiogram there are no signs of choroidal neovascularisa- tion (C) Transfoveal linear scan demonstrating undulating line of retinal pigment epithelium, subretinal fluid and relatively well preserved individual retinal layers 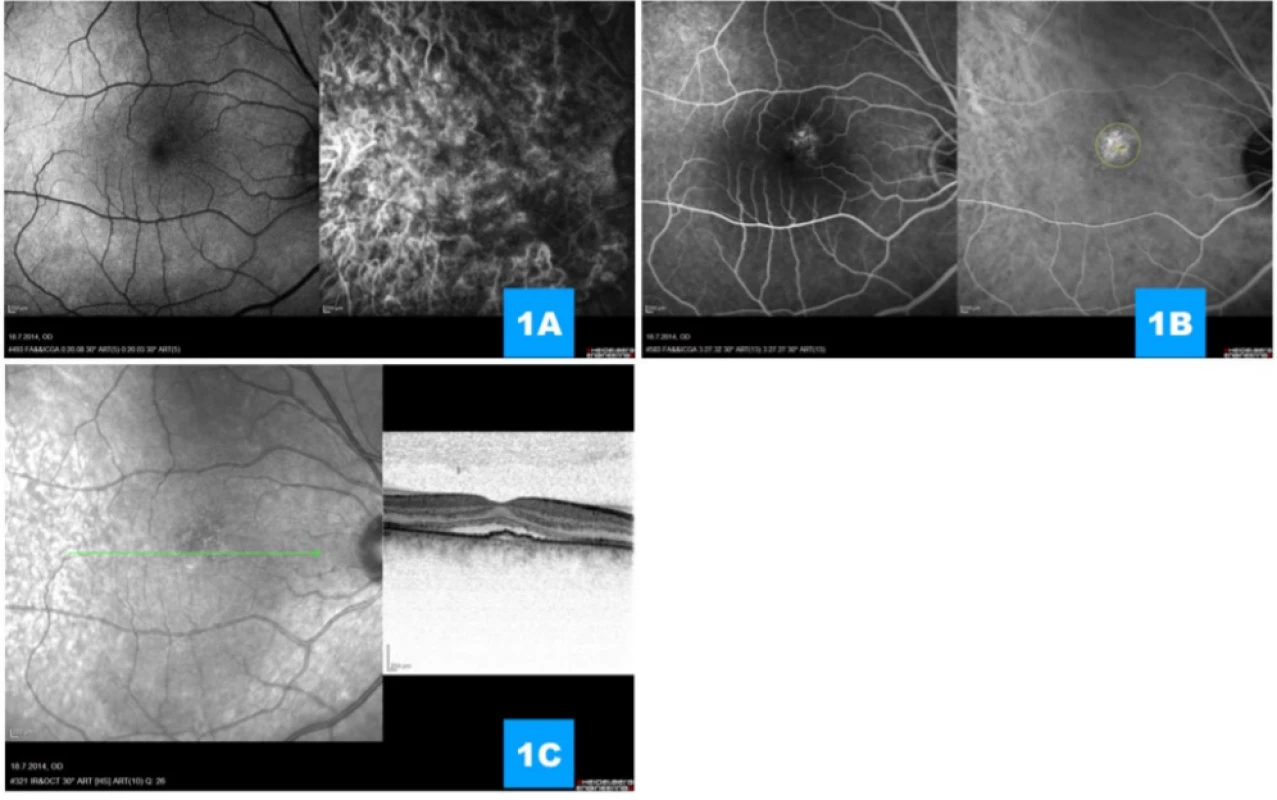
Fig. 2. (A) Complicating minor classic portion of secondary choroidal neovascularisation 1 month after performed photodynamic therapy (B, C, D) Demonstration of highly active choroidal neovascularisation on fluorescence angiography 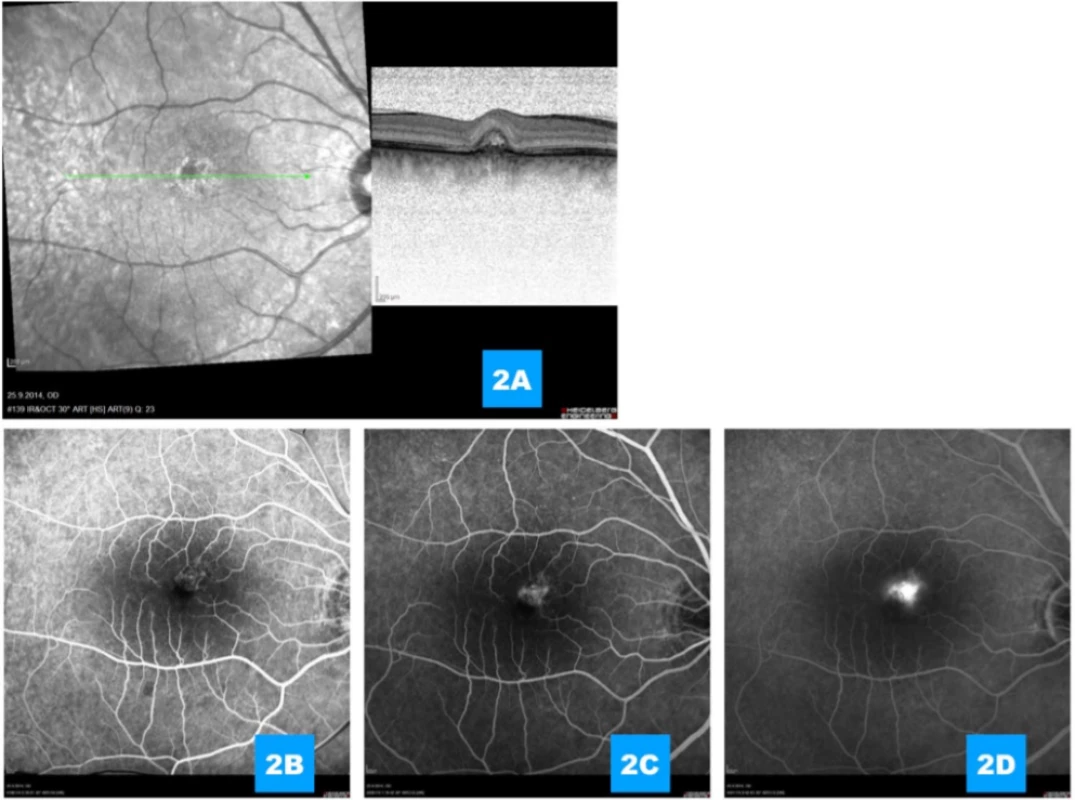
Fig. 3. (A, B) Progressive absolute regression of classic portion, regression of subretinal fluid, preservation of membrana limitans externa, renewal of interdigitation zone and intimation of extirpation of line of retinal pigment epithelium 1 month and 1 year respectively after application of anti-VEGF agent 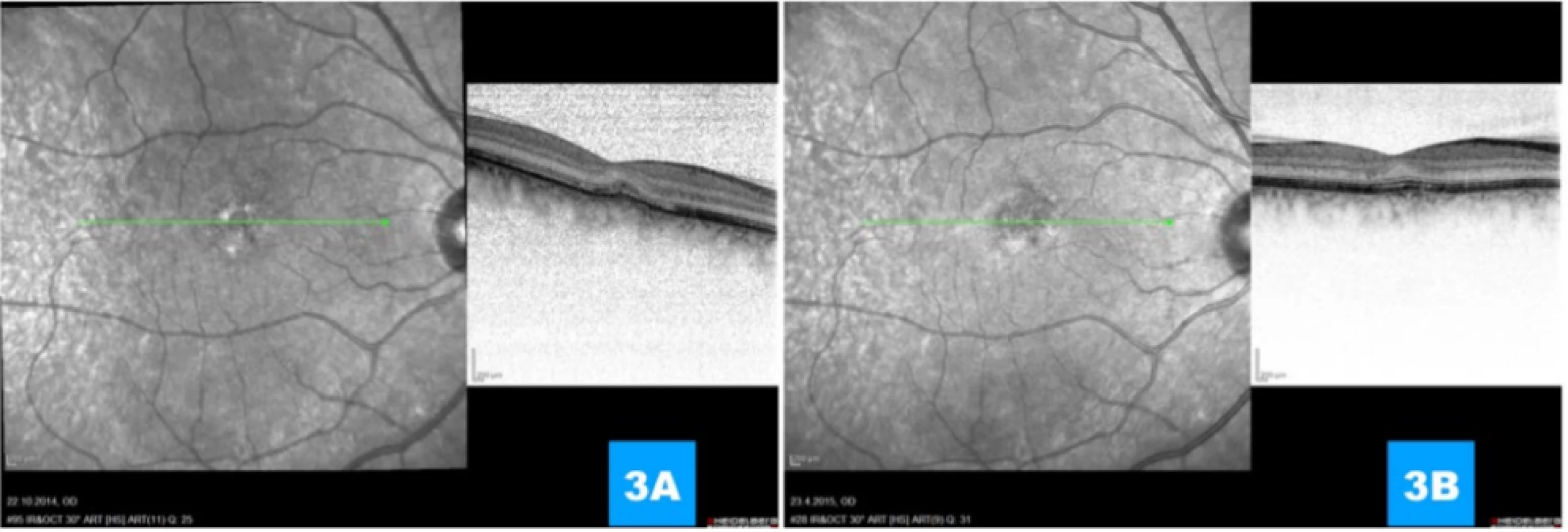
RESULTS
Anatomical results
The primary aim of this study was to evaluate the anatomical effect of HD-PDT in the treatment of cCSC, thus to assess quantitative changes of central retinal thickness (CRT) during the course of the observation period (Graph 1).
Graph 1. Box graph illustrating values of central retinal thickness at individual measurement times (before treatment and 1, 3, 6 and 12 months after treatment) 
In all eyes a positive effect was recorded after the performance of FAG/ICG-navigated HD-PDT, in the sense of a reduction of CRT during the course of the observation period. The average number of HD-PDT sessions was 1.07 (range 1-2). The mean size of the used laser beam was 1352 µm (at an interval from 800 µm to 2800 µm), in which we performed radiation by one beam in 45 cases and in the remaining 9 eyes we irradiated with 2 or more laser beams in a single session. Complete resorption of the subretinal fluid was achieved in 43 eyes (79.6%) at the end of the observation period (Fig. 4, 5).
Fig. 4. (A) Simultaneous image of fluorescence and indocyanine angiography illustrating late hyperfluorescence (pooling) beneath ablations of the retinal pigment epithelium, minor deposit of hyperfluorescence in the parapapillary region, indication of size and location of beams for photodynamic therapy (yellow outlining); there is clear gradation of the filling of the choroidea beneath serous ablations, at the same time the image excludes the presence of choroidal neovascularisation. 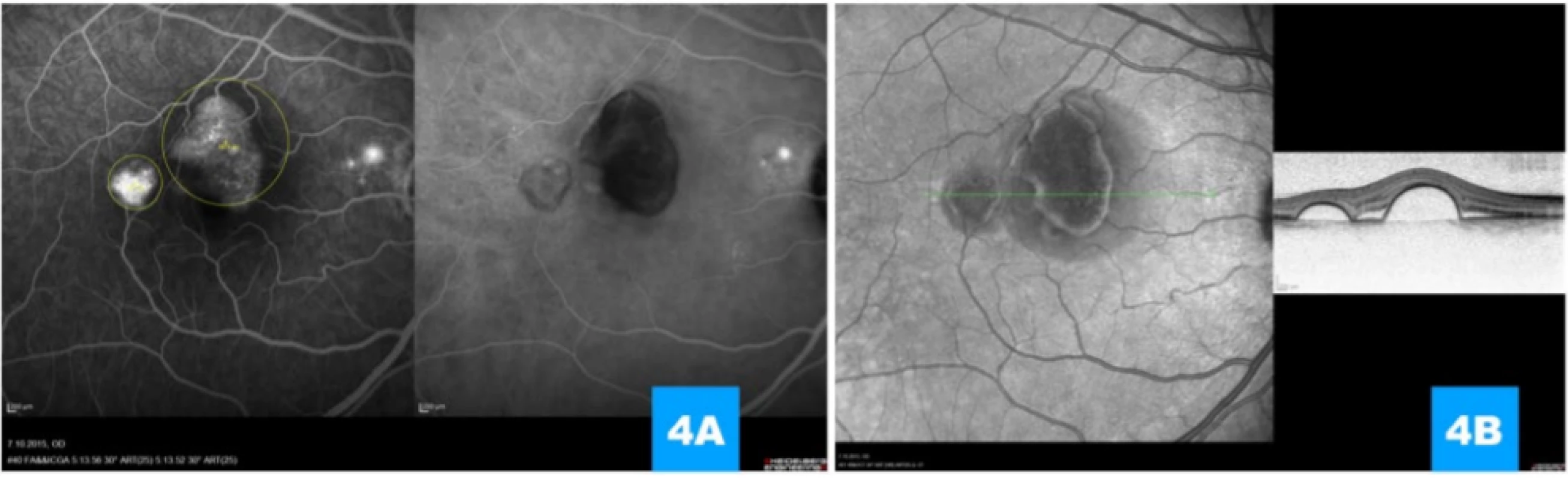
Fig. 5. (A) Regression of activity of pathology with progressive normalisation of retinal layers 1 month and (B) 1 year after performance of photodynamic therapy respectively 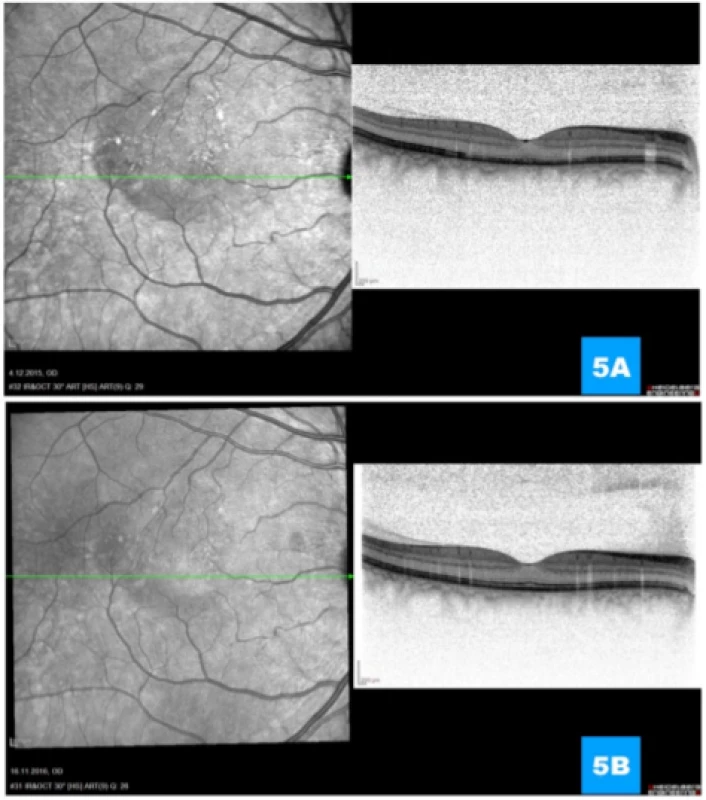
We recorded persistent SRF in the remaining 11 eyes (20.4%), for which additive therapy by intravitreal application of anti-VEGF was selected in 10 cases, in 4 eyes HD-PDT was repeated after an interval of 3 or more months, in which in 3 cases the procedure was combined with application of anti-VEGF into the vitreous body.
The mean value of baseline CRT was 385.3 ± 118.5 µm (median 367.5 µm; range 245-1000 µm). The first month after the performance of HD-PDT, a reduction of CRT was observed in 92.6% of eyes, with a mean reduction by 107.5 µm to mean CRT of 263 ± 50.5 µm (median 253 µm; range 158-423 µm). After three months mean CRT was 255.4 ± 46.5 µm (median 253 µm; range 156-393 µm). At the end of the observation period mean CRT was 263.5 ± 52 µm (median 258.5 µm; range 162-404 µm). Reduction of CRT at all measurement times was statistically significant in comparison with the baseline value (< 0.0001).
During the course of the observation period, cases of recurrence of the disease were recorded, with an increase of CRT by as much as 506 µm, for which HD-PDT was repeated on the basis of control FAG/ICG (total 4x), with a positive anatomical and functional effect at the end of the observation period. In 10 eyes intravitreal application of an anti-VEGF agent was indicated at an interval of 1-12 months (bevacizumab 2x, aflibercept 8x), due to a not entirely satisfactory effect of primary or repeated HD-PDT with residual subretinal fluid, of which in two cases an anti-VEGF agent was applied due to the development of secondary CNV (2 eyes, 3.7%) in connection with the performed treatment, with an interval of maximum 1 month after the performance of HD-PDT.
At the end of the observation period we achieved a satisfactory result without exudative activity in 49 eyes (90.7%). In two eyes recurrence was recorded even despite the initial good effect of treatment, as well as an increase in activity in the 12th month with discrete surface elevation of the neuroretina. In one patient there was a progression of changes in the vitreoretinal interface, with accentuation of traction of the epiretinal membrane (ERM), which evidently contributed to the presence of residual discrete SRF, and ultimately limited the resulting effect of HD-PDT. Patients with developed secondary CNV were treated with anti-VEGF therapy (aflibercept); in one case anatomical adjustment took place practically ad integrum, with regression of the entire neovascular complex and renewal of the OS/IS line and membrana limitans externa (MLE), in the second case a minor pacific fibrovascular juxtafoveal scar formed, with breach of the OS/IS line and MLE.
Functional results
The primary aim of this study was to evaluate also the functional effect of HD-PDT in the treatment of cCSC, thus to assess the change of best corrected visual acuity during the course of the observation period (Graph 2).
Graph 2. Box graph illustrating values of best corrected visual acuity at individual measurement times (before treatment and 1, 3, 6 and 12 months after treatment) 
Mean BCVA at the beginning of the observation period was 68.9 ± 10.5 ETDRS letters (median 71, range 35-85). Higher age and a higher value of subretinal fluid did not correlate with worse visual functions at the commencement of treatment. BCVA values progressively increased in the 1st, 3rd 6th and 12th month after treatment to 74.6 ± 12.3 ETDRS letters (median 77.5, range 33-89), 77.3 ± 12.4 ETDRS letters (median 83, range 35-95), 78.5 ± 11.1 ETDRS letters (median 83, range 38-95), and 79 ± 11 ETDRS letters (median 82, range 38-93). Improvement of BCVA before and after treatment was statistically significant in all measurements (p < 0.0001). Furthermore there is a significant difference between the values measured in the 1st and 6th or 12th month (p = 0.0089, p = 0.0002 respectively). On average there was a gain of 11 letters at the end of the observation period. Patient age did not correlate with relative change of BCVA at individual measurement times 1, 3, 6 or 12 months after the performance of HD-PDT (p = 0.8929, p = 0.04411, p = 0. 1336 and p = 0.4172 respectively).
Out of a total number of 54 eyes before the commencement of treatment with HD-PDT, 7 eyes (12.7%) had BCVA of at least 80 ETDRS letters, at the end of the observation period this had increased to 33 eyes (61.1%). At the baseline examination 14 eyes (25.9%) had BCVA worse than 60 ETDRS letters, at the end of the observation period this had been reduced to 4 eyes (7.5%).
At the end of the observation period we recorded an improvement of central visual acuity in 50 eyes (92.6%), wherein there was an improvement of BCVA by a minimum of 3 rows in 13 eyes (24.1%). In 4 eyes stabilisation of visual function was achieved after treatment, with preservation of baseline BCVA. In patients in whom secondary CNV occurred there was a temporary decrease of BCVA (not more than 1 row of ETDRS optotypes), with an adjustment of sight following intravitreal application of anti-VEGF therapy to a resulting 85 and 68 ETDRS letters respectively. In our cohort we did not record any cases of decrease of BCVA by 5 or more ETRDS at the end of the observation period.
After performed HD-PDT we did not record any significant atrophying changes of RPE in comparison with the baseline finding even one year after the performance of treatment. On the basis of an OCT analysis we consider irregular alignment of the retinal layers, atrophy of the RPE, presence of persistent dense subretinal hyperreflexive material (HRM), and the occurrence of CNV to constitute adverse factors for resulting BCVA. Even despite the highly satisfactory overall results of treatment, these changes showed a correlation with subjective perception of deteriorated vision, with a reduction of contrast sensitivity or discrete metamorphopsias.
DISCUSSION
Central serous chorioretinopaathy is characterised by idiopathic detachment of the neuroretina and/or retinal pigment epithelium on the posterior pole of the eye caused by seepage from pathologically altered choroidal capillaries. CSC occurs most frequently in younger people of productive age, manifested as an acute deterioration of visual acuity. In the great majority of cases spontaneous resorption of the subretinal fluid takes place, with a good prognosis quo ad visum [34]. However, in certain cases the pathology progresses to a chronic stage, in which exudation beneath the neuroretina and/or RPE is not absorbed within 3 months of the onset of the condition, or pathology has a recurrent character. Chronic form of CSC is associated with persistent subretinal exudation, progressive changes of the retina and a corresponding long-term deterioration of central visual acuity. Chronic or recurrent CSC may be a protracted condition, and as a result of long-term persistent separation of the neuroretina leads to irreversible functional changes, accompanied by dysfunction of the RPE [20], subretinal exudates and fibrosis [17], cystoid macular degeneration [18], and complicating CNV [4,30]. The precise cause of the origin of CSC still remains unclear, it is assumed that abnormal choroidal capillaries play a key role in the pathogenesis of this condition [36,42,47]. Increased thickness of the choroidea, perceptible on EDI-OCT [19], and increased choroidal permeability, demonstrated on ICG, suggest a conclusion that treatment targeted precisely at these deposits of pathologically altered choroidal capillaries may be effective in therapy [31,50]. Even despite convincing results of several prospective clinical trials, at present there is no gold standard in the treatment of this pathology. Opinions of experts differ markedly with regard to the commencement of treatment, how radical this should be, and the choice of the most appropriate therapeutic procedure.
In previous decades the standard of treatment for deposit lesions with extrafoveal seepage was conventional laser therapy. Although this treatment may accelerate the resorption of the subretinal fluid, it does not have an influence on choroidal hyperpermeability [44]. In 2003 studies were published demonstrating satisfactory results upon the use of photodynamic therapy [6,50]. The mechanism of verteporfin PDT in the irradiated localisation consists in the selective, non-thermal temporary or permanent occlusion of the choroidal channel, caused by singlets of oxygen molecules emitted from a photosensitive substance (verteporfin) following irradiation by laser energy (PDT, Visulas, ZEISS, Germany) [32,39]. Photodynamic therapy in the standard dosing regimen was the primary method of choice in the treatment of cCSC; although the anatomical and functional results are satisfactory in several international publications [6,34,50], the use of conventional PDT may lead to a more frequent incidence of complications – atrophy of RPE, hypoperfusion of the choriocapillaris, early hypoxia of chorioretinal tissue and occurrence of secondary CNV [6]. Some authors are of the opinion that the use of PDT in a more conservative regimen (reduced-dose, reduced-fluence or a mutual combination thereof) brings similar anatomical and functional results, but with a better safety profile and minimum of the aforementioned adverse effects [1,12,22]. They then view the principle of the effect of reduced PDT as consisting rather in the remodelling of the choroidal channel, with a reduction of seepage of choroidal capillaries, than in its occlusion [9].
The largest published cohort to date, published by Lim, covered an evaluation of a total of 256 eyes treated by standard or reduced PDT. Complete resorption of SRF took place in 81% of cases. Although the effectiveness of PDT treatment was demonstrated, its use even in reduced form may not be entirely harmless. Complications of this cohort were stated in 5.5% of cases, of which atrophy of the RPE occurred in 4% and severe acute decrease of BCVA in 1.5% of patients. In this case the reason for the decrease of visual functions was not stated [26]. With reference to the potential complications, a range of clinical trials have been conducted, confirming the safety and efficacy of PDT in modified dosing regimens. The authors Nicoló, Smretschnig and Rouvas in their studies published the effect of half-fluence PDT (25J/cm2, HF-PDT), which led to a complete resorption of SRF in 84-100% of eyes, with a degree of recurrence of 0-29% [37,45,46]. Similar results were presented by the authors Chan, Nicoló and Fujita upon the use of a reduced dose of verteporfin (3 mg/m2 HD-PDT), in which a resorption of SRF took place in 89.2-100% of cases, with a degree of recurrence of 0-17.2% [7,12,37]. Although Alkin and Kim did not record a significant difference in the anatomical and functional effect between the individual therapeutic modifications [1,22] Nicoló presented more rapid absorption of SRF and a longer-term in the case of HD-PDT in comparison with HF-PDT [37]. Fujita et al. published the largest cohort to date of patients with cCSC treated with HD-PDT at a single clinical centre. At the end of the observation period 89.2% out of 204 eyes recorded complete resorption of subretinal fluid, 5.4% eyes had persistent SRF and the degree of recurrence was 5.9% [12].
In our prospective non-randomised clinical trial we assessed the anatomical and functional results of 52 patients (54 eyes) treated at the Department of Ophthalmology at the 1st Faculty of Medicine, Charles University and Central Military Hospital-General University Hospital in Prague in the period of 2012-2018 for cCSC using the method of HD-PDT. In our cohort of patients, at the end of the observation period we attained an improvement or functional-anatomical stabilisation in 50 eyes (92.7%), while persistent SRF was recorded in 2 eyes (3.7%), and in 3 eyes (5.6%) we recorded recurrence over the course of one year, in which this occurred in 2 eyes at the end of the observation period. The mean age of the patients was 50.1 years (median 49.5, range 30-75), the mean number of HD-PDT sessions was 1.07 (range 1-2), mean size of used laser beam was 1352 µm (within an interval of 800 µm to 2800 µm).
Out of a total number of 54 eyes with cCSC, a complete resorption of subretinal fluid was achieved in 49 eyes (90.7%). The mean value of baseline CRT of 385.6 ± 118.5 µm (median 367.5 µm) was reduced to a value of 263.5 ± 52 µm (median 258.5 µm) at the end of the observation period. The degree of resorption of SRF following HD-PDT treatment in previous clinical trials is stated within the range of 85-100%, and thus our success rate of 90.7% of eyes is comparable with these trials [7,12,21,24,37].
During the course of the 12 month observation period, visual acuity improved significantly. The greatest gain of letters was observed in the 1st month (5.7 ± 6.1, median 5 ETDRS letters) after HD-PDT, with a gradual further improvement in the 3rd (8.4 ± 6.4, median 8 ETDRS letters) and 6th month (9.6 ± 7.6, median 10 ETDRS letters), stabilisation of BCVA was attained six months after treatment and was maintained until the end of the observation period, when the mean gain was 10.1 ± 7.2 ETDRS letters (median 10). The progressive further improvement of BCVA also following the reattachment of the neuroretina is probably due to the gradual improvement of the integrity of the retinal layers, the renewal of the interdigitation zone between the photoreceptors and the RPE cells [40]. At the end of the observation period, an improvement of central visual acuity was observed in our cohort in 50 out of 54 eyes (92.6%). We recorded an improvement of mean BCVA from an initial value of 68.9 ± 10.5 ETDRS letters (median 71) to a value of 79 ± 11 ETDRS letters (median 82) at the end of the observation period. In no case did we record a decrease of visual acuity by 5 or more ETDRS letters. We achieved an improvement of stabilisation of BCVA in a total of 50 eyes (92.6%), in 13 eyes (24.1%) BCVA improved by more than 3 rows, and in 53.7% by more than 2 rows of ETDRS optotypes. Our results are in congruence with the available literature [1,12,21,26,35].
However, photodynamic therapy may have potential side effects such as RPE atrophy, crack of RPE, sub-RPE haemorrhage, choroidal ischemia or secondary CNV [6,9,10]. The majority of patients in whom these complications were described were treated by PDT in the standard dosing regimen [26]. According to the available literature the anatomical and functional results are comparable between standard and HD-PDT, though it appears that the HD-PDT method is safer. Fujita et al. did not observe complicating CNV in any out of 204 eyes treated with the aid of HD-PDT [12], similarly to Karakus et al., who treated 27 eyes by the same method [21]. In contrast with these authors, Tseng describes complicating CNV in connection with HD-PDT in 2 out of 56 eyes (3.6%) [48]. In our study we also recorded the progression of manifestly demonstrated complicating CNV in 2 eyes (3.7%) within 1 month of the performance of treatment, in both cases this concerned CNV type II (thus minor classic CNV). The development of secondary CNV upon a background of cCSC has been described especially in connection with standard PDT or laser photocoagulation, though CNV may be a complication of cCSC itself [6,11,47]. On the assumption that only patients in whom the baseline examination (OCT/FAG/ICG) did not demonstrate the presence of CNV were included in our study, we consider the occurrence of CNV (type II) within one month of the performance of HD-PDT in our cohort to be a complication of this treatment. Another factor that convinces us of this is the fact that CNV occurring upon a background of long-term cCSC as a rule concerns type I neovascularisation, thus occult CNV [4].
With regard to the pathophysiology of the disease, the use of anti-VEGF preparations for the treatment of CSC is contentious. A range of clinical trials have provided ambiguous results of the effect of anti-VEGF preparations in the treatment of CSC [3,8,28]. The pathology itself is not linked with a higher level of VEGF in the vitreous body, and the effect of anti-VEGF treatment in CSC without the presence of CNV has altogether not been demonstrated [27]. However, the situation is different in complicated cases of secondary CNV upon a background of CSC. Previous clinical trials have demonstrated the anatomical and functional effect of bevacizumab, ranibizumab and aflibercept in the treatment of CNV associated with CSC [5,8,23]. However, there is only very limited data documenting the effectiveness and safety of combined therapy of PDT and anti-VEGF. Asahi demonstrated positive results of combined therapy of HD-PDT + aflibercept in patients not responding to conventional treatment (laser photocoagulation, monotherapy with PDT, monotherapy with anti-VEGF), recording more significant success above all in eyes complicated by CNV [2].
We consider the advantages of our study to be its prospective design, the complete one-year observation period for all patients, statistically assessable data, and the consistent relatively large cohort of patients treated and observed by 1 examiner at 1 clinical centre. A limiting factor is the absence of a control group (placebo or other therapeutic procedure), and this therefore does not constitute a randomised clinical evaluation.
CONCLUSIONS
Photodynamic therapy in a reduced-dose regimen is a safe and effective treatment for chronic forms of central serous chorioretinopathy. However, it is evident that the treatment of advanced diffuse and recurring forms of CSC is often very arduous, and the selection of only one therapeutic modality may not necessarily lead to a satisfactory anatomical-functional result. From this situation there follows the idea that combined therapy should possibly be reserved especially for complicated conditions of CSC, in which a conservative PDT regimen, in an ischemic terrain of a chronically damaged choriocapillaris and presence of CNV, may further enhance the expression of VEGF factors. In addition to reducing CSC activity, the application of anti-VEGF could neutralise this negative impact of PDT and thus reduce the risk of progression of CNV.
Today’s technical advances in the diagnosis and treatment of CNC in combination with the experiences of our centre represent a marked improvement of the prognosis of our patients with cCSC, for whom it is necessary to ensure not only correct diagnosis of the pathology based on multimodal imaging, but above all correct configuration and timing of adequate therapy. From the results we have so far it also ensues that the treatment of chronic forms of CSC should be commenced in the early stages so that we are able to attain the best possible anatomical and functional results, before irreversible changes are manifested, such as DRPE or complicating CNV.
Sworn declaration:
- The authors of the study declare that no conflict of interest exists in the compilation, theme and subsequent publication of this professional communication, and that it is not supported by any pharmaceuticals company.
- The authors of the study declare that this study has not been submitted to any other journal or printed elsewhere, with the exception of congress abstracts and recommended procedures.
MUDr. Kateřina Manethová, FEBOThomayerova nemocniceVídeňská 800, 140 59 Praha 4 – KrčReceived: 17. 4. 2019Accepted: 15. 1. 2020Available on-line: 20. 5. 2020
Zdroje
1. Alkin, Z., Perente, I., Ozkaya, A. et al.: Comparison of efficacy between low-fluence and half-dose verteporfin photodynamic therapy for chronic central serous chorioretinopathy. Clin Ophthalmol, 8; 2014 : 685–690.
2. Asahi, MG., Chon, AT., Gallemore, E. et al.: Photodynamic therapy combined with antivascular endothelial growth factor treatment for recalcitrant chronic central serous chorioretinopathy. Clin Ophthalmol, 11; 2017 : 2051–2056.
3. Bae, SH., Heo, J., Kim, C. et al.: Low-fluence photodynamic therapy versus ranibizumab for chronic central serous chorioretinopathy: one-year results of a randomized trial. Ophthalmology, 121; 2014 : 558–565.
4. Bonini Filho, MA., de Carlo, TE., Ferrara, D. et al.: Association of Choroidal Neovascularization and Central Serous Chorioretinopathy With Optical Coherence Tomography Angiography. JAMA Ophthalmol, 133; 2015 : 899–906.
5. Broadhead, GK., Chang, A.: Intravitreal aflibercept for choroidal neovascularisation complicating chronic central serous chorioretinopathy. Graefes Arch Clin Exp Ophthalmol, 253; 2015 : 979–981.
6. Cardillo Piccolino, F., Eandi, CM., Ventre, L. et al.: Photodynamic therapy for chronic central serous chorioretinopathy. Retina, 23; 2003 : 752–763.
7. Chan, WM., Lai, TY., Lai, RY. et al.: Safety enhanced photodynamic therapy for chronic central serous chorioretinopathy: one-year results of a prospective study. Retina, 28; 2008 : 85–93.
8. Chan, WM., Lai, TY., Liu, DT. et al.: Intravitreal bevacizumab (avastin) for choroidal neovascularization secondary to central serous chorioretinopathy, secondary to punctate inner choroidopathy, or of idiopathic origin. Am J Ophthalmol, 143; 2007 : 977–983.
9. Chan, WM., Lam, DS., Lai, TY. et al.: Choroidal vascular remodelling in central serous chorioretinopathy after indocyanine green guided photodynamic therapy with verteporfin: a novel treatment at the primary disease level. Br J Ophthalmol, 87; 2003 : 1453–1458.
10. Colucciello, M.: Choroidal neovascularization complicating photodynamic therapy for central serous retinopathy. Retina, 26; 2006 : 239–242.
11. Daruich, A., Matet, A., Dirani, A. et al.: Central serous chorioretinopathy: Recent findings and new physiopathology hypothesis. Prog Retin Eye Res, 48; 2015 : 82–118.
12. Fujita, K., Imamura, Y., Shinoda, K. et al.: One-year outcomes with half-dose verteporfin photodynamic therapy for chronic central serous chorioretinopathy. Ophthalmology, 122; 2015 : 555–561.
13. Gass, JDM.: Stereoscopic Atlas of Macular Diseases: Diagnosis and Treatment. 4th edition. St. Louis, Mosby, 1997, 1061 p.
14. Golshahi, A., Klingmuller, D., Holz, FG. et al.: Ketoconazole in the treatment of central serous chorioretinopathy: a pilot study. Acta Ophthalmol, 88; 2010 : 576–581.
15. Haimovici, R., Rumelt, S., Melby, J.: Endocrine abnormalities in patients with central serous chorioretinopathy. Ophthalmology, 110; 2003 : 698–703.
16. Huang, WC., Chen ,WL., Tsai, YY. et al.: Intravitreal bevacizumab for treatment of chronic central serous chorioretinopathy. Eye (Lond), 23; 2009 : 488–489.
17. Ie, D., Yannuzzi, LA., Spaide, RF. et al.: Subretinal exudative deposits in central serous chorioretinopathy. Br J Ophthalmol, 77; 1993 : 349–353.
18. Iida, T., Yannuzzi, LA., Spaide, RF. et al.: Cystoid macular degeneration in chronic central serous chorioretinopathy. Retina, 23; 2003 : 1–7; quiz 137–8.
19. Imamura, Y., Fujiwara, T., Margolis, R. et al.: Enhanced depth imaging optical coherence tomography of the choroid in central serous chorioretinopathy. Retina, 29; 2009 : 1469–1473.
20. Jalkh, AE., Jabbour, N., Avila, MP. et al.: Retinal pigment epithelium decompensation. I. Clinical features and natural course. Ophthalmology, 91; 1984 : 1544–1548.
21. Karakus, SH., Basarir, B., Pinarci, EY. et al.: Long-term results of half-dose photodynamic therapy for chronic central serous chorioretinopathy with contrast sensitivity changes. Eye (Lond), 27; 2013 : 612–620.
22. Kim, YK., Ryoo, NK., Woo, SJ. et al.: Comparison of visual and anatomical outcomes of half-fluence and half-dose photodynamic therapy in eyes with chronic central serous chorioretinopathy. Graefes Arch Clin Exp Ophthalmol, 253; 2015 : 2063–2073.
23. Konstantinidis, L., Mantel, I., Zografos, L. et al.: Intravitreal ranibizumab in the treatment of choroidal neovascularization associated with idiopathic central serous chorioretinopathy. Eur J Ophthalmol, 20; 2010 : 955–958.
24. Lai, TY., Chan, WM., Li, H. et al.: Safety enhanced photodynamic therapy with half dose verteporfin for chronic central serous chorioretinopathy: a short term pilot study. Br J Ophthalmol, 90; 2006 : 869–874.
25. Leaver, P., Williams, C.: Argon laser photocoagulation in the treatment of central serous retinopathy. Br J Ophthalmol, 63; 1979 : 674–677.
26. Lim, JI., Glassman, AR., Aiello, LP. et al.: Collaborative retrospective macula society study of photodynamic therapy for chronic central serous chorioretinopathy. Ophthalmology, 121; 2014 : 1073–1078.
27. Lim, JW., Kim, MU., Shin, MC.: Aqueous humor and plasma levels of vascular endothelial growth factor and interleukin-8 in patients with central serous chorioretinopathy. Retina, 30; 2010 : 1465–1471.
28. Lim, SJ., Roh, MI., Kwon, OW.: Intravitreal bevacizumab injection for central serous chorioretinopathy. Retina, 30; 2010 : 100–106.
29. L’Esperance, FA.: Ophthalmic lasers: Photocoagulation, photoradiation, and surgery. 2nd edition. St. Louis, Mosby, 1983, 606 p.
30. Manayath, GJ., Shah, VS., Saravanan, VR. et al.: Polypoidal choroidal vasculopathy associated with central serous chorioretinopathy: Pachychoroid Spectrum of Diseases. Retina, 38; 2018 : 1195–1204.
31. Maruko, I., Iida, T., Sekiryu, T. et al.: Morphologic changes in the outer layer of the detached retina in rhegmatogenous retinal detachment and central serous chorioretinopathy. Am J Ophthalmol, 147; 2009 : 489–494.
32. Michels, S., Hansmann, F., Geitzenauer, W. et al.: Influence of treatment parameters on selectivity of verteporfin therapy. Invest Ophthalmol Vis Sci, 47; 2006 : 371–376.
33. Moon, JW., Yu, HG., Kim, TW. et al.: Prognostic factors related to photodynamic therapy for central serous chorioretinopathy. Graefes Arch Clin Exp Ophthalmol, 247; 2009 : 1315–1323.
34. Mudvari, SS., Goff, MJ., Fu, AD. et al.: The natural history of pigment epithelial detachment associated with central serous chorioretinopathy. Retina, 27; 2007 : 1168–1173.
35. Naseripour, M., Falavarjani, KG., Sedaghat, A. et al.: Half-dose Photodynamic Therapy for Chronic Central Serous Chorioretinopathy. J Ophthalmic Vis Res, 11; 2016 : 66–69.
36. Nicholson, B., Noble, J., Forooghian, F. et al.: Central serous chorioretinopathy: update on pathophysiology and treatment. Surv Ophthalmol, 58; 2013 : 103–126.
37. Nicolo, M., Eandi, CM., Alovisi, C. et al.: Half-fluence versus half-dose photodynamic therapy in chronic central serous chorioretinopathy. Am J Ophthalmol, 157; 2014 : 1033–1037.
38. Nicolo, M., Zoli, D., Musolino, M. et al.: Association between the efficacy of half-dose photodynamic therapy with indocyanine green angiography and optical coherence tomography findings in the treatment of central serous chorioretinopathy. Am J Ophthalmol, 153; 2012 : 474–480.
39. Obana, A., Gohto, Y., Kaneda, K. et al.: Selective occlusion of choroidal neovascularization by photodynamic therapy with a water-soluble photosensitizer, ATX-S10. Lasers Surg Med, 24; 1999 : 209–222.
40. Piccolino, FC., de la Longrais, RR., Ravera, G. et al.: The foveal photoreceptor layer and visual acuity loss in central serous chorioretinopathy. Am J Ophthalmol, 139; 2005 : 87–99.
41. Pikkel, J., Beiran, I., Ophir, A. et al.: Acetazolamide for central serous retinopathy. Ophthalmology, 109; 2002 : 1723–1725.
42. Prunte, C., Flammer, J.: Choroidal capillary and venous congestion in central serous chorioretinopathy. Am J Ophthalmol, 121; 1996 : 26–34.
43. Reibaldi, M., Cardascia, N., Longo, A. et al.: Standard-fluence versus low-fluence photodynamic therapy in chronic central serous chorioretinopathy: a nonrandomized clinical trial. Am J Ophthalmol. 149; 2010 : 307–315.
44. Robertson, DM.: Argon laser photocoagulation treatment in central serous chorioretinopathy. Ophthalmology, 93; 1986 : 972–974.
45. Rouvas, A., Stavrakas, P., Theodossiadis, PG. et al.: Long-term results of half-fluence photodynamic therapy for chronic central serous chorioretinopathy. Eur J Ophthalmol, 22; 2012 : 417–422.
46. Smretschnig, E., Ansari-Shahrezaei, S., Hagen, S. et al.: Half-fluence photodynamic therapy in chronic central serous chorioretinopathy. Retina, 33; 2013 : 316–323.
47. Spaide, RF., Campeas, L., Haas, A. et al.: Central serous chorioretinopathy in younger and older adults. Ophthalmology, 103; 1996 : 2070–2079.
48. Tseng, CC., Chen, SN.: Long-term efficacy of half-dose photodynamic therapy on chronic central serous chorioretinopathy. Br J Ophthalmol, 99; 2015 : 1070–1077.
49. Uetani, R., Ito, Y., Oiwa, K. et al.: Half-dose vs one-third-dose photodynamic therapy for chronic central serous chorioretinopathy. Eye (Lond), 26; 2012 : 640–649.
50. Yannuzzi, LA., Slakter, JS., Gross, NE. et al.: Indocyanine green angiography-guided photodynamic therapy for treatment of chronic central serous chorioretinopathy: a pilot study. Retina, 23; 2003 : 288–298.
Prezentace: Dlouhodobé výsledky léčby chronických forem CSC v ÚVN (Manethová K, Ernest J, Hrevuš M, Hradcová Z), 19. Vejdovského olomoucký vědecký den, 17. 3. 2018, Olomouc.
Štítky
Oftalmologie
Článek vyšel v časopiseČeská a slovenská oftalmologie
Nejčtenější tento týden
2019 Číslo 6- Stillova choroba: vzácné a závažné systémové onemocnění
- Familiární středomořská horečka
- Diagnostický algoritmus při podezření na syndrom periodické horečky
- Možnosti využití přípravku Desodrop v terapii a prevenci oftalmologických onemocnění
- Normotenzní glaukom: prevalence a zásady terapie
-
Všechny články tohoto čísla
- Innovative strategies for treating retinal diseases
- Assessment of the efficacy of photodynamic therapy in patients with chronic central serous chorioretinopathy
- Sensitivity and specificity in methods for examination of the eye astigmatism
- Evaluation of retinal light scattering, visual acuity, refraction and subjective satisfaction in patients after Acrysof IQ PanOptix intraocular lens implantation
- Eyelid edema as a first sign of lymphoma
- Ocular Symptoms of Rosacea
- 100 let od narození prof. MUDr. Heleny Lomíčkové, DrSc. 40 let oční kliniky dětí a dospělých v Motole
- OCENĚNÍ ČLS JEP
- CENA PREZIDENTA ČLK
- Česká a slovenská oftalmologie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Ocular Symptoms of Rosacea
- Eyelid edema as a first sign of lymphoma
- Assessment of the efficacy of photodynamic therapy in patients with chronic central serous chorioretinopathy
- Innovative strategies for treating retinal diseases
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



