-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaONE-YEAR FOLLOW-UP OUTCOMES OF TREATMENT OF WET AGE-RELATED MACULAR DEGENERATION WITH AFLIBERCEPT
Authors: Y. M. Klimešová; M. Penčák; Z. Straňák; L. Lalinská; M. Veith
Authors place of work: Oftalmologická klinika FNKV a 3. LF UK, Praha, přednosta doc. MUDr. Pavel Studený, Ph. D., MHA
Published in the journal: Čes. a slov. Oftal., 74, 2018, No. 2, p. 47-52
Category: Původní práce
doi: https://doi.org/10.31348/2018/1/1-2-2018Summary
Purpose: To evaluate one-year follow-up outcomes of treatment with aflibercept in patients with newly diagnosed wet age-related macular degeneration (ARMD).
Method: Retrospective evaluation of treatment of 28 eyes of 28 patients with an average age of 74.2 years who were treated with aflibercept at the Department of Ophthalmology at Královské Vinohrady University Hospital. All patients were treated according to the summary of product characteristics (SPC), i.e. with an initial 3 injections at monthly intervals, followed by 4 injections every 2 months. We evaluated the change in best corrected visual acuity (BCVA) on Early Treatment of Diabetic Retinopathy Study (ETDRS) optotypes, and the change of central retinal thickness (CRT) with the aid of optical coherence tomography (OCT).
Results: The average initial BCVA value was 61.5 letters of ETDRS optotype. After the initial 3 injections, BCVA improved to 70.5 letters, after one year of treatment there was a slight decrease to 68.1 letters. Better or same visual acuity was recorded in 25 eyes (89.3%), a deterioration occurred in 3 eyes (10.7%). CRT was reduced from an initial average value of 360.9 µm to 253.3 µm after the initial phase and to 233.8 µm after one year of treatment. At the end of the observation period, 25 eyes (89.3%) were without signs of activity of the pathology. No complications of treatment were recorded.
Conclusion: In our cohort we confirm the efficacy and safety of the aflibercept preparation in patients with newly diagnosed wet form ARMD. By adhering to a fixed therapeutic regime it is possible to obtain similarly excellent results of treatment in real clinical practice as in clinical trials.
Key words: aflibercept, anti-VEGF therapy, optical coherence tomography, wet form ARMD
The authors of the study declare that no conflict of interest exists in the compilation, theme and subsequent publication of this professional communication, and that it is not supported by any pharmaceuticals company.
INTRODUCTION
Age-related macular degeneration (ARMD) ranks among degenerative and progressive pathologies affecting the central part of the retina. In developed countries it is the most frequent cause of practical blindness in persons older than 65 years (10). It concerns a multifactorial pathology, and its prevalence increases mainly with age. Further risk factors are currently considered to be smoking, insufficient intake of antioxidants, white race, UV radiation, diseases of the cardiovascular system including hypertension, diabetes mellitus and obesity. Of ocular factors, risk factors are pale colour of the iris and cataract surgery (15, 3). Due to the ageing population it is possible to expect that the prevalence and incidence of this pathology shall increase (9).
We distinguish between two forms of ARMD, dry and wet. In 85-90% of cases it concerns slowly developing dry (atrophic, non-exudative) form of ARMD, and in approximately 10-15% of cases rapidly progressing wet (neovascular, exudative) form develops, with the onset of choroidal neovascularisation (CNV), which in as many as 85% of cases leads to practical blindness. The pathology may be manifested by a combination of both forms (19, 9). As a rule it is initially manifested unilaterally, with the second eye afflicted in 50% of patients after 5 years of duration, and in all patients after 10 years. Both forms are typified by metamorphopsia, varying degrees of central scotoma and reduction of central visual acuity, in particular for near vision. ARMD itself does not lead to complete blindness, nevertheless, patients with very severe wet form, with visual acuity of less than 0.05, state a deterioration of quality of life in 63% of cases (6).
A key role in the pathogenesis of the origin of CNV is played by angiogenic factors – primarily vascular endothelial growth factor (VEGF) (13), as well as platelet-derived growth factor (PDGF) (18), transforming growth factor (TGF-ꞵ1), fibroblast growth factor (FGF), angiogenin and placental growth factor (PIGF) (22). For the proper functioning of the retina, it is necessary to have a balance between production and demand for VEGF. With increasing age, due to the influence of reduced permeability of the Bruch's membrane (BM), an overproduction of VEGF takes place, which stimulates the formation of neovascularisations. VEGF, specifically for vascular endothelial cells, is generated as a consequence of hypoxia by the cells of the retinal pigment epithelium (RPE). Inflammatory mediators and angiogenic cytokines produce choroidal neovascularisation and subsequent exudation (1, 21).
The purpose of treating ARMD is to retard or entirely halt the progression of this pathology, and thereby to prevent practical blindness. At present, the most effective treatment of wet form ARMD is intravitreal application of pharmaceuticals with a vascular endothelial growth factor blocker (anti-VEGF), which suppresses the growth of newly formed blood vessels (24). In the majority of patients, this treatment is able to stabilise the finding, and in some patients it can also improve visual acuity (12, 14, 23).
The first effective preparation against wet form ARMD was pegaptanib sodium (Macugen, Pfizer), modified RNA oligonucleotide, which binds with high affinity specifically only to isoform VEGF-A165, and blocks its binding to the receptor (12, 27). Due to its lower effectiveness in comparison with other anti-VEGF preparations, it is no longer used in the Czech Republic.
The preparation ranibizumab (Lucentis, Novartis) represents a fragment of humanised antibody against vascular growth factor. The baseline studies for determining the efficacy and safety of ranibizumab are the MARINA and ANCHOR studies, which have unequivocally demonstrated the positive effect of this treatment (7, 23).
To date the most recently registered preparation is aflibercept (Eylea, Bayer). This is recombinant fusion protein, composed of binding parts of VEGFR-1, VEGFR-2 and Fc parts of antibody IgG1 (8, 26). Similarly to ranibizumab, it binds all isoforms of VEGF-A. However, its affinity to molecule VEGF-A is 10-100 times higher. It acts as a false receptor, which binds VEGF-A, B and PIGF.
The aim of this study is a retrospective evaluation of one-year treatment of a group of patients with newly diagnosed wet form ARMD treated with aflibercept, and a comparison of our results with the results of clinical trials.
METHOD AND COHORT
Our own cohort comprised 28 eyes of 28 patients, of whom 11 were men and 17 women. In all cases it concerned patients with newly diagnosed wet form ARMD (i.e. without prior treatment). The treatment took place at the Department of Ophthalmology at Královské Vinohrady University Hospital in the period from January 2014 to November 2016. The average age of the patients was 74.2 years (range 54 - 88 years). In all patients only one eye was treated. 17 eyes (39.3%) were phakic, 11 eyes (60.7%) were aphakic. In 15 eyes (53.6%) this concerned treatment of the better eye.
All the patients in our cohort were treated according to the summary of product characteristics (SPC), i.e. by an initial 3 injections of 2 mg of aflibercept at intervals of one month, followed by 4 injections at intervals of 2 months. The observation period is 12 months.
In 10 patients, in whom there was no clear biomicroscopic or OCT finding, fluorescence angiography (FAG) was supplemented for verification of the presence and type of CNV. The cohort comprised 9 eyes (32.1%) with classic CNV, 2 eyes (7.2%) with minimally classic CNV and 17 eyes (60.7%) with occult CNV.
A condition for the commencement of treatment with aflibercept was the fulfilment of all the then valid indication recommendations for payment for the treatment of wet form ARMD, i.e. demonstrated CNV with wet form ARMD, initial visual acuity of 4/8 – 4/40, size of lesion maximum 8 PD (diameter of of optic nerve papilla), any applicable haemorrhage maximally up to 25% of lesion. In the case of occult and minimally classic membranes, the presence of hard exudates or haemorrhage was necessary, or deterioration of visual acuity by at least two rows of ETDRS optotypes within the course of two months. The treatment was terminated upon a loss of 15 and more letters of ETDRS optotypes within the course of 12 months.
We observed the change of best corrected visual acuity (BCVA) on ETDRS optotypes and the change of central retinal thickness (CRT) on the instrument Cirrus 4000 HD-OCT (Carl Zeiss Meditec, Dublin, CA). The stated parameters were evaluated after 3.5 and 7 applications and compared with the baseline values.
RESULTS
Average initial BCVA was 61.5 letters of ETDRS optotypes (range 40-70). After three initial doses of 2 mg of aflibercept, BCVA improved to 70.5 letters of ETDRS (range 50-85), the average gain was therefore +9.0 letters of ETDRS. A substantial improvement of >15 letters was attained in 9 eyes (32.1%), while 6 eyes (21.4%) improved by 10-14 letters, 4 eyes (14.3%) by 5-9 letters, and 8 eyes (28.6%) by 0-4 letters. In one eye (3.6%) there was a loss of 6 letters.
After the 5th application (i.e. immediately before the 6th injection) we recorded a slight decrease of visual acuity to 68.4 letters of ETDRS (range 40-83) and the average gain against the baseline corresponded to +8.4 letters. This slight decrease of BCVA can be explained by the fact that the application interval in this phase was now 2 months. An improvement of >15 letters was demonstrated in 2 eyes (11.8%), while 8 eyes (47.0%) had a gain of 10-14 letters and 2 eyes (11.8%) had a gain of 5-9 letters. In 4 eyes (23.5%) a minimal improvement by 0-4 letters was demonstrated, and in only one eye (5.9%) a loss of 2 letters was determined.
The resulting average best corrected visual acuity after the 7th application was 68.1 letters of ETDRS, in which 25 eyes (89.3%) attained the same or better visual acuity (graph 1). The average gain of letters as against the baseline examination was +7.1 letters after the 7th application (graph 2). 7 eyes improved by >15 letters. In 6 eyes (21.4%) visual acuity improved by 10-14 letters, in 5 eyes (17.9%) by 5-9 letters and in 7 eyes (25.0%) by 0-4 letters. We recorded a deterioration of visual acuity in 3 eyes (10.7%), in which this concerned a loss of less than 5 letters in 2 eyes and in one patient there was a loss of >15 letters after the 7th application as a consequence of the development of extensive atrophy of the RPE. This concerned the same patient who recorded a loss of letters after the three initial applications.
Average initial central retinal thickness (CRT) was 360.9 µm (range 218-640). After three applications of aflibercept CRT was reduced by 107.6 µm to the value of 253.3 µm (range 181-495). In 23 eyes (82.1%) the edema in the macula was completely absorbed, in the remaining 5 eyes (17.9%) activity of CNV was still perceptible on OCT. At further follow-up examinations the reduction of CRT was not so substantial. After the 5th application the CRT value was 250.2 µm (range 183-349). One month after the final 7th application CRT reached the outcome value of 233.8 µm (range 186-470). 25 eyes (89.3%) were without signs of CNV activity. The average CRT value as against the baseline value is illustrated by graph 3, while graph 4 shows the average change of absolute CRT values.
We did not record any inflammatory reactions, retinal detachment or any other complications in this observed cohort.
Graph 2. Average change in BCVA 
Graph 4. Average change in CRT 
Fig. 1. Initial fundus photography: patient – 75-year-old male, initial BCVA 63 ETDRS letters, CRT 348 μm, fluorescence angiography – minimally classic CNV. 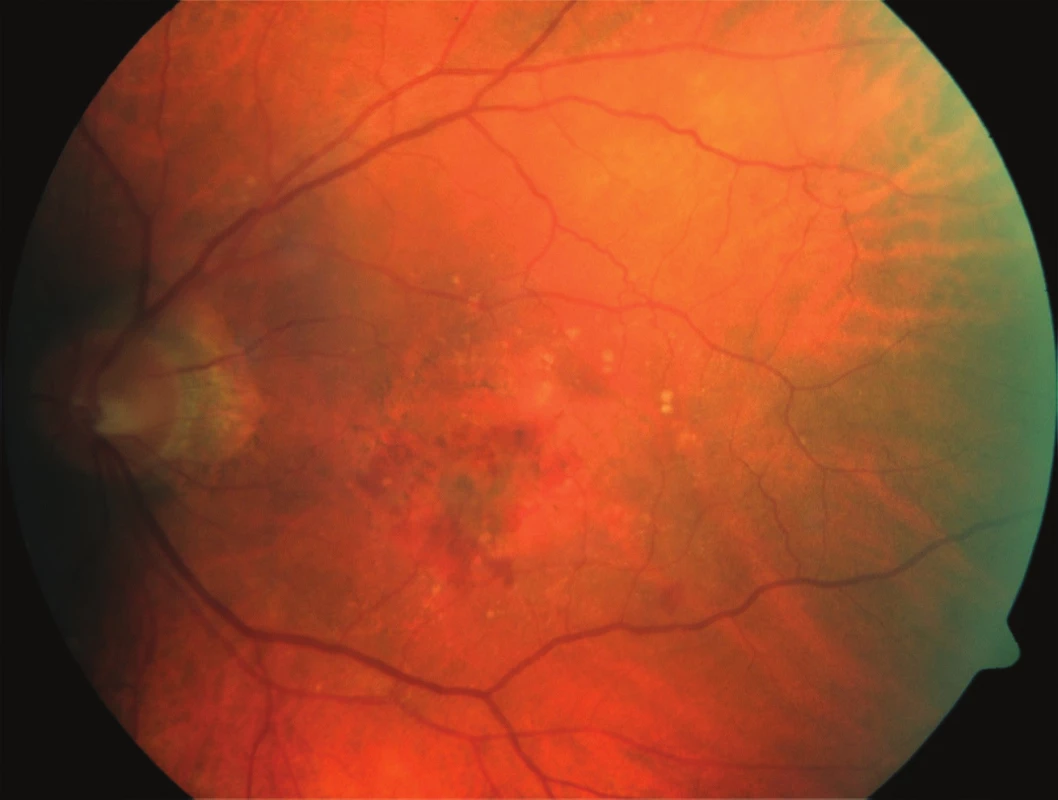
Fig. 2. Initial OCT – presence of fluid. 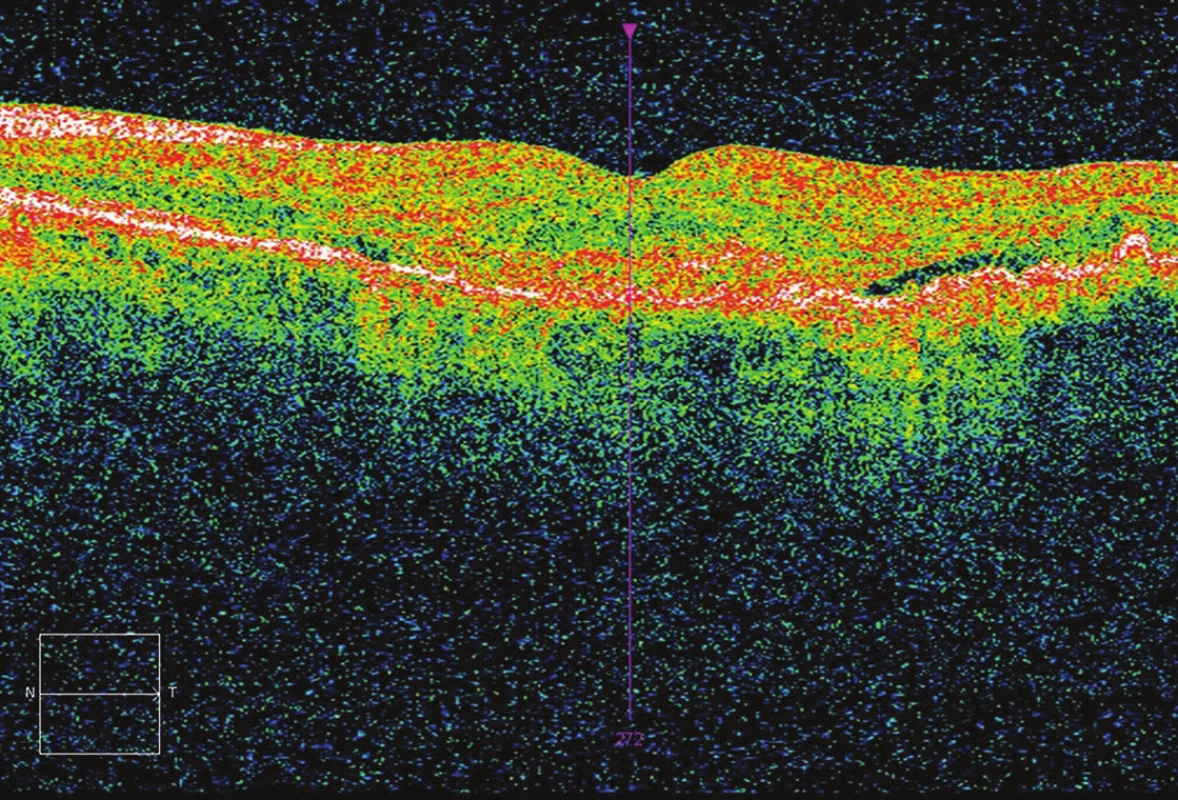
Fig. 3. OCT after 3 applications – CRT 233 μm, without fluid, BCVA 70 ETDRS letters (7 letters gain). 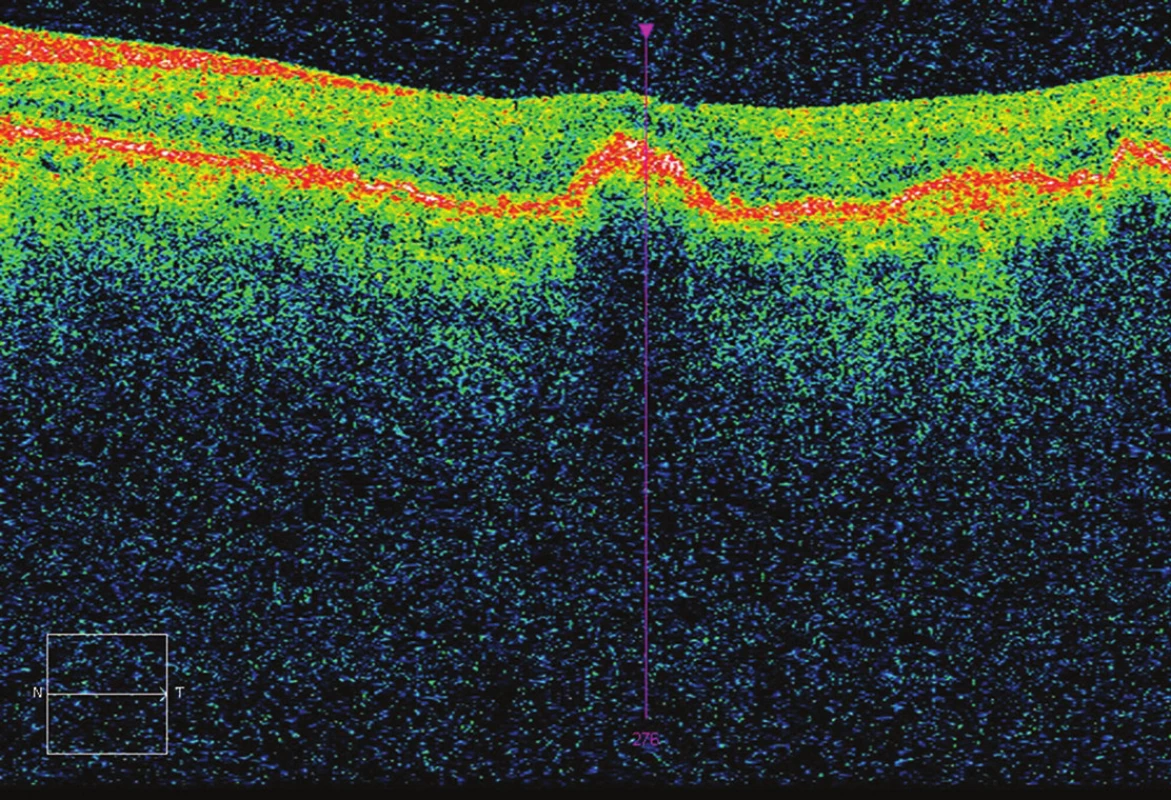
DISCUSSION
Anti-VEGF therapy is currently the method of first choice for patients with wet form ARMD. The most recent anti-VEGF preparation to receive registration and payment is aflibercept.
The effectiveness and safety of aflibercept in the treatment of wet form ARMD was verified by the clinical trials VIEW 1 and VIEW 2. This concerned a multicentric study in phase III, double-blind, prospective randomised trial, which actively monitored the clinical efficacy of aflibercept in comparison with ranibizumab. These were the largest controlled studies of the treatment of wet form ARMD with an anti-VEGF preparation (VIEW 1 n= 1 217, VIEW 2 n= 1 240 (14, 25).
Both trials had very similar characteristics of the cohort. Among the inclusion criteria were age of over 50 years, active subfoveal lesion on a background of ARMD, juxtafoveal lesion with infiltration in the fovea into the total size of the lesion up to 12 PD, CNV incorporating at minimum 50% of the size of the lesion, BCVA between 73 and 22 letters of ETDRS optotype. The exclusion criteria included any previous systemic treatment of ARMD (an exception was food supplements), intraocular inflammation or glaucoma in anamnesis.
The patients in these trials were divided into 4 groups: 3 different dosing regimes of aflibercept – 0.5 mg per month, 2 mg per month, 2 mg per 2 months after 3 initial monthly doses (therefore as in our cohort according to SPC), in the fourth group the patients were treated with ranibizumab (2 mg per month). According to the type of CNV, in the VIEW 1 trial the cohort comprised classic CNV in 23.6% of cases, minimally classic in 36.5%, occult in 39.2%, and in the VIEW 2 trial in the same order 28.8%, 34.6% and 35.9%.
In our cohort 28 eyes were observed. Average initial BCVA in our cohort was somewhat better – 61.5 letters of ETDRS (VIEW 1 55.7 and VIEW 2 51.6), while by contrast the initial CRT value was worse – 360.9 µm (VIEW 1 324.4 µm and VIEW 2 342.6 µm). The reason for this could be the different representation of the type of CNV, since in our cohort the occult type predominated – 60.7%.
The average gain of letters after 12 months of treatment in the VIEW 1 trial was +7.9 letters, and +8.9 letters in the VIEW 2 trial. A gain of > 15 letters was attained in the VIEW 1 trial by 30.6% of patients, and in VIEW 2 by 31.4% of patients. Our cohort had a slightly lower average gain of letters, i.e. +7.1 letters, and less patients attained a gain of > 15 letters – 25%. This fact can be attributed to the better initial value of BCVA in our cohort, where there was less room for a more substantial improvement during the course of treatment.
The reduction of CRT after 12 months of treatment was 128.5 µm in the VIEW 1 trial, and 149.2 µm in the VIEW 2 trial. In our cohort the reduction of CRT was analogous – 127.1 µm. In the VIEW 1 trial 63.4% of patients were without signs of activity of the pathology, and in VIEW 2 71.9% of patients, in our cohort 89.3%. This difference may have been caused by the different representation of the types of CNV and also by an error of small numbers.
In addition to these two large clinical trials, there are various publications evaluating the effect of aflibercept in regular clinical practice. This predominantly concerns publications comparing the effectiveness of treatment using aflibercept with other anti-VEGF preparations, or evaluates its effect in patients following prior treatment with another preparation (2, 4, 11, 20, 31). There are less studies available publishing the results of treatment of “naïve” patients, i.e. patients without any prior treatment (5, 29, 30). As an example, it is possible to present the publication by Warwick et al. - one-year results of fixed treatment with aflibercept in naïve patients (30). In their cohort they evaluated 58 eyes of 51 patients with an average age of 82.8 years. The average initial BCVA value was 54.2 letters of ETDRS and CRT of 284.2 µm. After 12 months of treatment by 7 doses they attained a gain of +4.67 letters of ETDRS, and CRT decreased by only 35.6 µm. The better anatomical and functional results in our cohort can be explained by the different representation of the type of CNV and higher age of the patients in Warwick's study. The higher age of the patients may have been responsible primarily for atrophic changes of the retina.
The Czech national register AMADEUS has also evaluated the effect of anti-VEGF treatment in real clinical practice. The register has recorded the results of treatment of more than 8000 patients in the Czech Republic. An analysis thereof demonstrates the significance of sufficient frequency of follow-up examinations, and in particular of applications. If patients are treated sufficiently intensively it is possible in clinical practice to approach the excellent results of treatment attained in clinical trials (16, 17, 28).
Fig. 4. OCT after 5 applications – CRT 248 μm, presence of fluid, BCVA 73 ETDRS letters (10 letters gain). 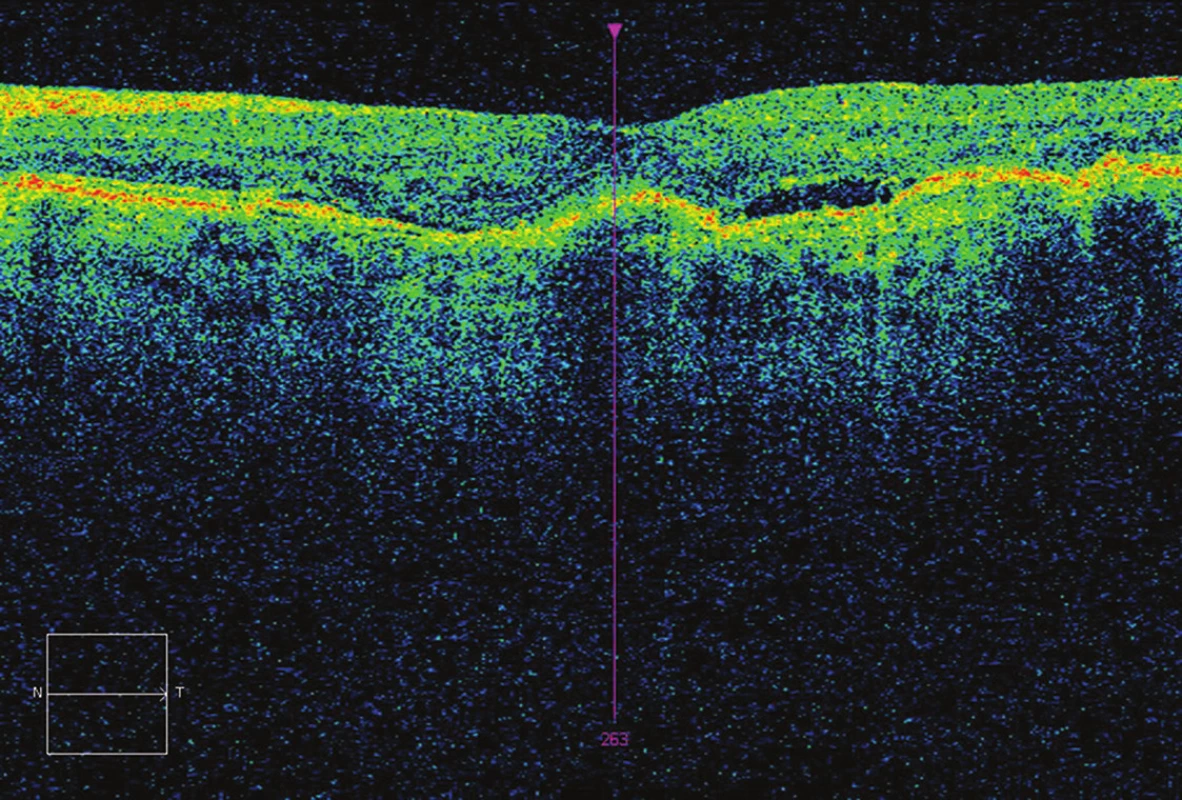
Tab. 1. OCT after 7 applications – CRT 221 μm, without fluid, BCVA 75 ETDRS letters (12 letters gain). 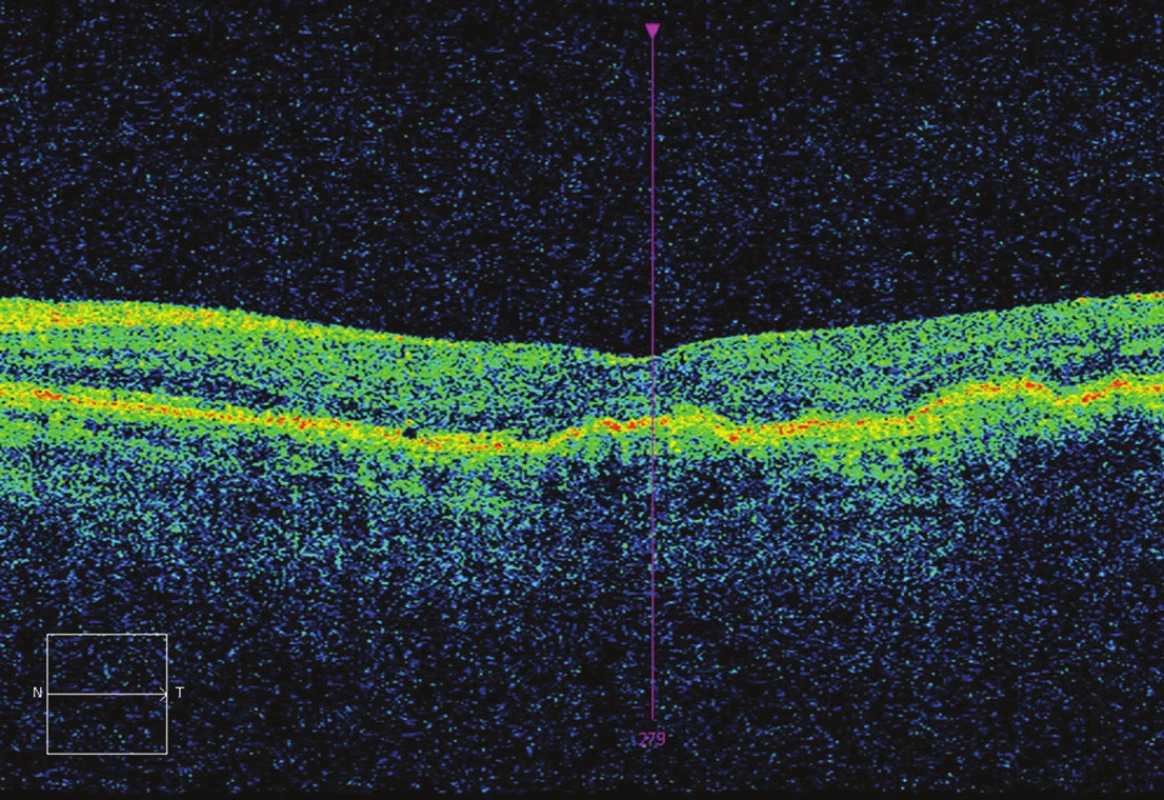
CONCLUSION
The results we attained in the treatment of wet form ARMD with aflibercept are comparable with the results of clinical trials. By adhering to a fixed dosing regime, we ensured sufficient intensity of treatment, which in the majority of patients enabled us to preserve stable visual acuity.
MUDr. Klimešová
Oftalmologická klinika FNKV a 3. LF UK, Praha
Zdroje
1. Ambati, J., Fowler, BJ.: Mechanisms of age-related macular degeneration. Neuron, 75; 2012 : 26-39.
2. Arcinue, CA., Ma, F., Barteselli, G. et al.: One-year outcomes of aflibercept in recurrent or persistent neovascular age-related macular degeneration. Am J Ophthalmol, 159; 2015 : 426 – 436.
3. AREDS Research Group: Risk factors associated with age-related macular degeneration. A case-control study in the age-related eye disease study: Age-Related Eye Disease Study Report Numer 3. Ophthalmology, 107; 2000 : 2224–2232.
4. Bakall, B., Folk, JC., Boldt, HC. et al.: Aflibercept therapy for exudative age-related macular degeneration resistant to bevacizumab and ranibizumab. Am J Ophthalmol, 156; 2013 : 15–22.
5. Balaratnasingam, C., Dhrami-Gavazi, E., McCann, JT. et al.: Aflibercept: a review of its use in the treatment of choroidal neovascularization due to age-related macular degeneration. Clin Ophthalmol, 19; 2015 : 2355 – 71.
6. Brown, MM., Brown, GC., Stein, JD. et al.: Age-related macular degeneration: economic burden and value-based medicine analysis. Can J Ophthalmol, 40; 2005 : 277 - 87.
7. Brown, DM., Michels, M., Kaiser, PK. et al.: Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: Two-year results of the ANCHOR study. Ophthalmology, 116; 2009 : 57–65.
8. Dixon, JA., Olivek, S. C., Olson, JL. et al.: VEGF trap-eye for the treatment of neovascular age-related macular degeneration. Expert Opin Investig Drugs. 18; 2009 : 1573–1580.
9. Ernest, J.: Makulární degenerace: Trendy v léčbě věkem podmíněné makulární degenerace. Praha: Mladá fronta, 2010. (ISBN 978-80-204-2363-4) 249s.
10. Friedman, DS., O’Colmain, BJ., Munoz, B. et al.: Eye Diseases Prevalence Research Group. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol, 122;2004 : 564–572.
11. Gharbiya, M., Iannetti, L., Parisi, F. et al.: Visual and anatomical outcomes of intravitreal aflibercept for treatment-resistant neovascular age-related macular degeneration. Biomed Res Int, 2014;2014 : 273754.
12. Gragoudas, ES., Adami, AP., Cunningham, ET. et al.: VEGF Inhibition Study in Ocular Neovascular Clinical Trial Group. Pegaptanib for neovascular age-related macular degeneration. N Engl J Med, 351; 2004 : 2805–16.
13. Grisanti, S., Tatar, O.: The role of vascular endothelial growth factor and other endogenous interplayers in age-related macular degeneration. Prog Retin Eye Res, 27; 2008 : 372–390.
14. Heier, JS., Brown, DM., Chiny, V. et al.: Intravitreal Aflibercept (VEGF Trap-Eye) in Wet Age-related Macular Degeneration. Ophthalmology, 119;2012 : 2537–2548.
15. Hyman, L., HE, O., Grimson, E. et al. Risk factors for age related maculopathy. Incest Ophthalmol Vis Sci, 33; 1992 : 801.
16. Chrapek, O., Jarkovský, J., Studnička, J. et al.: The efficacy of ranibizumab treatment in clinical practice in patients with the wet form of age-related macular degeneration. The results of the Czech National Registry. Biomedical Papers – Olomouc 2015; 159(3): 407-412.
17. Chrapek, O., Pitrová, Š., Dušek, L. et al.: Výsledky léčby vlhké formy věkem podmíněné makulární degenerace u pacientů evidovaných v celonárodním registru AMADEUS. Česká a slovenská oftalmologie 2010, 66(3):110-118.
18. Jo, N., Mailhos, C., Ju, M. et al.: Inhibition of platelet-derived growth factor B signaling enhances the efficacy of anti-vascular endothelial growth factor therapy in multiple models of ocular neovascularization. Am J Pathol, 168; 2006 : 2036–2053.
19. Kolář, P.: Věkem podmíněná makulární degenerace. Praha: Grada Publishing, a.s., 2008. (ISBN 978-80-247-6760-4) 148s.
20. Messenger, WB., Campbell, JP., Faridi, A., et al.: Injection frequency and anatomic outcomes 1 year following conversion to aflibercept in patients with neovascular age-related macular degeneration. Br J Ophthalmol, 98; 2014 : 1205–1207.
21. Neufeld, G., Cohen, T., Gengrinovitch S. et al.: Vascular endothelial growth factor (VEGF) and its receptors. FASEB J., 13; 1999 : 9–22.
22. Rakic, JM., Lambert, V., Devy, L. et al. Placental growth factor, a member of the VEGF family, contributes to the development of choroidal neovascularization. Incest Ophthalmol Vis Sci, 44; 2003 : 3186 – 3193.
23. Rosenfeld, PJ., Brown, DM., Heier, JS. et al.: Ranibizumab for neovascular age-related macular degeneration. N Engl J Med, 355; 2006 : 1419 – 1431.
24. Salomon, SD., Lindsley, K., Vedula, SS. et al.: Anti-vascular endothelial growth factor for neovascular age-related macular degeneration. Cochrane Database Syst Rev 2014; 8: CD005139
25. Schmidt-Erfurth, U., Kaiser, PK., Korobelnik, J-F. et al.: Intravitreal Aflibercept Injection for Neovascular Age-related Macular Degeneration. Ophthalmology, 121; 2014 : 193–201.
26. Semeraro, F., Morescalchi, F., Duse, S. et al.: Aflibercept in wet AMD: specific role and optima use. Drug Des Devel Ther, 7; 2013 : 711–722.
27. Singerman, LJ., Masonson, H., Patel, M. et al.: Pegaptanib sodium for neovascular age-related macular degeneration: third-year safety results of the VEGF Inhibition Study in Ocular Neovascularisation (VISION) trial. Br J Ophthalmol, 92; 2008 : 1606–1611.
28. Studnička, J., Říhová, B., Rencová, E. et al.: Cost and effectiveness of therapy for wet age-related macular degeneration in routine clinical practice. Ophthalmologica 2013; 230(1): 34-42.
29. Udaondo, P., Salom, D., García-Delpech, S. et al.: Aflibercept as First-Line Therapy in Patients with Treatment-Naïve Neovascular Age-Related Macular Degeneration: Prospective Case Series Analysis in Real-Life Clinical Practice., 236; 2016 : 29-35.
30. Warwick, AN., Leaver, HH., Lotery, AJ.: Fixed bimonthly aflibercept in naïve and switched neovascular age-related macular degeneration patients: one year outcomes. Int J Ophthalmol, 9; 2016 : 1156–1162.
31. Wykoff, CC., Brown, DM., Maldonado, ME. et al.: Aflibercept treatment for patients with exudative age-related macular degeneration who were incomplete responders to multiple ranibizumab injections (TURF trial) Br J Ophthalmol, 98; 2014 : 951–955.
Štítky
Oftalmologie
Článek Zemřela Jaroslava Vladyková
Článek vyšel v časopiseČeská a slovenská oftalmologie
Nejčtenější tento týden
2018 Číslo 2- Stillova choroba: vzácné a závažné systémové onemocnění
- Familiární středomořská horečka
- Diagnostický algoritmus při podezření na syndrom periodické horečky
- Možnosti využití přípravku Desodrop v terapii a prevenci oftalmologických onemocnění
- Normotenzní glaukom: prevalence a zásady terapie
-
Všechny články tohoto čísla
- ONE-YEAR FOLLOW-UP OUTCOMES OF TREATMENT OF WET AGE-RELATED MACULAR DEGENERATION WITH AFLIBERCEPT
- The use of micropulse laser in patients with diabetic macular edema at the Department of Ophthalmology, Faculty Hospital Hradec Králové
- Results of the first 12 months treatment of macular edema complicating BRVO in patients treated with ranibizumab
- ACUTE MYOPIA WITH ELEVATION OF INTRAOCULAR TENSION AS AN ADVERSE SIDE EFFECT OF ANTIDEPRESSANT MEDICATION
- CHANGE OF CENTRAL AND PERIPHERAL VISION IN PATIENT WITH SYMPTOMATIC CYST OF RATHKE'S CLEFT FOLLOWING TRANSSPHENOIDAL RESECTION.
- Patnáct let Lionského oftalmologického edukačního centra oční kliniky Fakultní nemocnice Královské Vinohrady v Praze
- 19. Vejdovského olomoucký vědecký den
- Zemřela Jaroslava Vladyková
- Česká a slovenská oftalmologie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- CHANGE OF CENTRAL AND PERIPHERAL VISION IN PATIENT WITH SYMPTOMATIC CYST OF RATHKE'S CLEFT FOLLOWING TRANSSPHENOIDAL RESECTION.
- The use of micropulse laser in patients with diabetic macular edema at the Department of Ophthalmology, Faculty Hospital Hradec Králové
- Results of the first 12 months treatment of macular edema complicating BRVO in patients treated with ranibizumab
- ACUTE MYOPIA WITH ELEVATION OF INTRAOCULAR TENSION AS AN ADVERSE SIDE EFFECT OF ANTIDEPRESSANT MEDICATION
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání





