-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Screening, Treatment and Long-term Observation of Retinopathy of Prematurely Born Children in the Czech Republic
Authors: A. Zobanová 1; P. Brychcínová 1; R. Autrata 2; K. Šenková 2
Authors place of work: Soukromá oční ordinace, Kršková 807, Praha 5, 152 00 1; Dětská oční klinika, LF MU a FN Brno, Černopolní 9, 613 00 Brno, Přednosta: prof. MUDr. Rudolf Autrata, CSc., MBA 2
Published in the journal: Čes. a slov. Oftal., 74, 2018, No. 6, p. 253-264
Category: Doporučené postupy
doi: https://doi.org/10.31348/2018/6/7Binding regulations for treatment of ROP, recommended procedure of the Czech Society for Children's Ophthalmology and Strabology (CSCOS) under the patronage of the Czech Ophthalmology Society (COS)
INTRODUCTION
Retinopathy of Prematurity (ROP) is a vasoproliferative pathology affecting the development of vascularisation of the retina in prematurely born children. Its incidence is closely correlated with low gestation age and birth weight [19, 22]. Despite all the advances that have taken place in diagnosis and treatment, ROP remains one of the main causes of blindness in children [5].
In countries with a high standard of perinatal and neonatal care, thus including the Czech Republic, the pathology ROP occurs practically exclusively within the category of very premature newborns (birth between 28th and 32nd week of pregnancy, mostly with a birth weight of 1000-1500 g) and extremely premature newborns (birth before 28th week of pregnancy, mostly with a birth weight below 1000 g). During the last two decades, the quality of care of the pregnant mother, foetus and newborn has improved substantially within the Czech Republic. As a result, both general and specific newborn mortality has significantly decreased, the spectrum and lethality of neonatal morbidity had changed, and the quality of life of the surviving prematurely born children has been improved [51].
DEVELOPMENT OF RETINAL VASCULARISATION
In the human foetus, up to the 4th month of pregnancy the immature retina is without capillaries, and receives nutrition by diffusion from the choriocapillaris. The vascular system of the retina begins to develop after the 16th week of pregnancy. The growing capillaries branch out from the hyaloid artery in places of the optic nerve, and proceed centrifugally in the direction toward the periphery up to the ora serrata. At the time of birth, under physiological conditions vascularisation of the retina is complete. In a healthy eye there remains a non-vascularised strip 1-3 mm wide beyond the ora serrata even in adults. The growing capillary is formed by proliferating mesenchymal spindle cells, i.e. precursors of the endothelial cells. These grow throughout the inner layer of the retina in a direction toward the periphery and form into columns, which luminise and progressively differentiate into the aforementioned endothelial cells, thereby forming the primary retinal capillary network. The remaining mesenchymal cells die off.
PATHOGENESIS OF ROP
The fundamental observations about ROP and its development originate from the 1950s, and are based on histological findings on samples of tissue of the afflicted retina. Within the framework of development of ROP, two connecting phases have been described. The classic theory [4, 37] emphasises initial arteriolar vasoconstriction, which at first is reversible, after which there follows irreversible vaso-obliteration, in which only the main vascular stems survive. After breach of the endothelium of primitive capillaries, the surviving mature capillaries and accompanying mesenchymal tissue form pathological mesenchymal A-V shunts and thus replace the destroyed capillary network. The shunts are localised on the transition of the vascular and avascular part of the retina. Hyperoxia prolongs vasoconstriction. In the second phase a vasoproliferative response to retinal ischemia is generated, leading to neovascularisation [6, 38]. The second “gap junctions” theory [23] accents the role of hyperoxia, specifically oxidation stress with the generation of abnormal connections referred to as “gap junctions” (pathological links between neighbouring spindle cells), which impair normal cellular migration and the formation of capillaries.
Expanding knowledge about the pathogenesis of ROP has progressively revealed further mechanisms in connection with the origin and progression of changes after premature birth leading to pathological vascularisation of the immature retina. It has long been known that oxygen plays a key role in the etiopathogenesis of ROP. Low or high levels of PaO2 regulate the normal or abnormal production of hypoxia-inducible factor 1 (HIF-1) and vascular endothelial growth factors (VEGF), which are decisive regulators of angiogenesis of the retina [36, 49]. Despite to the fact that it has been demonstrated that carefully monitored low saturation by oxygen reduces the risk of occurrence of severe ROP, its optimal level remains unclear. The actual development of the pathology, as stated above, takes place in two phases – the initial “avascular” phase leading to chronic hypoxia of the retina and the secondary “proliferative” phase, inducing vasodilation and angiogenesis. The main modulators of the progression of ROP during both phases are “insulin-like growth factor 1” (IGF-1) and erythropoietin (EPO) [26, 42]. In addition to this, the etiopathogenesis of ROP is contributed to by a whole cascade of further factors such as nitric oxide, adenosine, noradrenalin, β-adrenergic receptors, genetic factors and others [9]. All these factors altogether function as mediators of the progression from the avascular to the proliferative phase, and a deeper understanding of their role in the origin and development of ROP may help improve its effective and safe treatment.
DESCRIPTION OF CLINICAL FINDING AND CLASSIFICATION OF ROP
ROP was first described by Terry in 1942 as retrolental fibroplasia (RLF), which represents only its most severe 5th stage [46]. In the 1950s a two-phase classification of ROP emerged, differentiating the active and cicatricial phase. In the Czech Republic the issue of ROP was the lifelong focus of the work of professor Lomíčková [28, 29], whose publications were almost 30 years ahead of their time. At present the International Classification of Retinopathy of Prematurity (ICROP) from 1984 is used [12], revised and supplemented in 2005 with further specification of the evaluation of vascular changes [45].
The ICROP classification evaluates:
- a) localisation of changes on the retina according to defined zones I-III
- b) extent of affliction of the retina according to 30 grade sectors corresponding to a clock face
- c) stage of severity of the pathology according to structural changes on the border of the vascularised and avascular part of the retina
- d) presence or absence of abnormally spread and coiled capillaries on the posterior pole of the eye, referred to as plus signs of the disease
It also defines aggressive posterior form of ROP (APROP), afflicting in particular extremely premature newborns with an extremely low birth weight [45].
Localisation of changes
Localisation of changes on the retina (fig. 1) is of fundamental significance from the perspective of evaluating the prognosis and differences in timing therapeutic intervention in the individual forms of ROP. In general it applies that the closer the changes are to the posterior pole of the eye, the worse the prognosis and the sooner the need for treatment.
Fig. 1. Classification of retinopathy of prematurity (ROP) according to localisation 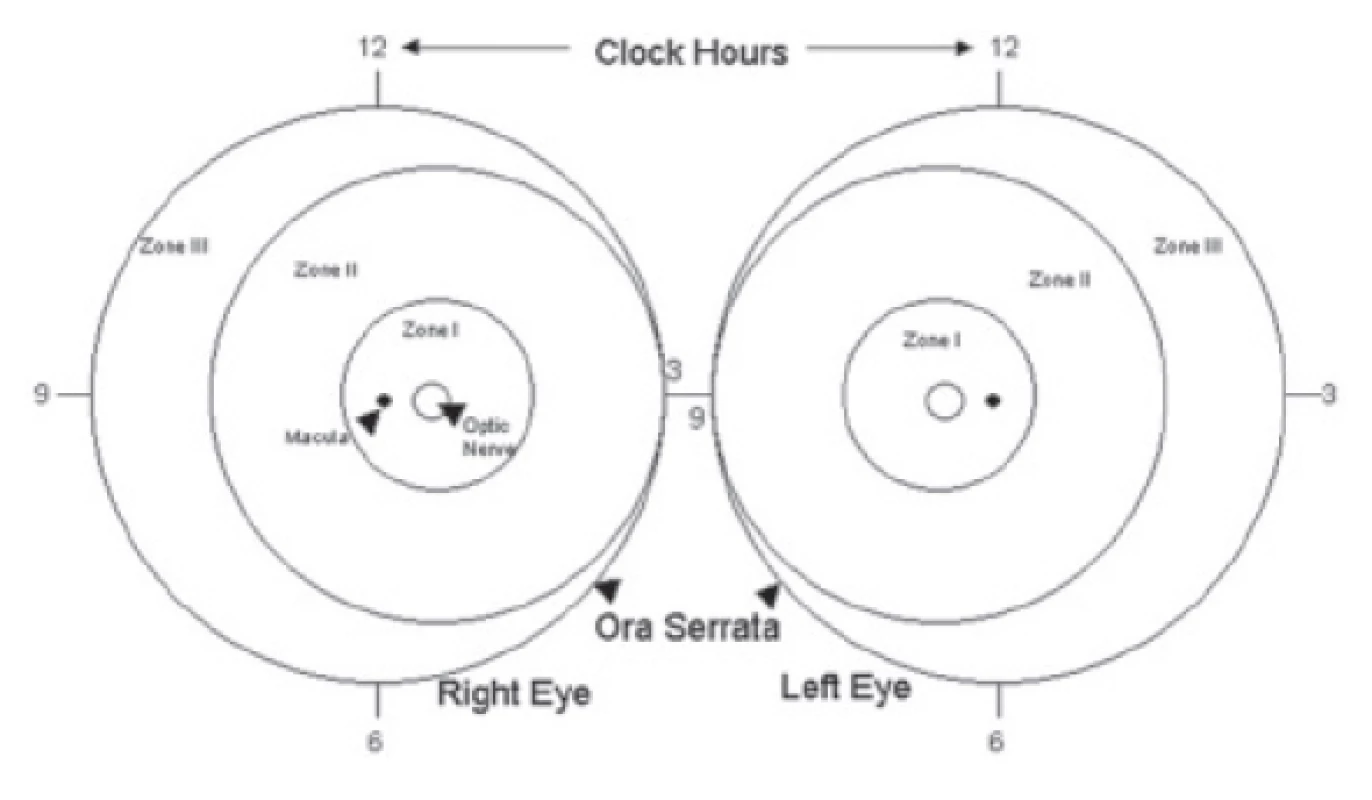
Zone I – circular zone of posterior pole with centre in the middle of the disc of the optic nerve, the radius of the circle determining the border of the zone is twice the distance between the optic nerve and the macula (fig. 1). From a practical perspective it is possible to evaluate the extent of the first zone referentially with the aid of indirect ophthalmoscopy with the use of a 28 D lens, in which we centre the nasal edge of the visual field to the middle of the disc of the optic nerve. The temporal edge of the visual field of the lens then expresses the outer limit of zone I.
Zone II – circular band with centre in the disc of the optic nerve. It connects to zone I, the outer limit is nasally the edge of the ora serrata, temporally it corresponds anatomically approximately to the elevator of the eye (fig. 1). A clinical aid for evaluating the temporal limit of zone II may in some cases be the exit of the vorticose veins from the eyeball. If it is visible, it forms a characteristic appearance of a rosette on the ocular fundus.
Zone III – remaining sector of crescent shape connecting temporally to zone II (fig. 1). This is the most remote part of the retina, where the development of vascularisation takes longer and ends latest due to the nasal shift of the optic nerve.
The zone with the most advanced changes in the given eye determines the classification of ROP into the indication criteria for the therapy of this eye.
Extent of affliction of retina
The extent of changes on the retina is traditionally evaluated with the aid of 30 grade sectors according to a clock face of 1-12. In the classic form of the development of ROP, pathological changes on the interface of the vascularised and avascular part of the retina appear first of all in the temporal part of the retina. At present, however, we are also encountering less typical forms of ROP, in which the changes are localised first of all nasally. ROP very often progresses rapidly with changes in this localisation.
Stages of ROP
Retinopathy of prematurity takes place in five developmental stages, which are characterised by structural changes ensuing on the border of the already vascularised and avascular part of the retina.
Stage 1 – demarcation: State in which growing capillaries suddenly end at the line separating the choroid and the avascular zone. This is a relatively fleeting stage, with the possibility of both progression and regression, and normalisation of the further development within a few weeks. Morphologically this concerns hyperplastic mesenchymal tissue of the spindle cells, which through its expansion and increase in thickness becomes visible. Upon progression to higher stages this tissue forms a wide, translucent surface before the fornix, or up to the ora serrata.
Stage 2 – intraretinal ring – fornix - ridge: The demarcation line grows in breadth and thickness, and then rises as a ridge above the level of the retina. It may be of white or pink colour. Morphologically, in addition to hyperplasia of mesenchyma it concerns a differentiation and organisation of the endothelial cells, and as a result the fornix has two clear contours with a relatively hypocellular zone in the middle. The absence of fibrovascular proliferation differentiates the 2nd stage from the 3rd stage. In this stage small isolated clusters of fibrovascular tissue may appear in the region of the posterior fornix, lying on the surface of the retina, referred to as “popcorn”. They do not yet represent the beginning of the 3rd degree of ROP, but their presence indicates a markedly higher risk of further progression of the pathology [48].
Stage 3 – extraretinal fibrovascular proliferation: In this stage extraretinal fibrovascular proliferation ensues from the anterior and posterior contour of the fornix, spreading from the surface of the retina into the vitreous body. Contraction and scarring of these accompanying fibrous changes later occurs, which by pulling cause tractional retinal detachment and thereby transition to further more severe stages of ROP.
Stage 4 – partial amotio: This concerns partial tractional retinal detachment (4a – without affliction of the macula, 4b – including affliction of the macula). Tractional retinal detachment may later be complicated by subretinal exudation, which further worsens the condition.
Stage 5 – total amotio: Complete retinal detachment, formerly referred to as retrolental fibroplasia (RLF)/ retrolental fibrovascular membrane.
The stage of ROP in the given eye is determined by the place with the most advanced changes in this eye.
Plus form of ROP
According to the revised classification from 2005 [45], the condition of the capillaries is a component of the evaluation of ROP, and this is referred to as “Plus” form or “Pre-plus” form. This is defined as increased dilation and tortuosity of the capillaries of the posterior pole of the eye. Accompanying manifestations of the Plus form of the pathology may be rigidity of the pupil, dilation of the iris capillaries or vitreal haze and retinal haemorrhages. It is important to differentiate between “pre-plus” and “plus” signs, which characterise the activity and progression of the pathology.
“Plus” signs are divided into three levels of progression: mild, medium severe and severe.
“Pre-plus” signs are signs of pathology defined as vascular abnormalities of the posterior pole, which are insufficient for a diagnosis of “plus form” of ROP, but document greater arterial tortuosity and venous dilation than a usual physiological finding.
“Plus” represents a florid, more active form of ROP, in which the possibility of spontaneous regression is negligible, and such a finding is a signal of a significantly worse prognosis.
Aggressive posterior form of ROP (APROP)
This term is reserved for a finding of structural changes on the retina, which cannot be unequivocally classified into any of the above-presented stages of ROP, and was previously referred to as “rush form”. It afflicts the posterior part of the retina, i.e. zone I to zone II posterior. It is considered the most severe and rapidly progressing form of ROP. It does not pass through the classic stages, there is frequently not even a present demarcation line, only discrete, easily overlooked signs in the region of the transition between the vascular and avascular part of the retina. Rapidly developing “Plus” signs dominate in all four quadrants, in the case of severe “Plus” signs it is not possible to distinguish the artery from the veins due to the influence of pronounced vasodilation and tortuosity. Arteriovenous shunts may appear not only on the boundary of the vascularised and avascular part of the retina, but also between the capillaries in the vascularised part. Haemorrhages may also be present here. In ROP of zone I, flat, inconspicuous retinal proliferation develops, without perceptible propagation into the vitreous body, so a conventional fornix does not form. APROP may thus jump within the course of a few days from stage 1 to stage 3, without the possibility of distinguishing a typical stage 2 of ROP. The image of the early phase of stage 3 ROP in posterior zone II may be so innocuous that an inexperienced doctor may be lulled into a false sense of security and overlook the development of stage 3 ROP, with simultaneously incipient tractional amotio.
Untreated APROP always progresses to the final stage of ROP. After the first laser treatment it may regress, but usually reactivates. Reactivation is characterised by a return of “Plus” form of the pathology, progressive contraction of the posterior vitreous membrane and incipient posterior tractional retinal detachment. The reason is that there is a deficiency of orientation points for precise laser treatment in the surface of the imperceptible transition between the vascularised and avascular retina. Although ROP appears to be regressing, the untreated parts of the avascular retina persist as a source of VEGF, and continue to influence the course of ROP. Furthermore, although laser photocoagulation of the retina destroys the cells producing VEGF, it does not influence the already existing level of VEGF in the vitreous body. VEGF therefore continues to act despite the timely and virtually complete destruction of its source. This explains the usual failure of transpupillary laser treatment in the case of APROP. From this perspective it appears logical to use anti-VEGF preparations as the first choice in the treatment of these refractory and fulminant forms of ROP [15, 35]. Another reason for the administration of anti-VEGF preparations for APROP in zone 1 is the time which we gain until any applicable further reactivation of ROP. During this time, the development of vascularisation continues in a direction toward zone II, and the surface of the retina requiring destruction by photocoagulation in the next therapeutic procedure, if necessary, is markedly smaller.
SCREENING OF ROP
Screening is performed by an experienced ophthalmologist trained in this specialisation, using indirect ophthalmoscopy. The examination can be supplemented by photo documentation with the aid of a portable wide-angle digital fundus camera RetCam.
Recommended equipment: Indirect binocular ophthalmoscope, convex lens, wide-angle digital fundus camera RetCam, eyelid speculum, scleral depressor or muscle hook (commonly used for strabismus operations) for applicable indentation.
The fundamental prerequisite for screening examination is flawless artificial mydriasis of the pupil. Mydriatic eye drops are either parasympathetic blockers (e.g. homatropine, tropicamide, cylopenatolate), which have an influence on the muscles of the pupillary sphincter, or sympathetic stimulants (e.g. phenylephrine hydrochloride), which influence the muscles of the pupil dilator. For this reason, for quality mydriasis we use a combination of both types of mydriatics. No serious adverse systemic effects have been reported. Fluctuations of heart rhythm due to pressure of the speculum and hook during a protracted and unsparing examination should not be confused with side effects of mydriatics.
Local anaesthesia with the aid of local anaesthetic eye drops (oxybuprocaine hydrochloride, 1 or 2 drops, 30-60 seconds before examination) becomes more important especially in situation in which we consider the possibility of using a speculum or if we envisage the use of scleral indentation during the examination, and also before the use of a fundus camera RetCam.
Screening of ROP is performed on all newborn children born up to the 31st week of gestation inclusive, or with a birth weight of up to 1500 g inclusive. At least one of the above criteria is sufficient for inclusion. Also included for screening are newborns with a birth weight of 1500-2000 g of birth after the 31st week of pregnancy, who due to post-natal instability required cardiorespiratory support [3]. All children born before the 32nd week of gestation or with a birth weight of 1 500 g or lower should undergo at least one screening examination for ROP before being discharged [52]. The onset of ROP symptoms correlates better with post-conception age (i.e. gestation age at birth + chronological age after birth of child). As a result, the commencement of ROP screening should be governed according to post-conception age [3]. The timing of the first ROP screening is presented in table 1.
Tab. 1. Designation of first screening examination for ROP 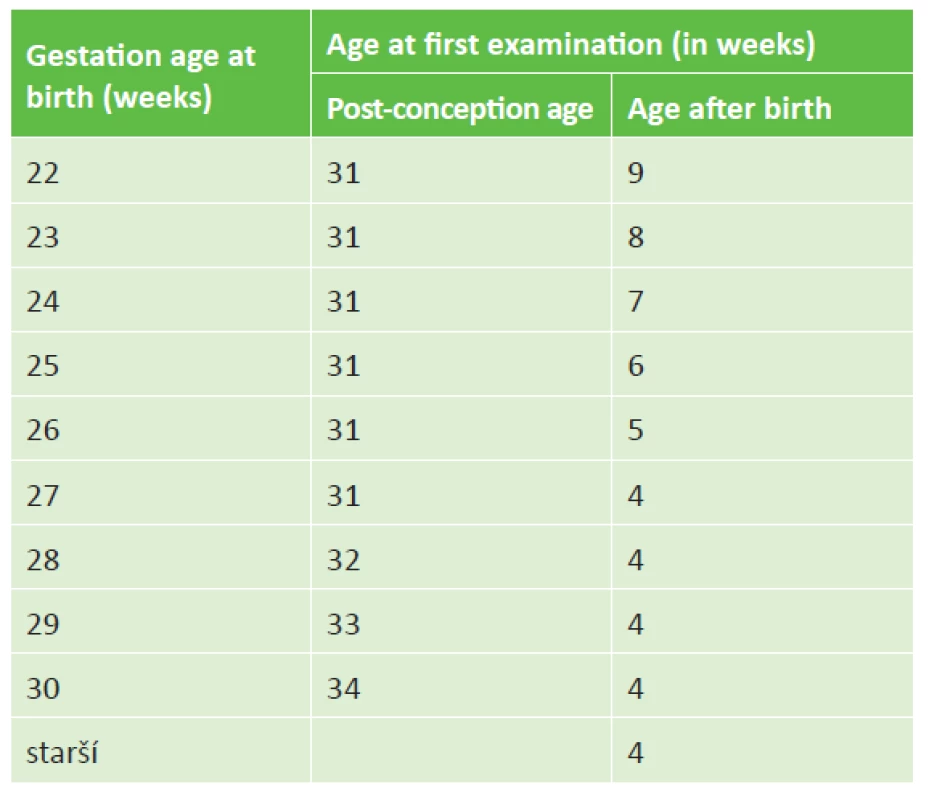
We perform screening examinations at an interval of 1-2 weeks according to the finding on the retina and the age of the child. A minimum frequency of screening is set at 1x per week in the case that the capillaries end in zone I or posteriorly in zone II, or if any signs of “Pre-plus” or “Plus” form of the pathology are perceptible, or if any 3rd stage of ROP is present, in any zone. From the moment of origin of signs of ROP, the frequency of examinations is set by the ophthalmologist individually according to the current finding.
ROP screening is performed up to the end of vascularisation of zone III of the retina, usually until the 40th post-conception week of age of the child, thus up to the originally expected date of birth. Screening may also be concluded earlier if the child is beyond the risk of development of ROP. This occurs if the development of vascularisation progresses into zone III, in children without the development of ROP usually after the end of the 36th week of gestation.
We monitor children with ROP until complete regression of the finding on the retina, either spontaneously or following a therapeutic procedure (decrease of “staging” and continuation of vascularisation up to the periphery), often in outpatient conditions, but always at centres engaged in the treatment of ROP.
After discharge for home care, the child is transferred into the system of long-term observation [40]. The neonatal centre, following an agreement with a consulting ophthalmologist, is obliged to ensure subsequent care nominally with an experienced ophthalmologist. In the case of reactivation of ROP or doubts concerning ongoing regression, the child is transferred back into the care of the consulting ophthalmologist of the original parent centre. The interval of examinations in long-term observations is determined by the severity of the course of the pathology, its origin, extent of treatment and regression of changes.
INVOLUTION RESULTS OF ROP ACCORDING TO ICROP 2005 [45]
ROP stage 1 and 2 regresses without structural consequences, ROP stage 3 regresses spontaneously in 50% of cases, but may leave some of the following after effects:
Changes on posterior pole
A. Vascular
- Persisting tortuosity
- Raising of temporal arcades
- Sharp angle at interval of temporal arcades
B. Retinal- Pigment changes
- Distortion and dislocation of macula
- Pulling and folding of macular region in direction toward periphery
- Changes on vitreoretinal interface
- Vitreous fibrous changes
- Retinal traction drawing across papilla or deforming papilla
- Tractional-rhegmatogenous retinal detachment
Changes in retinal periphery
A. Vascular
- Failure of development of peripheral vascularisation and its premature termination leaves a wide avascular band before the ora serrata
- Abnormal, non-dichotomous branching of retinal capillaries
- Abnormal conjunctivas between vascular arcades
- Teleangiectasia
B. Retinal- Pigment changes
- Changes on vitreoretinal interface
- Vitreous fibrous changes with or without adhesion to retina
- Peripheral folding of retina
- Lattice-like degeneration
- Thinning of retina
- Retinal cracks
- Tractional-rhegmatogenous retinal detachment
SUMMARY OF RECOMMENDATIONS
In order to achieve sufficient mydriasis of the pupil we apply 3x phenylephrine 2.5% (Neosynephrine 10% diluted by Aqua for injection 1 : 3) and homatropine 2.0% (magistraliter) progressively, always at an interval of approx. 5-10 minutes, 1 hour before the examination.
Local anaesthesia before the use of a speculum and fundus camera – oxybuprocaine hydrochloride 0.4% (Benoxi gtt, 1 or 2 drops, 30-60 seconds before examination).
It is necessary to make a record of the finding after every screening examination. In the case of a suspect or positive finding of developing ROP, the afflicted zone, extent and degree of changes and the condition of the capillaries must be defined. The conclusion must contain an evaluation of the finding in the sense of the physiological finding, suspect changes or clear diagnosis of ROP. It must also contain the timing of the next ocular examination or indication for surgical solution, or information about termination of screening, and finally the signature of the doctor who performed the examination.
The zone with the most advanced changes in the given eye determines its inclusion in the indication criteria for the therapy of that eye.
The stage of ROP in the given eye is determined by the place with the most advanced changes in that eye.
If RetCam is a component of the equipment of the ophthalmological or neonatal centre, it is necessary to obtain photo documentation in ROP stage 2 and higher at least once before discharge or transfer to another centre.
Every child from the risk group must have at least one ocular screening examination at the parent perinatal centre, and any applicable transfer to another, intermediary centre is possible only after ensuring regular ocular follow-up examinations by an experienced ophthalmologist. In the case of discovery of signs of progressing ROP, it is necessary to transfer the child back to a centre which is capable of ensuring the appropriate therapy.
INDICATION FOR TREATMENT OF ROP
In 2003 the Early Treatment for Retinopathy of Prematurity (ETROP) study demonstrated that early coagulation therapy is linked with better structural and functional results 9 months after treatment in comparison with the commencement of therapy in the later stages of ROP [17]. This study newly defines Type 1 ROP with a high risk of the development of retinal detachment and subsequent blindness, which requires an immediate therapeutic procedure, and by contrast Type 2 ROP, with a realistic possibility of spontaneous regression. Although this second group of ROP is not indicated for an urgent surgical procedure, it requires further careful observation, either leading to later therapy or until signs of regression of the finding. The revised results of the ETROP study are today the worldwide basis for the recommended procedures for the therapy of retinopathy of prematurity (table 2).
Tab. 2. Indication criteria for treatment of ROP 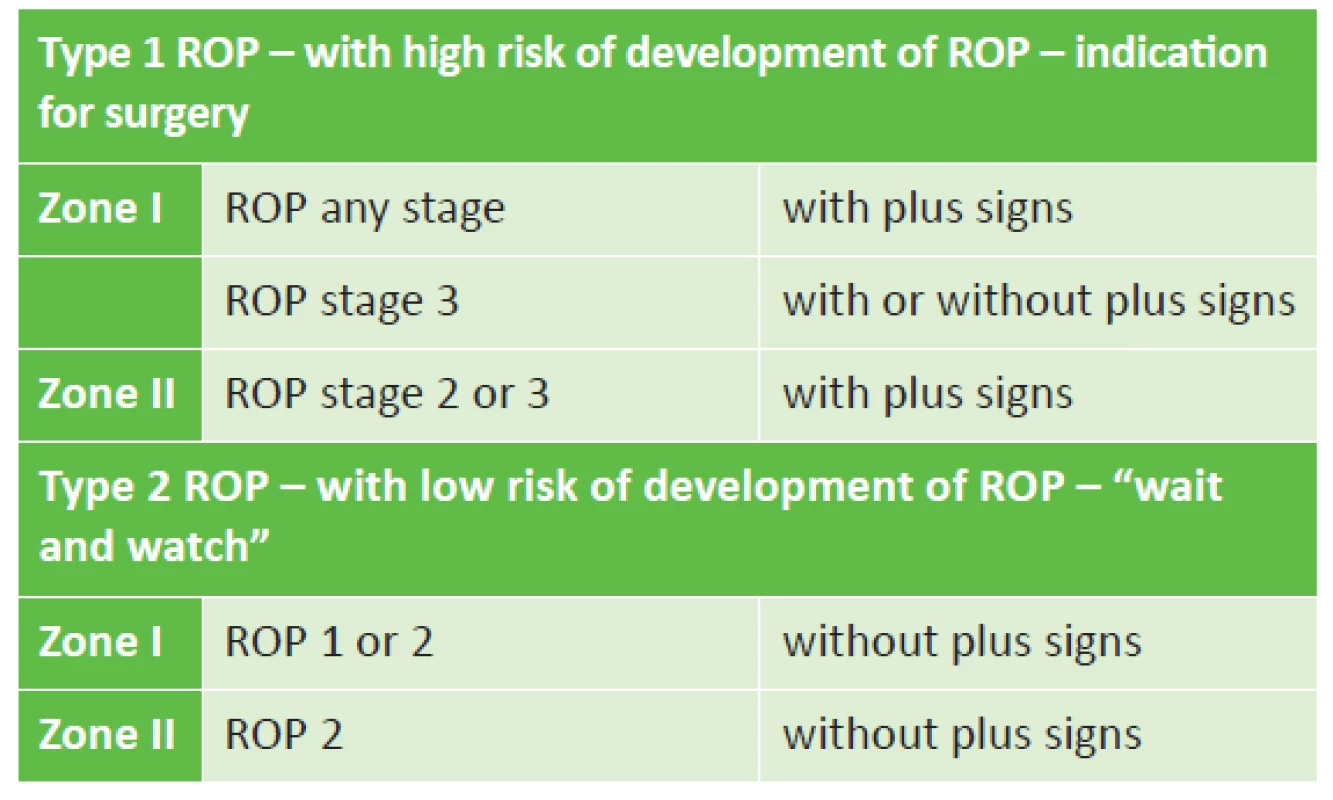
The revised ETROP study from 2003 [16] replaces the original conclusions of the multicentric CRYO-ROP study from 1988 [34], which indicates treatment on the basis of the “threshold” stage, i.e. the extent of change of stage 3+ of ROP in five connected sectors of the clock face or in a total number of eight sectors on the clock face. The “pre-threshold” stage is considered to be the extent of changes of stage 3, which does not reach the threshold stage. The ETROP study [16] considers whether this concerns type 1 ROP with a high risk of progression of ROP, which is an immediate indication for surgery, or whether it concerns type 2 ROP with a low risk of progression, which requires thorough observation (table 2). In the case of a borderline finding between the two types, the indication and decision on the method of treatment is a matter for the ophthalmologist.
TREATMENT OF ROP
The principle of the only successful treatment of ROP to date consists in reducing the level of VEGF in the eye. We can achieve a reduction of VEGF either through a reduction of the surface of the avascular retina, which produces VEGF, i.e. by thermocoagulation (photocoagulation or cryocoagulation) and/or unbinding of already generated VEGF with the aid of intravitreal application of its antibodies, i.e. “anti-VEGF” preparations.
According to the customs of different centres, treatment of retinopathy of prematurity is performed under regular analgosedation and local anaesthesia or under general anaesthesia of the premature newborn under the supervision of an experienced neonatologist or anaesthetist. In the ideal case this concerns close mutual co-operation of both specialists. For the possibility of the correct performance of the therapeutic procedure, it is necessary to ensure maximum artificial mydriasis, which we attain by means of a combination of drops of phenylephrine hydrochloride (2.5%) and homatropine (2%), or if applicable tropicamide (0.5%). Eyelid speculums are used for opening the eyes.
RETINAL THERMOCOAGULATION
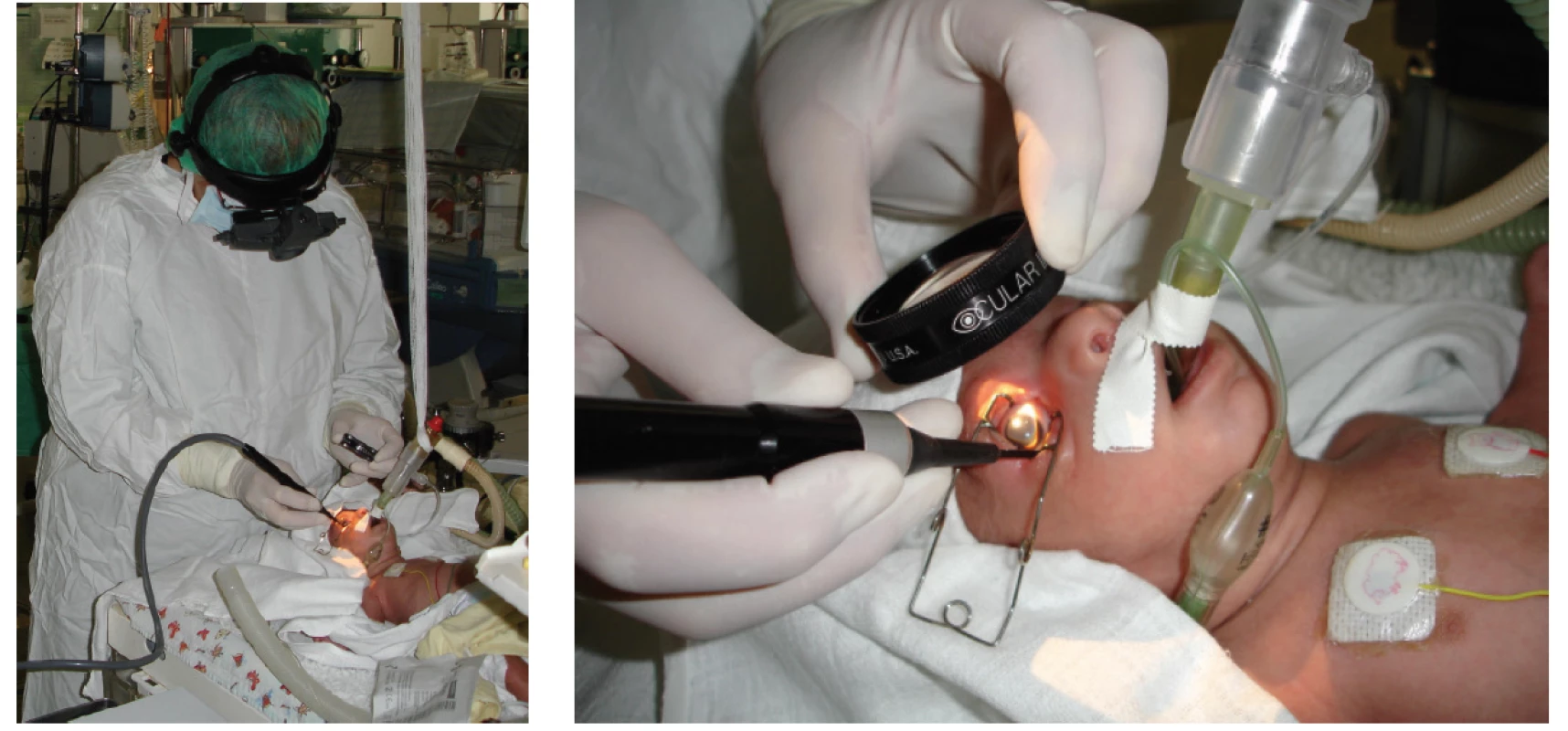
Transpupillary laser photocoagulation of the retina (fig. 2) is considered the “gold standard” of treatment. In comparison with cryotherapy it is connected with better long-term results, both structural and functional [13]. It is performed by means of a diode laser placed on the head of the operating surgeon, under general anaesthesia or regular analgosedation supplemented by local anaesthesia under ophthalmoscopic supervision. 400-1000 points are applied to each eye via the pupil into the avascular region of the retina. The effectiveness of the correctly targeted laser ray is confirmed by the formation of a white point on the retina. The advantage of transpupillary photocoagulation is the possibility of flawless treatment of the entire band of the avascular retina on the interface of the vascularised and avascular part. It is not possible to perform the operation with a narrow, rigid pupil, upon filling of the capillaries of the pupillary membrane, upon greater thickening of the avascular part or upon greater opacity of the optic media. As a result, it is not possible to wait so long with the indication of the method as with cryotherapy. After the procedure, local application is commenced with a combined preparation of antibiotics with corticoids 4x per day over a period of 3-4 days as prevention against the onset of postoperative uveal reactions.
Fig. 2. Transpupillary surgery on the retina with diode laser, with indirect ophthalmoscopy 
Transscleral laser photocoagulation by diode laser is a procedure requiring less time than transpupillary photocoagulation. For the treatment of the avascular part of the retina it is usually necessary to apply within the range of 300-500 laser points. It can also be performed upon relatively worse transparency of the optic media, and is more sparing to the ocular tissues than cryotherapy. Despite this, the performance of transscleral laser photocoagulation of the retina is hampered by the same risk of imperfect treatment of changes of ROP in posterior zone II.
The main difference between both basic techniques of laser treatment (transpupillary and transscleral photocoagulation of the retina) is determined by the energy and time of action of the laser ray on the ocular tissue. In the case of transpupillary laser photocoagulation this concerns energy regularly within the range of 200-500 mW at times of around 200 ms in comparison with transscleral laser photocoagulation, in which we work with higher energy, usually in an interval of 500-1500 mW at times of 400-800 ms.
Cryotherapy of the retina (fig. 3) to date ranks among the standard therapeutic procedures for progressing ROP. It is performed with the aid of a specially adapted retinal probe for infants with a temperature of -80 °C at the end of the tip, via the conjunctiva transsclerally under general anaesthesia or under regular analgosedation supplemented by local anaesthesia under ophthalmoscopic supervision. The exposure time is until indication of whitening of the retina. It is possible to perform cryotherapy also upon partial opacity of the optic media and upon higher degrees of ROP with larger edema of the periphery of the retina. 20-50 points are applied (according to the size of the probe used) to each eye circularly into the avascular region of the retina, copying the line of the fornix. Cryotherapy is a technically simpler and faster method. However it causes more destruction to the retina and is not capable of correctly treating the region close to the posterior pole of the eye. Frequent postoperative relative complications of this technique include haematoma and swelling of the eyelids, chemosis and subconjunctival suffusion, laceration of the conjunctiva, vitreous or retinal haemorrhage, and above all excessive or undesirable retinal scarring in the postoperative period. From the long-term perspective, another complication of cryotherapy is the higher incidence of short-sightedness in prematurely born children treated by this technique. As a result, today this treatment is recommended only in isolated cases. After the procedure local application of antibiotics with corticoids is launched 4x per day for a period of 3-4 days as prevention against the onset of postoperative uveal reactions and infection.
Both methods of transscleral thermocoagulation of the retina have their advantages and drawbacks. Upon treatment of the threshold stage of ROP in zone II-III, both methods are practically identically effective. Both methods can be used in combination with transpupillary laser photocoagulation in order to attain optimal therapeutic treatment of the retina upon localisation of ROP on the interface of zones I and II if it is necessary to shorten the time of the procedure under general anaesthesia.
Correctly timed and performed retinal thermocoagulation is irreplaceable for preserving an as yet unaltered, lying retina. Further surgical interventions then only anatomically and functionally repair the severely afflicted intraocular structures.
An exception and a separate chapter in the indications of therapy is formed by the above-described APROP (aggressive posterior form of ROP) and ROP in zone I, which are primarily indicated for intravitreal application of anti-VEGF preparations.
Before the procedure, photo documentation of the finding is always obtained with the aid of a fundus camera RetCam (if this is a component of the equipment of the ophthalmological or neonatal centre).
TREATMENT BY INTRAVITREAL APPLICATION OF ANTI-VEGF PREPARATIONS
It ensues from the results of the studies that vascular endothelial growth factor (VECF) produced by retinal cells of the avascular retina plays an important role in the pathogenesis of ROP [43]. Therapeutic procedures used to date, i.e. laser photocoagulation and cryotherapy, in their effect directly destroy these cells. In recent years, however, there is an increasing amount of evidence supporting the use of targeted pharmacological inhibition of already formed VEGF in the treatment of ROP [1, 2, 10, 11, 14, 18, 20, 21, 24, 25, 27, 31-33, 38, 41, 44, 47, 50]. It has been demonstrated that treatment with anti-VEGF preparations enables an appropriate development of vascularisation of the retina, and is linked with a lower incidence of refractive errors and better visual acuity.
Intravitreal application of anti-VEGF (fig. 4) is unequivocally indicated upon APROP and ROP type 1 (table 2) in zone I, and is often considered the method of first choice in the case of rapidly progressing ROP in posterior zone II, with expressed signs of “Plus” form of the pathology. Before intravitreal application, the parents must sign an informed consent form, since this concerns “off-label” treatment. For intravitreal application of anti-VEGF it is possible to use either originally filled syringes or sterile syringes prepared by a pharmacy, used for the application of insulin. The dosing depends on the type of preparation used. Before the procedure, photo documentation of the finding on the retina is obtained with the aid of a portable wide-angle fundus camera RetCam. The anti-VEGF preparation is applied intravitreally under general anaesthesia, under strictly sterile conditions with rinsing of the conjunctival sac with Betadine (dilution 1 : 10) for a period of 90 s, using a needle 30 G ½ 0.3 x 13 mm. The injection is made at a distance of 1.0 to 2.0 mm from the limbus (taking into account the age and size of the child, specifically the size of the eyeball) most often by no. 6 on the clock face. After application, local antibiotic prophylaxis with fluoroquinolones (ofloxacin, ciprofloxacin) is commenced 4x per day for a period of 3-4 days. The advantages of intravitreal treatment consist in its fast application and thereby the reduction of the burden of long general anaesthesia. After application of the antibody, in the following days we monitor not only the subsidence of the severity of ROP, but also often the restoration of the natural development of vascularisation up to the periphery of the retina. A fundamental benefit is the fact that the treatment does not generate secondary structural changes in the posterior pole of the eye, especially in the macular region (deformation of papilla, dislocation or degenerative changes of macula). Supplementary laser therapy if applied destroys the already reduced surface of the peripheral part of the retina [6-8, 31].
Fig. 4. Intravitreal application of anti-VEGF 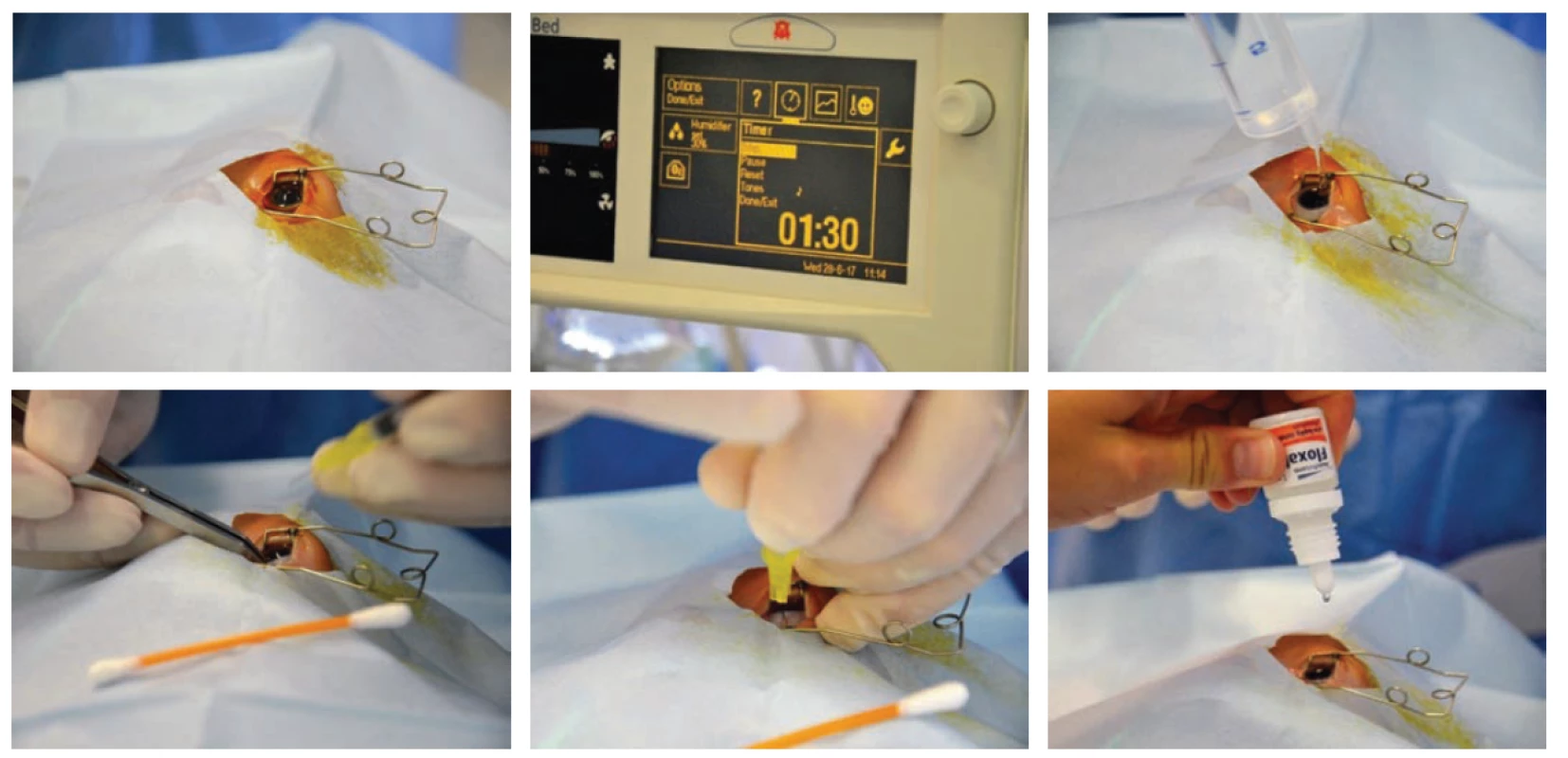
The exclusion criteria for intravitreal treatment with anti-VEGF are eye infections over the course of 5 days before application [39].
PHOTO DOCUMENTATION OF FINDINGS ON OCULAR FUNDUS OF NEWBORNS
Photo documentation of abnormal findings on an immature retina (fig. 5) should at present be a standard component of the healthcare documentation of the child. Digital photo documentation using a portable wide-angle fundus camera RetCam is becoming available in all regional centres of perinatal and neonatal care. Photo documentation enables not only archiving of findings, but above all provides the possibility of assessing larger sections of the retina continually, to focus precisely and compare the extent of the pathological findings and their changes over time, and enables distance consultation of the finding. Last but not least, it serves as a document of correct indication of treatment of ROP.
Fig. 5. Fotodokumentace pomocí Retcam 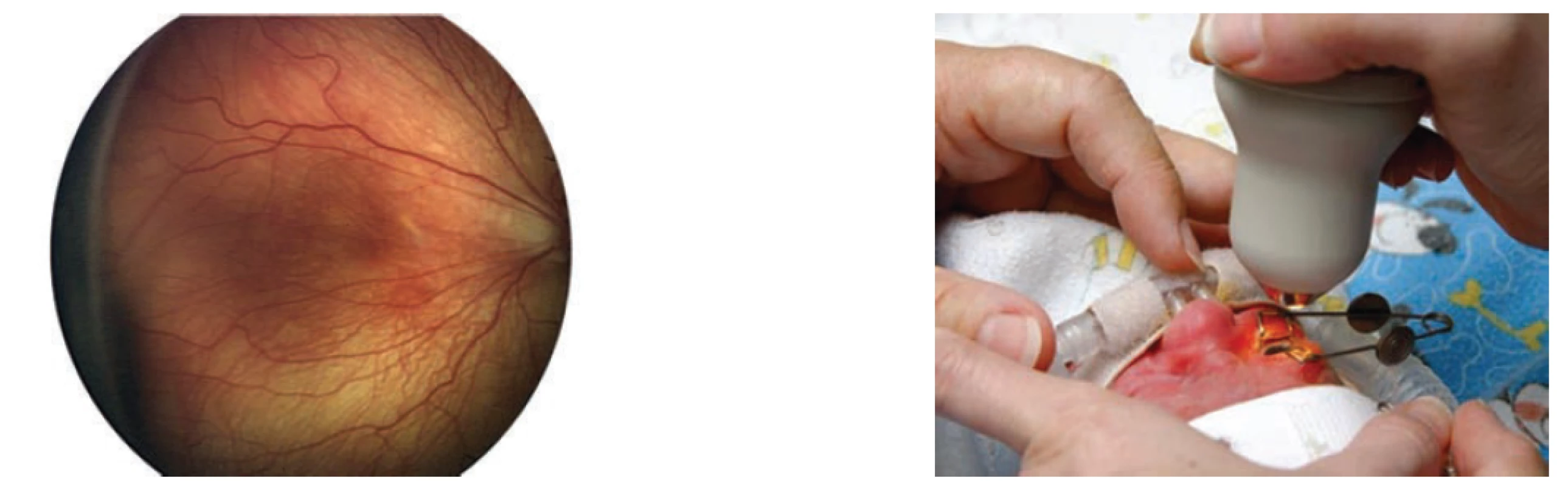
The sensitivity of digital display systems is highly dependent on the quality of the obtained photo documentation. From this perspective, digital display systems therefore do not replace conventional methods of examination by indirect ophthalmoscope, but can be understood as an appropriate supplement to the conventional method of examination.
Summary of recommendations:
It is necessary to clearly define what type of ROP this concerns (type 1 or 2).
In the case of necessity of a therapeutic procedure, the recommended time for its performance is 24-72 hours from the determination of the diagnosis meeting the indication criteria, but it is up to the ophthalmologist to assess whether this concerns an urgent surgical procedure, or whether a certain delay is admissible. The commencement of treatment in the case of APROP is necessary as soon as possible, within 48 hours at the latest, as is the case in type 1 ROP.
The choice of the first therapeutic treatment of ROP is determined by the zone of ROP. Upon treatment of ROP in zone II-III, transpupillary laser photocoagulation is the method of first choice (if a head laser is a component of the equipment of the ophthalmological or neonatal centre). Photocoagulation is also preferred upon localisation of ROP in posterior zone II. An exception is a finding of APROP, ROP in zone I and on the interface of zone I and posterior zone II, which are primarily indicated for intravitreal treatment with anti-VEGF.
Cryotherapy is a technically simpler and faster method. However, it causes more destruction to the retina and is unable to correctly treat the region close to the posterior pole, and as a result we use it only in the case that it is not possible to perform transpupillary laser photocoagulation as the method of first choice. An alternative therapeutic choice is transscleral laser photocoagulation.
Before each surgical procedure the parents sign an informed consent form, and photo documentation of the finding on the retina is obtained with the aid of a portable wide-angle fundus camera RetCam (if this is a component of the equipment of the ophthalmological or neonatal centre). It is necessary to obtain photo documentation of ROP in order to document the correct indication of the surgical procedure. Further photo documentation is obtained by the ophthalmologist according to his/her own discretion, but always upon worsening of the finding and before discharge of the child.
The exclusion criteria for intravitreal treatment with anti-VEGF are ocular infections over the course of 5 days before application.
Follow-up examinations after surgical procedure (day of performance of procedure is defined as day 0):
- a) After transpupillary laser photocoagulation: 5th - 7th day after procedure and then according to the finding and discretion of ophthalmologist until regression of finding.
- b) After transscleral laser photocoagulation / cryotherapy of retina: 5th - 7th day after procedure and then according to finding and discretion of ophthalmologist until regression of finding.
- c) After intravitreal treatment with anti-VEGF: 1st, 3rd - 4th day, 7th day and then according to finding and discretion of ophthalmologist until regression of finding.
SUPPLEMENTARY TREATMENT
In the case of an unsatisfactory response to the treatment of first choice, it is necessary to perform a further therapeutic procedure, i.e. “re-treatment”. It is possible to supplement the first type of treatment used or to transfer the patient to the second type of procedure. Upon insufficient or zero regression, the choice of supplementary therapy is entirely in the hands of the ophthalmologist. Patients after anti-VEGF can be transferred for laser photocoagulation and vice versa after laser photocoagulation to anti-VEGF therapy (this may relate to both eyes or only one eye) [39].
Signs of regression are considered to cover regression of changes in the capillaries, i.e. thinning or normalisation of width of capillaries, subsidence or disappearance of their tortuosity and visible or full reabsorption of haemorrhages. Regression on the periphery is attested to by a reduction, thinning or disappearance of the fornix, subsidence or disappearance of pre-retinal fibrotic changes.
Choice of therapy after treatment with anti-VEGF is governed by type of regression
If no regression takes place, or if the regression of the finding in the capillaries is minimal in comparison with the finding on the periphery, this attests to persistent high aggressivity of the pathology, and it is suitable to use a second application of anti-VEGF. This treatment shall be an advantage also due to the faster response in comparison with photocoagulation. Upon application of the injection we select a different quadrant. In the case of at least partial regression caused by the reduction of activity of ROP and persistent changes in the periphery, it is suitable to apply supplementary therapy by photocoagulation, in which we achieve a combined effect of treatment of ROP.
Patients after treatment with anti-VEGF (transfer to laser photocoagulation or possibility of 2nd application of anti-VEGF)
- a) the finding is unchanged or worsened at the follow-up examination on the 4th day (comparison with finding before treatment)
- b) the finding shows minimal improvement, without change or worsened at the follow-up examination on the 7th day (comparison with finding before treatment)
- c) the finding is worsened at any time between the 7th and 28th day (comparison with finding before treatment)
- d) worsening of ROP in comparison with previous examination at any time after the 28th day
After the first and second repeated injection it is possible to transfer the patient for laser photocoagulation of the retina according to the discretion of the ophthalmologist.
Patients after treatment by transpupillary laser photocoagulation (transfer to anti-VEGF therapy)
- a) the finding is unchanged or worsened at the follow-up examination on the 5th - 7th day (comparison with finding before treatment)
- b) the finding shows minimal improvement, without change or worsened at the follow-up examination on the 14th day (comparison with finding before treatment)
- c) the finding is worsened at any time after the 14th day (comparison with finding before treatment)
An injection can be applied to one or both eyes depending on the acute clinical finding on the retina.Patients after treatment by transscleral thermocoagulation (laser photocoagulation / cryocoagulation) (transfer to anti-VEGF therapy)
- a) the finding is unchanged or worsened at the follow-up examination on the 5th - 7th day (comparison with finding before treatment)
- b) the finding shows minimal improvement, without change or worsened at the follow-up examination on the 14th day (comparison with finding before treatment)
- c) the finding is worsened at any time after the 14th day (comparison with finding before treatment)
An injection can be applied to one or both eyes depending on the acute clinical finding on the retina.
Supplementation of laser therapy comes into consideration only in the case of insufficient primary treatment of the avascular surface of the retina by coagulation.
PARS PLANA VITRECTOMY
Upon progression of the pathology to further more severe stages of ROP (stages 4 and 5), the finding is no longer suitable for primary treatment or supplementary treatment according to the recommended procedures.
Vitreoretinal procedures are currently reserved only for the solution of these terminal stages of ROP and their complications, which are characterised by scarry and tractional changes on the retina, with all their negative consequences in the sense of partial or complete retinal detachment.
The recommended technique of treatment for ROP in the fourth and fifth stages is lens treating pars plana vitrectomy with subsequent tamponade of the eye with silicone oil or expansive gas. The indication for the performance of this type of operation is strictly individual, with reference to the local retinal finding, the general condition of the child and the experience of the vitreoretinal surgeon performing the given operation. This usually concerns an operation of a longer duration, which places a large burden on the organism of the premature newborn infant.
However, at this point it is necessary to emphasise that the anatomical successes of this type of treatment are usually in large contrast with the minimal functional results.
LONG-TERM OBSERVATION OF SEVERELY PREMATURE CHILDREN
Programmes of long-term observation of prematurely born children, not only with ROP, have been in existence in the Czech Republic since 1997, thus for almost 20 years. They have a uniform professional content, form and regulations. This concerns quality inter-disciplinary co-operation.
Severely premature children without ROP have a high risk of occurrence of refractive errors and strabismus. Mild and medium degree of ROP with spontaneous regression also has an adverse influence on the development of the retina, and practically never takes place without consequences for vision.
Factors limiting resulting visual acuity
Immaturity may have an influence on the development of normal foveolar cellular and vascular architecture. Studies using optical coherence tomography (OCT) demonstrate that the foveolar depression in prematurely born children may be ovoid and shallow, and the fovea is generally thickened [30]. This abnormality in the depth of the foveolar depression originates as a consequence of reduced migration of cells from the foveolar region, caused by an imbalance of the levels of VEGF and their influence on vascular remodelling. Limited foveolar differentiation reduces the quality of incipent visual potentials. If a foveolar shift takes place through distorsion or dislocation (disorganisation of retinal elements of the fovea by traction) as consequences of ROP, the quality of vision decreases beneath the level of purblindness, despite the fact that the retina is not detached. Furthermore, the situation is worsened by absent or late application of glasses correction. The correct development of the immature brain is impaired as a result of untreated anisometropia or strabismus, and leads to the occurrence of amblyopia.
LONG-TERM OUTPATIENT OBSERVATION BY OPHTHALMOLOGIST
Within the framework of long-term observation, it is important to ensure timely determination of the quality of visual acuity, to ensure objective size of refraction and assess the capacity and strength of accommodation [54]. If possible, we attempt to map the defects in the visual field of the child and determine the quality of contrast sensitivity (table 3).
Tab. 3. Long-term ophthalmological observation of risk children in the Czech Republic 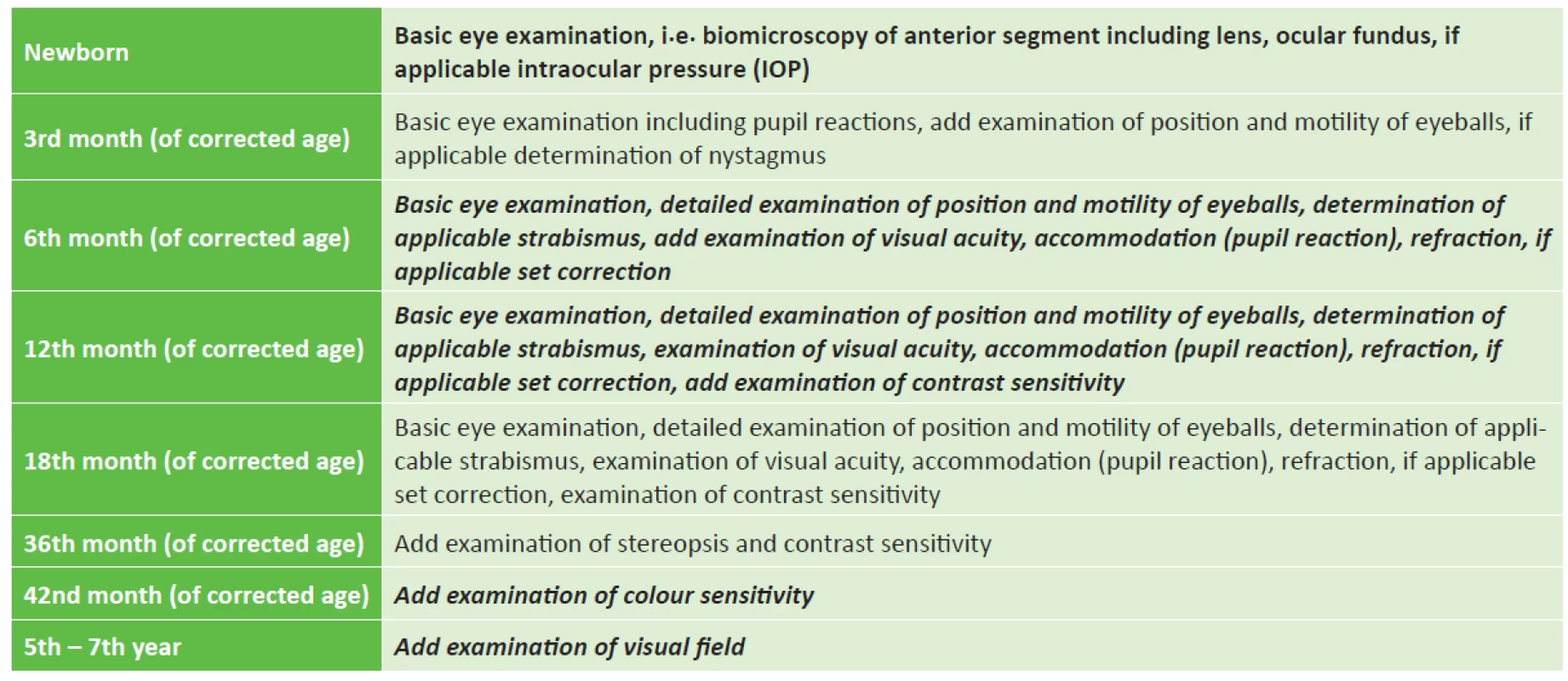
(this concerns the standard scope of ophthalmological examination conducted by an experienced paediatric ophthalmologist) On the basis of the obtained data, we have the possibility of designing a therapeutic and visual rehabilitation plan for the development of visual functions, sometimes long before the complete regression of ROP and definitive determination of the degree of visual affliction. Timely referral of the child for an ocular examination is a condition for the success of the possible treatment.
The most common complication following mild or medium forms of ROP is refractive errors (myopia, astigmatism, anisometropia), with an incidence of 32-74%, strabismus with an incidence of 30 % (most frequently esotropia in 13-21 %). Pseudo-strabismus also frequently occurs following pulling to folding of the retina, with or without a shift of the macula (pseudo-exotropia), and amblyopia on the basis of refracted errors not distinguished and treated sufficiently early [53].
The late effects include the development of complicated cataract or secondary glaucoma, which may be a complication in scarring forms of ROP. Local corticoids are combined in treatment with anti-glaucomatous agents in order to slow the course of scarring. In the later period, as much as several years after undergoing ROP, these patients are at risk of the development of ciliary block and acute glaucoma outbreak. These conditions frequently require surgical solution with the aid of laser iridectomy. In the case of the final 5th stage of ROP, atrophy of the eyeball gradually takes place, which is accompanied by a pronounced drop of intraocular pressure, irritation and pain of the eyeball, and requires local application of corticoids. In the case of the onset of zonular keratopathy we protect the surface of the cornea by long-term application of artificial tears.
With regard to the critical periods of development of sight, we insist upon precise treatment, i.e. adequate correction of refractive error and always full correction of astigmatism, as well as addition or hypercorrection in the case of afflictions connected with accommodation defect. It is also important to ensure correct indication and a regime of occlusion therapy in the case of strabismus, amblyopia or pronounced asymmetry of determined visual acuity. The entire treatment is conducted with respect to the ophthalmological finding. A sensitively chosen surgical solution for strabismus has a significant influence on the development of motor functions, not only in children with a motor handicap. Any severe visual disability (including consequences of ROP) always negatively influences the development of motor, cognitive and communication skills of the child, especially in pre-school age. Some perinatal damages to the brain may lead to CVI (Cerebral Visual Impairment), which is a central visual defect. Complete long-term care of the child also covers patient, careful visual stimulation and rehabilitation in co-operation with a visual therapist, and may include support for the development of binocular vision with the aid of orthopaedic exercise. We are able to judge the results of work with affected children only after a time interval, often of several years. These results are sufficiently encouraging that it pays to devote the maximum endeavour to such children.
Received by the Editorial Department on: 30.11.2018
Accepted for printing on: 17.12.2018
Corresponding author:
MUDr. Anna Zobanová
Private Eye Clinic, Kršková 807, Prague 5, 152 00
Zdroje
1. Ahmed, AE., Channa, R., Durrani, J. et al.: Early experience with intravitreal bevacizumab combined with laser treatment for retinopathy of prematurity. Middle East Afr J Ophthalmol, 17; 2010 : 264-247.
2. Altinsoy, HI., Mutlu, FM., Gungor, R. et al.: Combination of laser photocoagulation and intravitreal bevacizumab in aggressive posterior retinopathy of prematurity. Ophthalmic Surg Lasers Imaging, 42; 2010: E1-E5.
3. American Academy of Pediatrics. Section on Ophthalmology: Screening examination of premature infants for retinopathy of prematurity. Pediatrics, 131; 2013 : 189-195.
4. Ashton, N., Ward, B., Serpell, G.: Role of oxygen in the genesis of retrolental fibroplasia; a preliminary report. Br J Ophthalmol, 37; 1953 : 513-520.
5. Augestad, LB., Klingenberg, O., Fosse, P.: Braile use among Norwegian children from 1967 to 2007: trends in the underlying causes. Acta Ophthalmol, 90; 2012 : 428-434.
6. Autrata, R., Šenková, K., Holoušová, M. et al.: Combined treatment with laser photocoagulation and cryotherapy for threshold retinopathy of prematurity. Eur J Ophthalmol, 18; 2008 : 112-117.
7. Autrata, R., Šenková, K., Holoušová, M. et al.: Effects of intravitreal pegaptanib or bevacizumab and laser in treatment of threshold retinopathy of prematurity in zone I and posterior zone II-four years results. Cesk Slov Oftalmol, 68; 2012 : 29-36.
8. Autrata, R., Krejčířová, I., Šenková, K. et al.: Intravitreal pegaptanib combined with diode laser therapy for stage 3+ retinopathy of prematurity in zone I and posterior zone II. Eur J Ophthalmol, 22; 2012 : 687-694.
9. Carvallaro, G., Filippi, L., Bagnoli, P. et al.: The pathophysiology of retinopathy of prematurity: an update of previous and recent knowledge. Acta Ophthalmol, 92; 2014 : 2-20.
10. Castellanos, MA., Schwartz, S., García-Aguirre G. et al.: Short-term outcome after intravitreal ranibizumab injections for the treatment of retinopathy of prematurity. Br J Ophthalmol, 97; 2013 : 816-819.
11. Chung, EJ., Kim, JH., Ahn, HS. et al.: Combination of laser photocoagulation and intravitreal bevacizumab (Avastin) for aggressive zone I retinopathy of prematurity. Graefes Arch Clin Exp Ophthalmol, 245; 2007 : 1727-1730.
12. Committee for the Classification of Retinopathy of Prematurity: an international classification of retinopathy of prematurity. Arch Ophthalmol, 102; 1984 : 1130-1134.
13. Connolly, BP., Ng, EY., McNamara, JA. et al.: A comparison of laser photocoagulation with cryotherapy for threshold retinopathy of prematurity at 10 years. Part 2. Refractive outcome. Ophthalmology, 109; 2002 : 936-941.
14. Dorta, P., Kychenthal, A.: Treatment of type 1 retinopathy of prematurity with intravitreal bevacizumab (Avastin). Retina, 30 (4 Suppl); 2010: S24-S31.
15. Drenser, KA., Trese, MT., Capone, A., Jr.: Aggressive posterior retinopathy of prematurity. Retina, 30 (4 Suppl); 2010: S37-S40.
16. Early Treatment for Retinopathy of Prematurity Cooperative Group: Revised indications for the treatment of retinopathy of prematurity: results of the early treatment for retinopathy of prematurity randomized trial. Arch Ophthalmol, 121; 2003 : 1684-1694.
17. Good, WV., Early Treatment for Retinopathy of Prematurity Cooperative Group: Final results of the Early Treatment for Retinopathy of Prematurity (ETROP) randomized trial. Trans Am Ophthalmol Soc, 102; 2004 : 233-248.
18. Harder, BC., von Baltz S., Jonas, JB., et al.: Intravitreal bevacizumab for retinopathy of prematurity. J Ocul Pharmacol Ther, 27; 2011 : 623-627.
19. Hartnett, ME., Penn, JS.: Mechanisms and management of retinopathy of prematurity. N Engl J Med, 367; 2012 : 2515-2526.
20. Honda, S., Hirabayashi, H., Tsukahara, Y. et al.: Acute contraction of the proliferative membrane after an intravitreal injection of bevacizumab for advanced retinopathy of prematurity. Graefes Arch Clin Exp Ophthalmol, 246; 2008 : 1061-1063
21. Kim, J., Kim,SJ., Chang, YS. et al.: Combined intravitreal bevacizumab injection and zone I sparing laser photocoagulation in patients with zone I retinopathy of prematurity. Retina, 34; 2014 : 77-82.
22. Koo, KY., Kim, JE., Lee, SM. et al.: Effect of severe neonatal morbidities on long term outcome in extremely low birth-weight infants. Korean J Pediatr, 53; 2010 : 694-700.
23. Kretzer, FL., Hittner, HM.: Human retinal development: relationship to the pathogenesis of retinopathy of prematurity. In: McPherson, AR., Hittner, HM., Kretzer, FL. (Eds), Retinopathy of prematurity: current concepts and controversies. Toronto, Decker, 1986, 27-52.
24. Kusaka, S., Shima, C., Wada, K. et al.: Efficacy of intravitreal injection of bevacizumab for severe retinopathy of prematurity: a pilot study. Br J Ophthalmol, 92; 2008 : 1450-1455.
25. Lalwani, GA., Berrocal, AM., Murray, TG. et al.: Off-label use of intravitreal bevacizumab (Avastin) for salvage treatment in progressive threshold retinopathy of prematurity. Retina, 28(3 Suppl); 2008: S13-S18.
26. Langford, K., Nicolaides, K., Miell, JP.: Maternal and fetal insulin-like growth factor and their binding proteins in the second and third trimesters of human pregnancy. Hum Reprod, 13; 1998 : 1389-1393.
27. Lin, CJ., Chen, SN., Hwang, JF.: Intravitreal ranibizumab as salvage therapy in an extremely low-birth-weight infant with rush type retinopathy of prematurity. Oman J Ophthalmol, 5; 2012 : 184-186.
28. Lomíčková, H.: Léčení retrolentální fibroplasie kyslíkem. Čs Oftalmol, 14; 1958 : 138-146.
29. Lomíčková, H., Odehnal, M., Zobanová, A. et al.: Kryokoagulace v léčbě retinopatie nedonošených. Čs Oftalmol, 46; 1990 : 1-8.
30. Miki, A., Honda, S., Inoue, Y.,Yamada,Y. et al.: Foveal depression and related factors in patients with a history of retinopathy of prematurity. Ophthalmologica, 240; 2018 : 106-110.
31. Mintz-Hittner, HA., Kennedy, KA., Chuang, AZ., BEAT-ROP Cooperative Group: Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. N Engl J Med, 364; 2011 : 603-615.
32. Mintz-Hittner, HA., Kuffel, RR., Jr.: Intravitreal injection of bevacizumab (Avastin) for treatment of stage 3 retinopathy of prematurity in zone I or posterior zone II. Retina, 28; 2008 : 831-838.
33. Mota, A., Carneiro, A., Breda, J. et al.: Combination of intravitreal ranibizumab and laser photocoagulation for aggressive posterior retinopathy of prematurity. Case Rep Ophthalmol, 3; 2012 : 136-141.
34. Multicenter trial of cryotherapy for retinopathy of prematurity: Preliminary results. Cryotherapy for Retinopathy of Prematurity Cooperative Group. Arch Ophthalmol, 106; 1988 : 471-479.
35. Nicoară, SD., Stefanut AC., Nascutzy C. et al.: Regression rates following the treatment of aggressive posterior retinopathy of prematurity with bevacizumab versus laser: 8-year retrospective analysis. Med Sci Monit, 22; 2016 : 1192-1209.
36. Olofsson, B., Korpelainen, E., Pepper, MS. et al.: Vascular endothelial growth factor B (VEGF-B) binds to VEGF receptor-1 and regulates plasminogen activator activity in endothelial cells. Proc Natl Acad Sci USA, 95; 1998 : 11709-11714.
37. Patz, A., Hoeck, LE., De LaCruz, E.: Studies on the effect of high oxygen administration in retrolental fibroplasia. I. Nursery observations. Am J Ophthalmol, 35; 1952 : 1248-1253.
38. Quiroz-Mercado, H., Martinez-Castellanos, MA., Hernandez-Rojas, ML. et al.: Antiangiogenic therapy with intravitreal bevacizumab for retinopathy of prematurity. Retina, 28(3 Suppl); 2008: S19-S25.
39. RAINBOW study: A randomized, controlled study evaluating the efficacy and safety of Ranibizumab compared with laser therapy for the treatment of infants born prematurely with retinopathy of prematurity – Protocol.
40. Royal College of Ophthalmologists, Royal College of Paediatrics and Child Health, British Association of Perinatal Medicine & BLISS: Guidelines for the Screening and Treatment of Retinopathy of Prematurity, UK Retinopathy of Prematurity Guideline. Royal College of Paediatrics and Child Health, 2008.
41. Sato, T., Kusaka, S., Shimojo, H. et al.: Vitreous levels of erythropoietin and vascular endothelial growth factor in eyes with retinopathy of prematurity. Ophthalmology, 116; 2009 : 1599-1603.
42. Sautina, L., Sautin, Y., Beem, E. et al.: Induction of nitric oxide by erythropoetin is mediated by the β common receptor and requires interaction with VEGF receptor 2. Blood, 115; 2010 : 896-905.
43. Smith, LE.: Through the eyes of a child: understanding retinopathy through ROP. The Friedenwald lecture. Invest Ophthalmol Vis Sci, 49; 2008 : 5177-5182.
44. Sonmez, K., Drenser, KA., Capone, A., Jr. et al.: Vitreous levels of stromal cell-derived factor 1 and vascular endothelial growth factor in patients with retinopathy of prematurity. Ophthalmology, 115; 2008 : 1065-1070.
45. The International Committee for the Classification of Retinopathy of Prematurity: The international classification of retinopathy of prematurity revised. Arch Ophthalmol, 123; 2005 : 991-999.
46. Terry, TL.: Extreme prematurity and fibroplastic overgrowth of persistent vascular sheath behind each crystaline lens. I. Preliminary report, Am J Ophthalmol, 25; 1942 : 203-204.
47. Travassos, A., Teixeira, S., Ferreira, P. et al.: Intravitreal bevacizumab in aggressive posterior retinopathy of prematurity. Ophthalmic Surg Lasers Imaging, 38; 2007 : 233-237.
48. Wallace, DK., Kylstra, JA., Greenman, DB. et al.: Significance of isolated neovascular tufts (“popcorn”) in retinopathy of prematurity. J AAPOS, 2; 1998 : 52-56.
49. Wood, SM., Gleadle, JM., Pugh, CW. et al.: The role of the aryl hydrocarbon receptor nuclear translocator (ARNT) in hypoxic induction of gene expression. Studies in ARNT-deficient cells. J Biol Chem, 271; 1996 : 15117-15123.
50. Wu, WC., Yeh, PT., Chen, SN. et al.: Effects and complications of bevacizumab use in patients with retinopathy of prematurity: a multicenter study in Taiwan. Ophthalmology, 2011; 118(1): 176-83.
51. Zoban, P.: Výskyt dlouhodobé morbidity u rizikových skupin dětské populace. In Štembera, Z., Dittrichová, J., Sobotková, D. et al. (Eds), Perinatální neuropsychická morbidita dítěte, Praha, Nakladatelství Karolinum, 2014, 518-529.
52. Zobanova, A.: Koordinace péče o poruchy vidění ve spolupráci dětský lékař a oftalmolog. Pediatrie pro praxi, 5; 2004 : 236-237.
53. Zobanová, A.: Současný pohled na retinopatii předčasně narozených dětí. Pediatrie pro praxi, 17; 2016 : 279-284.
Štítky
Oftalmologie
Článek Silent Sinus Syndrome
Článek vyšel v časopiseČeská a slovenská oftalmologie
Nejčtenější tento týden
2018 Číslo 6- Stillova choroba: vzácné a závažné systémové onemocnění
- Familiární středomořská horečka
- První schválený léčivý přípravek pro terapii Leberovy hereditární optické neuropatie dostupný rovněž v ČR
- Diagnostický algoritmus při podezření na syndrom periodické horečky
- Možnosti využití přípravku Desodrop v terapii a prevenci oftalmologických onemocnění
-
Všechny články tohoto čísla
- Virtiol – Simulation of Quality of Vision with Multifocal and Edof Intraocular Lenses
- Corticosteroid Induced Posterior Subcapsular Cataract
- Ocular Manifestations in Patients with HIV infection
- The Importance of Evaluating the Development of Oct Findings During Conservative Treatment of Vitreomacular Traction Complicated by Macular Hole Formation
- Silent Sinus Syndrome
- Idiopathic Chodoidal Neovascular Membrane in a 12-year-old Girl
- Screening, Treatment and Long-term Observation of Retinopathy of Prematurely Born Children in the Czech Republic
- OČNÍ KLINIKA 1. LÉKAŘSKÉ FAKULTY UNIVERZITY KARLOVY A VŠEOBECNÉ FAKULTNÍ NEMOCNICE V PRAZE SLAVÍ 200 LET OD SVÉHO ZALOŽENÍ
- Vážený a milý pán doc. MUDr. Tomáš Mazalán, CSc.
- Česká a slovenská oftalmologie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Ocular Manifestations in Patients with HIV infection
- Silent Sinus Syndrome
- Virtiol – Simulation of Quality of Vision with Multifocal and Edof Intraocular Lenses
- Corticosteroid Induced Posterior Subcapsular Cataract
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání