-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Investigation of the anabolic activity of dry extracts of Iris hungarica leaves and rhizomes on the model of hydrocortisone-induced protein catabolism
Studium anabolické aktivity suchých extraktů listů a oddenků Iris hungarica na modelu hydrokortizonem navozeného katabolismu bílkovin
Článek představuje výsledky studie anabolického účinku suchých extraktů listů a oddenků Iris hungarica na modelu katabolismu proteinů indukovaného hydrokortizonem. Předchozí studie prokázaly přítomnost anabolické aktivity suchých extraktů z listů a oddenků Iris hungarica u intaktních zvířat. Bylo proto odůvodněné studovat účinek experimentálních extraktů na stav metabolismu bílkovin, který je regulován glukokortikoidy. Model katabolismu proteinů indukovaného hydrokortizonem byl použit ke stanovení anabolické aktivity pro suché extrakty z listů a oddenků Iris hungarica v dávce 150 mg/kg sledováním obnovení tělesné hmotnosti a zvýšení celkového proteinu v srdečním svalu krys a v homogenátu svalové tkáně, jehož cílem je podpora myofibrilární hypertrofie. Suchý extrakt z oddenků Iris hungarica snižoval exkreci močoviny, normalizoval metabolismus, obnovoval dusíkovou rovnováhu a inhiboval katabolismus bílkovin. Výsledky ukazují, že suchý extrakt z Iris hungarica má schopnost korigovat metabolismus bílkovin, který je částečně regulován glukokortikoidy, vzhledem k vysokému obsahu isoflavonoidů a aminokyselin, a naznačují, že tento rostlinný produkt může být potenciálně použit ve vývoji nového léku zaměřeného na úpravu metabolismu bílkovin a svalové atrofie.
Klíčová slova:
anabolická aktivita – hydrokortizon – orotát draselný – Iris hungarica
Authors: Viktoria Rybak 1; Gunel Kerimova 1; Viktoria Korol 2
Authors place of work: Department of Physiology and Pathological Physiology, National University of Pharmacy, Ukraine 1; Department of Chemistry of Natural Compounds and Nutritionology, National University of Pharmacy, Ukraine 2
Published in the journal: Čes. slov. Farm., 2021; 70, 59-65
Category: Původní práce
doi: https://doi.org/10.5817/CSF2021-2-59Summary
This article presents the results of the study of the anabolic effect of dry extracts of Iris hungarica leaves and rhizomes on the model of hydrocortisoneinduced protein catabolism. Previous studies have established the presence of anabolic activity of dry extracts of Iris hungarica leaves and rhizomes in intact animals. Therefore, it was reasonable to study the effect of the experimental extracts on the state of protein metabolism, which is regulated by glucocorticoids. The model of hydrocortisoneinduced protein catabolism was used to determine anabolic activity for dry extracts of Iris hungarica leaves and rhizomes at a dose of 150 mg/kg by monitoring the recovery of body weight and the increase in the total protein in the cardiac muscle of rats and in muscle tissue homogenate, which is aimed to promote myofibrillar hypertrophy. Dry extract of Iris hungarica rhizomes reduced urea excretion, normalized metabolism, restored nitrogen balance, and inhibited protein catabolism. The results indicate that dry extract of Iris hungarica has the ability to correct protein metabolism, which is regulated in part by glucocorticoids, due to the high content of isoflavonoids and amino acids, and suggest that there is a potential use for this herbal product in the development of a new drug aimed at correcting protein metabolism and muscular atrophy.
Keywords:
nabolic activity – hydrocortisone – potassium orotate – extract – Iris hungarica
Introduction
The importance of proteins for the body is defined by its vital functions, such as: structural, plastic, energy, transport, regulatory, etc. Protein hormones regulate all biological functions in the body, its growth and development, reproduction. By chemical structure, the adrenal cortex hormones are steroids. Glucocorticoids take part in the regulation of carbohydrate and protein metabolism, affect the permeability of cell membranes for glucose and amino acids, exert anti-inflammatory and desensitizing action. Glucocorticoids stimulate gluconeogenesis, enhance glycogenesis, increase the mobilization of lipids from fat depots and the lipolytic action of somatotropin and catecholamines (permissive effect of glucocorticoids). They have a predominantly anabolic effect in the liver, and a catabolic one in extra-hepatic tissues (lipid, muscle, connective tissue). The molecular mechanism of anabolic action involves stimulation of transcription and, therefore, translation of specific proteins (liver enzymes, plasma albumins)1, 2).
Glucocorticoids suppress synthesis of proteins and promote their breakdown by gluconeogenesis in the lymphoid tissue and somatic compartment, while enhancing biosynthesis of many globulins and transaminases in the liver. This is manifested in the transfer of amino acids from the somatic to the visceral compartment during stress, starvation, acute-phase response, or systemic inflammatory response syndrome, and traumatic injuries. The synthesis of liver proteins balances their breakdown in the somatic compartment, which is why hypercorticism manifests as a negative nitrogen balance, hyperaminoacidemia, and aminoaciduria. This mechanism causes the clinical symptom complex: muscle atrophy, osteoporosis, thinning of the skin, hypoplasia of the thymic-lymphatic system3–5).
In clinical practice, glucocorticoids are used in combination with other drugs for the treatment of many acute and chronic diseases: Addison’s disease, arthritis, dermatitis, neurodermatitis, eczema, severe allergic reactions, anaphylactic shock, angioneurotic oedema, serum sickness, status asthmaticus, shocks of various nature; traumatic brain injury, stroke, liver disease, glomerulonephritis, blood diseases (haemolytic anaemia, acute lymphocytic and myeloblastic leukaemia), and in transplantation2, 4, 5).
Steroids also include a variety of compounds of plant (phytosterols, aglycones of steroidal saponins and cardiotonic glycosides, withanolides, steroidal alkaloids) and animal (vitamin D) origin. They have different pharmacological activity and are widely used in medicine6, 7).
Iris hungarica Waldst. et Kit, genus Iris, from the family Iridaceae, which has a wide range of pharmacological actions, including effects on metabolic processes, one aspect of which is protein metabolism7, 8).
Given the above, and the fact that dry extract of leaves and rhizomes of Iris hungarica showed an anabolic effect in intact animals8, 9), it was considered reasonable to investigate the state of protein metabolism, which is regulated by glucocorticosteroids.
In this study, we investigated the anabolic activity of dry extracts of leaves and rhizomes of Iris hungarica on a model of hydrocortisone-induced protein catabolism.
Experimental part
Plant material
The subject of pharmacological investigation was the dry extract of Iris hungarica leaves and rhizomes. Raw materials were collected in April 2018 on the territory of the Botanical Garden of the V. N. Karazin University (Kharkiv).
In order to directly obtain dry extract, 1000 mL of ethanol (70% v/v) was added to the ground raw material (100 g), and heated under reflux in a water bath for 2 hours. The extraction was repeated twice. The resulting extracts were combined, filtered through a Buchner funnel and evaporated to dryness on a rotary evaporator.
The study was performed in 30 white outbred male rats weighing 230–240 g, divided into 6 study groups (5 animals per group) according to the following design. The first group of animals is the Intact Control, the second is the Control Pathology (without treatment), the third comprised animals that received potassium orotate tablets at a dose of 100 mg/kg, the fourth received dry extract of maral root rhizomes at a dose of 150 mg/kg, the fifth received dry extract of Iris hungarica leaves at a dose of 150 mg/kg, and the sixth received dry extract of Iris hungarica rhizomes at a dose of 150 mg/kg.
The comparison drugs selected, which are pharmacological alternatives, were: potassium orotate tablets (Borschagivskyi Khimiko-Farmatsevtychnyi Zavod Scientific and Production Centre CJSC, Kyiv, Ukraine), which belongs to the group of non-steroidal anabolic agents, and dry extract of maral root rhizomes (Soyuz Afghan Private Enterprise, Ukraine).
The herbal comparison drug – dry extract of maral root rhizomes – was selected based on the ability to enhance protein synthesis in the body, which is a part of the drug Ekdysten (ecdysterone), natural compounds of a steroid structure.
Experimental model of protein metabolism disorders
This was reproduced by intramuscular injection of 2.5% suspension of hydrocortisone acetate (Farmak JSC, Ukraine) at a dose of 100 mg/kg during the last eight days of the experiment6, 10).
Dry extract of Iris hungarica leaves and rhizomes and comparison drugs – potassium orotate and dry extract of maral root rhizomes – were used in the treatment and prevention regimen, i.e. administered once intragastrically for four weeks under fasting conditions in the form of Tween 80-stabilised aqueous suspension one hour before the administration of hydrocortisone acetate at a dose of 100 mg/kg intramuscularly, which interspersed with the period of reproduction of model pathology. Animals of the intact control group received equivalent amount of drinking water by weight.
The anabolic effect of the study drugs and comparison drugs was evaluated by the following indicators: body weight gain of animals; relative weight of internal organs – heart, gastrocnemius muscle, testes; total protein in blood serum, gastrocnemius and cardiac muscle (Miller GL, 1959); volume and content of urea in the urine; serum and urea glucose content during the experiment11, 12).
Animals were weighed fasted using laboratory electronic scales AD 300 (Axis, Poland). After euthanasia of the animals in the CO2 box, blood serum was collected, internal organs sensitive to anabolic agents (heart, gastrocnemius muscle, testes) were removed and weighed, and they were used to produce homogenates for further biochemical studies10, 11).
Quantitative protein content in blood serum
This was determined by the biuret method using a standard set of reagents “Total protein (Biuret with calibrator)” HP010.01 (Filisit-Diagnostyka Scientific and Production Enterprise LLC, Ukraine) according to the instructions for use.
Quantitative protein content in tissue homogenates
This was determined by the Hartree-Lowry assay. The concentration was determined photometrically against the blank at 650 nm on a photoelectrocolorimeter KFK-313).
Glucose content in blood serum
This was determined colorimetrically, using glucose oxidase method with a standard set of reagents “Glucose-F (Glucose oxidase with calibrator)” HP009.02 (Filisit-Diagnostyka Scientific and Production Enterprise LLC, Ukraine) according to the instructions for use.
Statistical analysis of results
It was carried out using the standard software package STATISTICA 6.0. Given the sample size, the Shapiro-Wilk test was used to determine the distribution characteristics, which showed an arbitrary distribution of data. Since the distribution is arbitrary in nature, statistical analysis was performed using the Kruskal-Wallis test and Newman-Keuls test.
The animals were kept in the vivarium of the Central Research Laboratory of the National University of Pharmacy certified by the State Enterprise State Expert Centre of the Ministry of Health of Ukraine as a base for research on experimental pharmacology according to sanitary regulations and on the necessary diet14, 15).
The study protocol is consistent with bioethical standards and complies with the “General Ethical Principles of Animal Experiments” (Ukraine, 2001)16), and does not contradict the European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes (Strasbourg, 1986, as amended in 1998) and the Law of Ukraine No. 3447-IV of 21.02.2006 with amendments “On the protection of animals from cruelty”, and Order of the Ministry of Education and Science, Youth and Sports of Ukraine No. 249 of 01.03.2012. “The procedure of conducting animal experiments by scientific institutions”.
Results and discussion
The key indicator in the assessment of the anabolic effect is nitrogen balance: anabolic processes are predominant when it is positive, and catabolic when it is negative. One of the ways to stimulate protein synthesis processes is to use anabolic drugs12).
Hydrocortisone is a metabolically active glucocorticoid produced by adrenal hormone that has a metabolic effect on the liver due to amino acid retention, increased RNA synthesis, enzyme proteosynthesis, gluconeogenesis and other processes10).
Modelling of hydrocortisone-induced protein catabolism in rats is appropriate for investigating the anabolic effects of new drugs, because glucocorticoids in large doses or in long-term use can cause metabolic disorders, which results in a decrease in anabolic processes and activation of catabolic ones. The mechanism of action of glucocorticoids is associated with the interaction with cytosolic receptors in the cells of target organs. Thus, a complex (glucocorticoid receptor) is formed, which penetrates the nucleus and causes expression or depression of messenger RNA, which leads to inhibition of protein synthesis and development of catabolic processes6).
The study found that dry extract of Iris hungarica leaves and rhizomes at a dose of 150 mg/kg and reference drugs – potassium orotate at a dose of 100 mg/kg and dry extract of maral root rhizomes at a dose of 150 mg/kg – had little effect on body weight changes in the intact animals (Days 1 to 22 of the experiment) (Table 1).
Tab. 1. The effect of dry extracts of Iris hungarica leaves and rhizomes on body weight changes in animals with hydrocortisone-induced protein catabolism, n = 5, (±) 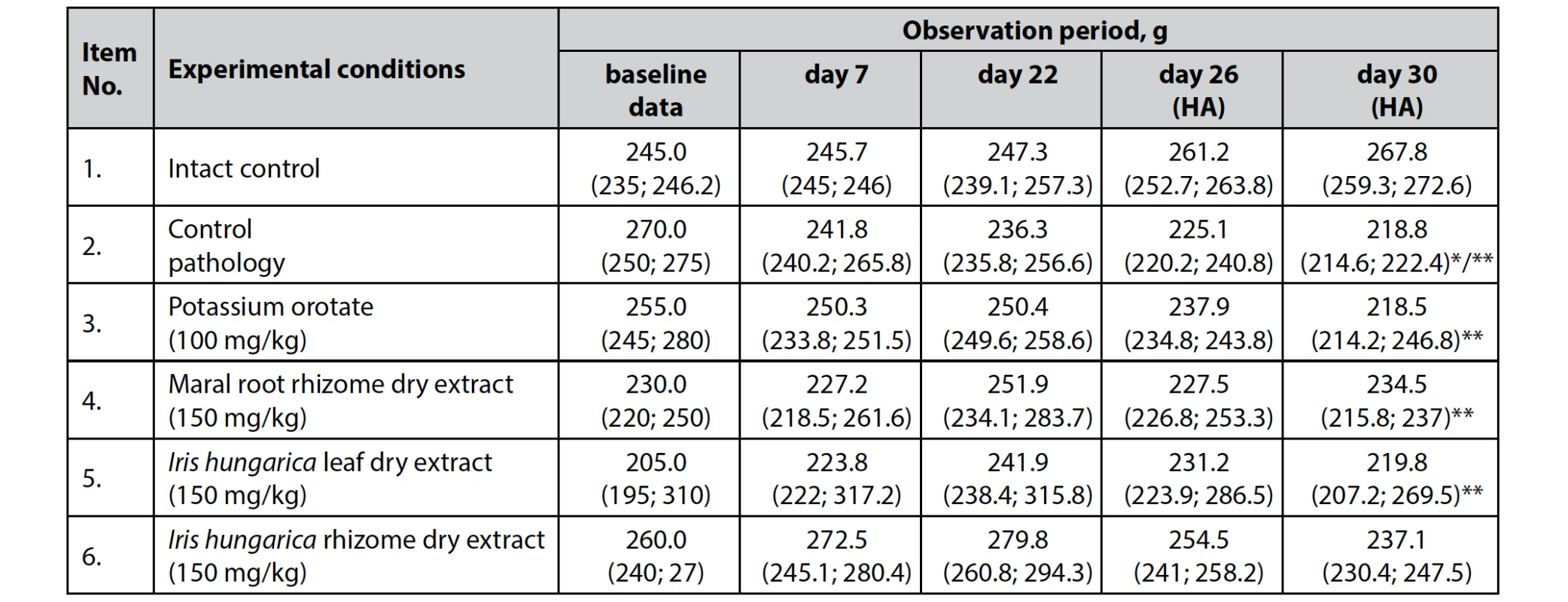
HA – hydrocortisone acetate 100 mg/kg (observation during the reproduction of the experimental model)
*p < 0.05 – differences are statistically significant relative to the baseline
**p < 0.05 – differences are statistically significant for the intact control animals
However, on the last day of the study, which coincided with the last administration of hydrocortisone acetate, the body weight of animals started notably decreasing under the influence of high doses of glucocorticoid.
Thus, body weight at the last observation was significantly lower in the group of animals of control pathology (without treatment), both compared with the corresponding indicator in the intact control (p < 0.05) and compared with the personal baseline value (p < 0.05), which indicates supraphysiologically activated catabolic processes and dystrophy.
The use of dry extract of Iris hungarica leaves, potassium orotate and dry extract of maral root rhizomes somewhat slowed down the course of catabolic processes and dystrophy, which was reflected in the absence of significant differences in mean body weight of animals at Day 30 from the baseline body weight in the corresponding groups (р > 0.05). However, it should be noted that the final values of the average weight of animals in these groups were still significantly lower than in the intact control (p < 0.05).
That said, the average weight of animals in the group receiving dry extract of Iris hungarica rhizomes was within the age norm and did not differ statistically from the intact control, indicating a significant inhibition of catabolic processes caused by the administration of hydrocortisone acetate (Table 1).
Despite the presence of significant changes in the total weight of animals in certain groups, no significant differences were observed in the relative weight of the heart, gastrocnemius muscle and testes among the experimental groups (Table 2).
Tab. 2. The effect of dry extracts of Iris hungarica leaves and rhizomes on the relative weight of the internal organs of rats with hydrocortisone-induced protein catabolism, n = 5, (±) 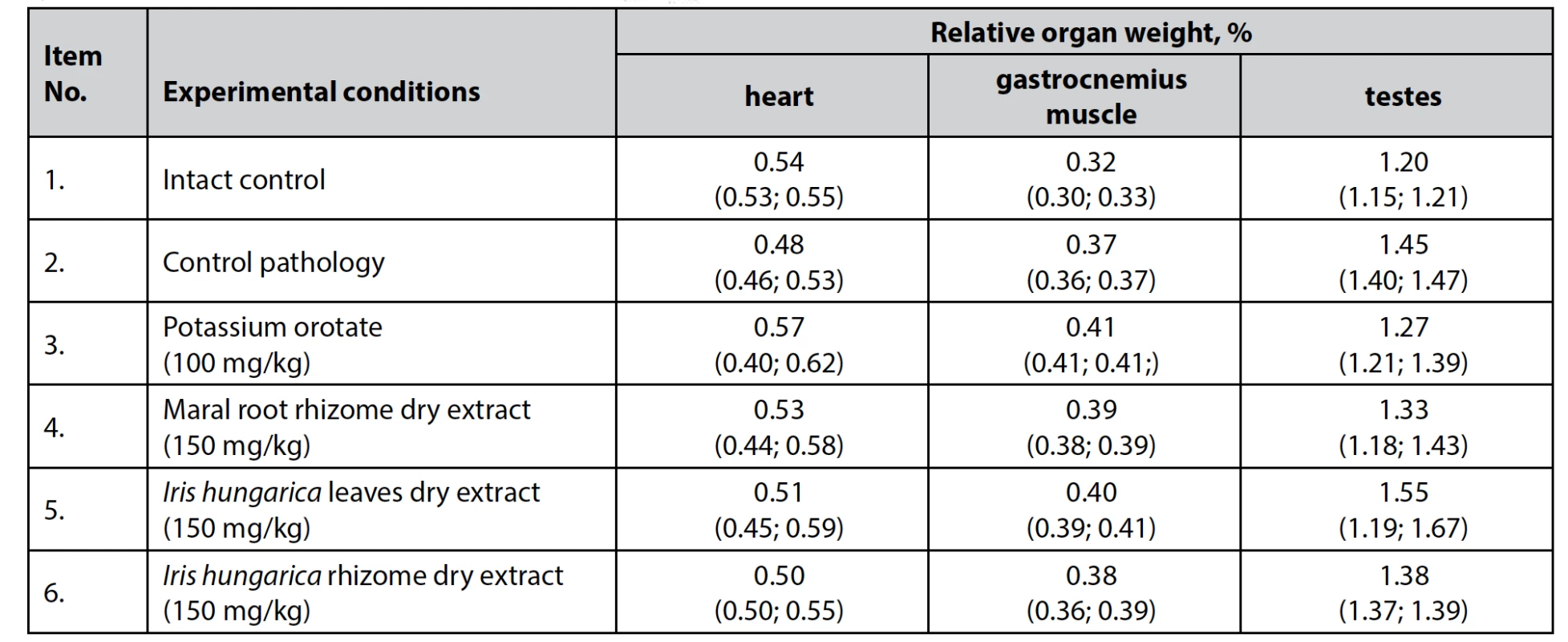
Emphasizing the intensity of anabolic and catabolic processes, as mentioned above, none of the products, including dry extract of Iris hungarica leaves and rhizomes at a dose of 150 mg/kg, potassium orotate at a dose of 100 mg/kg, or dry extract of maral root rhizomes at a dose of 150 in mg/kg, caused an increase in muscle mass, and the level of total protein in the blood serum also showed no change (Table 3).
Tab. 3. The effect of dry extracts of Iris hungarica leaves and rhizomes on the total protein content in rats with hydrocortiso-ne-induced protein catabolism, n = 5, (±) 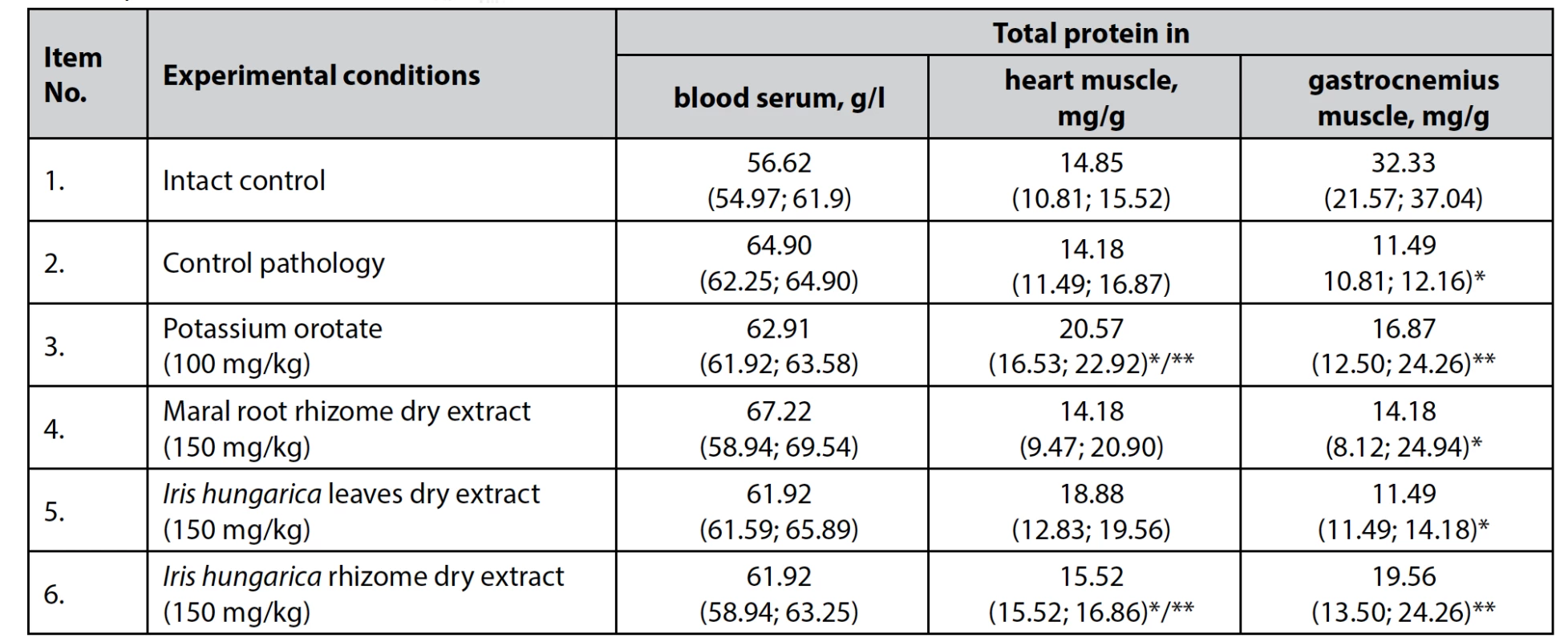
*p < 0.05 – differences are statistically significant for the intact control animals
**p < 0.05 – differences are statistically significant for the control pathology animalsDry extract of Iris hungarica rhizomes at a dose of 150 mg/kg and potassium orotate at a dose of 100 mg/kg significantly increased (p < 0.05) the content of total protein in the heart muscle, relative to the indicators of animals intact control and control pathology, while no statistically significant differences were found when exposed to dry extract of Iris hungarica leaves and dry extract of maral root rhizomes at a dose of 150 mg/kg.
However, the concentration of protein in the muscle tissue homogenate in animals treated with dry extract of Iris hungarica rhizomes and potassium orotate increased significantly (by 41.3% and 31.9%), indicating the presence of an anabolic effect directed specifically at muscle tissue (Table 3).
This indicates the development of myofibrillar rather than sarcoplasmic hypertrophy in animals, increasing the content of myosin and actin, which are contractile proteins in myofibrils. Myofibrillar hypertrophy is associated with an increase in muscle fibers and an increase in muscle strength with a moderate increase in volume17).
Regarding the analysis of urine, it was determined that the average urinary volume during a 3-hour diuresis decreased significantly (p < 0.05) by 62.0% in the group of animals receiving dry extract of Iris hungarica rhizomes at a dose of 150 mg/kg, by 35.9% in animals receiving dry extract of Iris hungarica leaves at a dose of 150 mg/kg, and by 48.9% in animals receiving the comparison drug potassium orotate at a dose of 100 mg/kg,
when compared with control pathology, indicating a general decrease in excreted urea and inhibition of protein catabolism (Table 4).Tab. 4. The effect of dry extracts of Iris hungarica leaves and rhizomes on the urine in rats with hydrocortisone-induced protein catabolism, n = 5, (±) 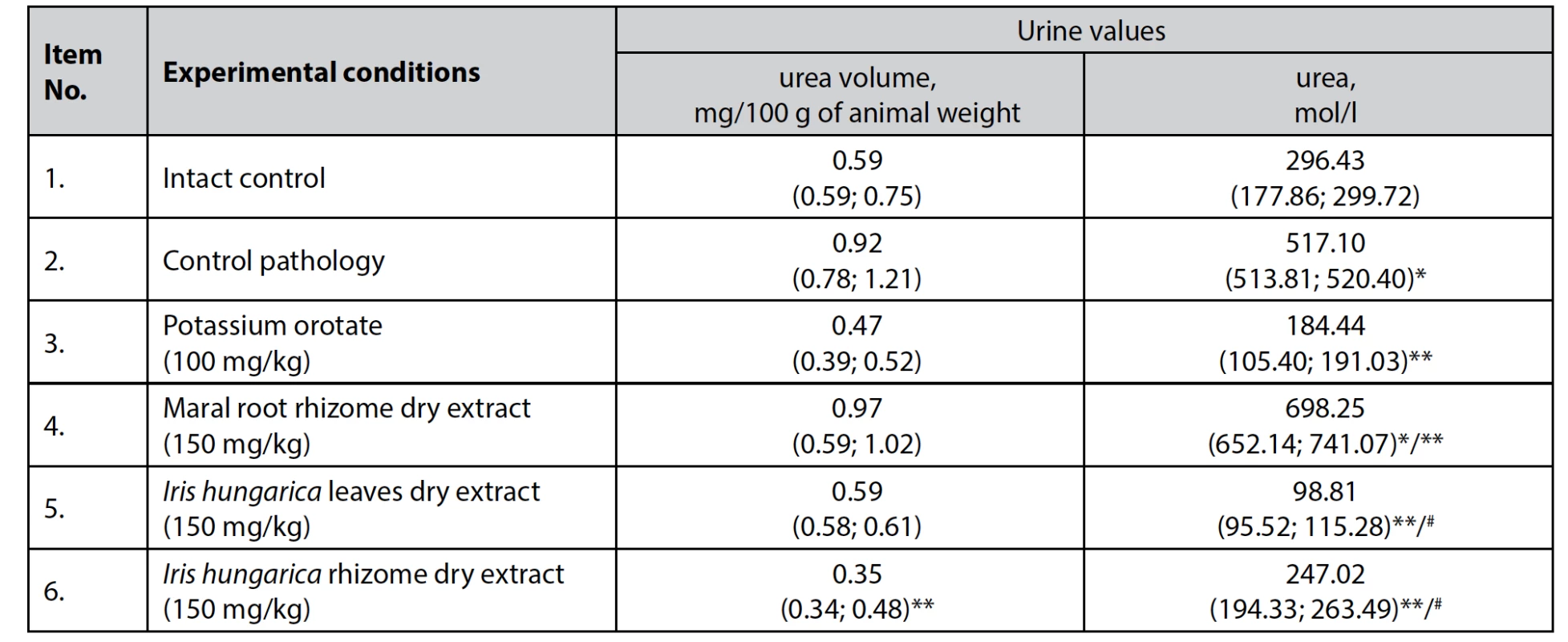
*p < 0.05 – differences are statistically significant for the intact control animals
**p < 0.05 – differences are statistically significant for the control pathology animals
#p < 0.05 – differences are statistically significant for the group of animals that received dry extract of the maral root rhizomesIn addition, dry extract of Iris hungarica rhizomes can be predicted to have a capacity to affect mineral and water metabolism.
The herbal comparison drug – dry extract of maral root rhizomes at a dose of 150 mg/kg – was not found to reduce the level of excreted urea (Table 4).
There was also a significant decrease in the level of excreted urea (p < 0.05) in the model of hydrocortisone-induced protein catabolism: by 80.9% in animals receiving dry extract of Iris hungarica leaves at a dose of 150 mg/kg, by 52.2% in animals receiving dry extract of Iris hungarica rhizomes at a dose of 150 mg/kg, and by 64.3% in animals receiving the comparison drug (potassium orotate at a dose of 100 mg/kg) compared with the control pathology. This indicates the potential normalization of protein metabolism, restoration of nitrogen balance and inhibition of protein catabolism caused by the administration of hydrocortisone.
A particularly pronounced effect was shown by dry extract of Iris hungarica leaves, which is 16.6% greater than the effect of the synthetic comparison drug, potassium orotate.
Analysis of animal blood showed that the urea content in the blood serum had no significant differences in animals of all experimental groups, indicating a sufficient function of the glomerular apparatus of the kidneys to remove excess urea, even under increased protein catabolism (Table 5).
Tab. 5. The effect of dry extracts of Iris hungarica leaves and rhizomes on the serum in rats with hydrocortisone-induced pro-tein catabolism, n = 5, (±) 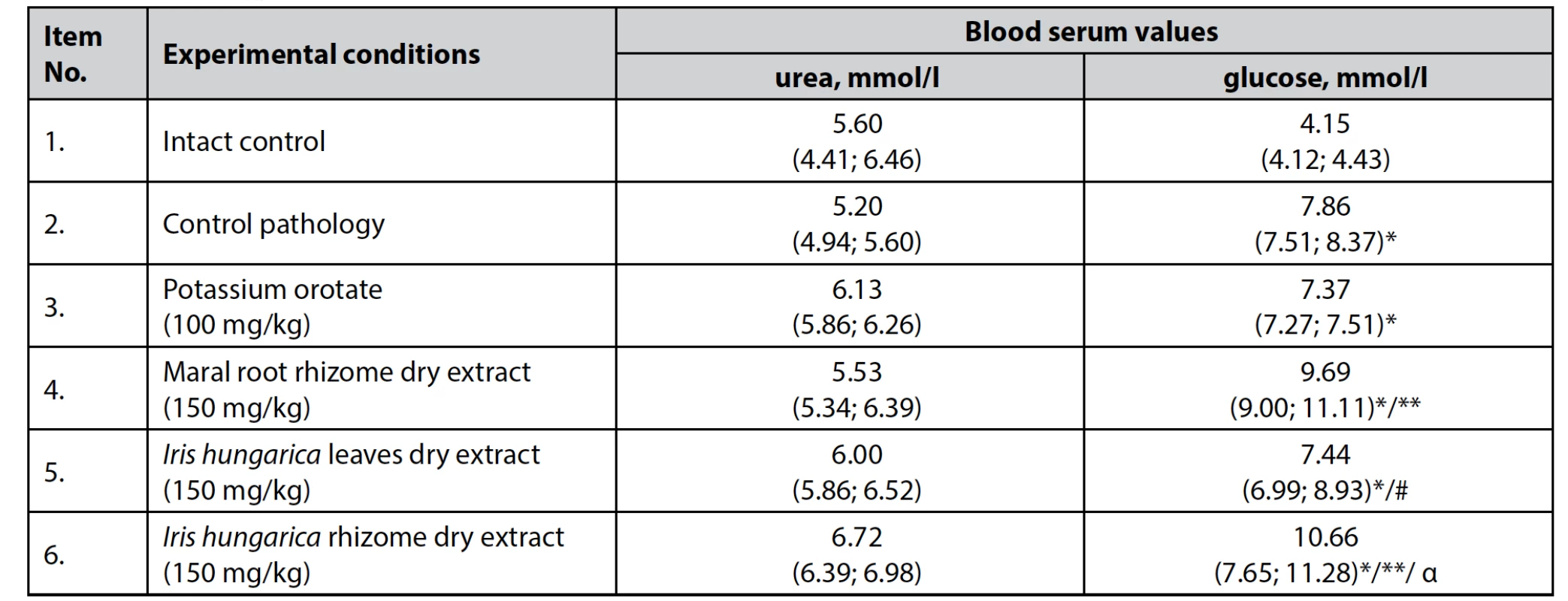
*p < 0.05 – differences are statistically significant for the intact control animals
**p < 0.05 – differences are statistically significant for the control pathology animals
#p < 0.05 – differences are statistically significant for the group of animals that were administered dry extract of Iris hunga-rica rhizomes
αp < 0.05 – differences are statistically significant for the group of animals that were administered potassium orotateMeasurements of glucose in the blood of animals treated with dry extract of Iris hungarica rhizomes showed an increase in the serum concentration, even when compared with the control group (p < 0.05). This indicates, on the one hand, that the effect of the drug on carbohydrate metabolism is almost absent, and, on the other hand, that the muscle mass is preserved due to protein rather than glycogen.
Serum glucose was significantly lower in the group of animals treated with dry extract of Iris hungarica leaves than in the group treated with dry extract of Iris hungarica rhizomes, which may indicate that the muscle mass is preserved due to partial inhibition of glycogen breakdown.
Conclusion
- The model of hydrocortisone-induced protein catabolism allowed us to determine the therapeutic and prophylactic effect of the study extracts (dry extract of Iris hungarica leaves and rhizomes) and comparison drugs (potassium orotate and dry extract of maral root rhizomes) on the body weight of animals (restoration to intact control values).
- When exposed to the study extracts and comparison drugs on the model of hydrocortisone-induced protein catabolism, there were no significant differences in the relative mass of the heart, gastrocnemius muscle and testes, as well as the total protein in the blood serum.
- The hydrocortisone-induced protein catabolism model showed the presence of anabolic action in dry extract of Iris hungarica rhizomes with a significant increase (p < 0.05) in the total protein content in rat cardiac muscle and in muscle tissue homogenate (9.4% higher than the comparison drug, potassium orotate), which is aimed to promote myofibrillar hypertrophy.
- Dry extract of Iris hungarica rhizomes significantly reduced (p < 0.05) urea excretion (by 62%), normalized protein metabolism, restored nitrogen balance, and inhibited protein catabolism caused by the administration of hydrocortisone while exceeding the effect of the comparison drug by 13.1% for potassium orotate and 26.1% for dry extract of Iris hungarica leaves. It also caused no changes to urea content in the blood of animals (which is provided by a sufficient function of the glomerular apparatus of the kidneys), but increased glucose content in the blood of animals (indicating that the muscle mass is preserved due to protein rather than glycogen).
- The results indicate that dry extract of Iris hungarica has the ability to correct protein metabolism, which is regulated in part by glucocorticoids, due to the high content of isoflavonoids and amino acids, and suggest that there is a potential use for this herbal product in the development of a new drug aimed at correcting protein metabolism and muscular atrophy.
Conflict of interest: none.
Zdroje
- Jadhav A. N., Rafiq M., Devanathan R., et al. Ketosteroid standardized cissus quadrangularis L. Extract and its anabolic activity: Time to look beyond ketosteroid? Pharmacogn. Mag. 2016; 12(46), 213–217.
- Zheng W., Hemker M. L., Xie M., et al. Anabolic activity of a soy extract and three major isoflavones in C2C12 myotubes. Planta med. 2018; 84(14), 1022–1029.
- Esposito D., Rathinasabapathy T., Poulev A., et al. Akt-dependent anabolic activity of natural and synthetic brassinosteroids in rat skeletal muscle cells. J. Med. Chem. 2011; 54(12), 4057–4066.
- Bansode F. W., Arya K. R., Singh R. K., et al. Dose-dependent effects of Asparagus adscendens root (AARR) extract on the anabolic, reproductive, and sexual behavioral activity in rats. Pharm. Biol. 2015; 53(2), 192–200.
- Gorelick J., Iraqi R. H., Bernstein N. Ecdysteroid content and therapeutic activity in elicited spinach accessions. Plants (Basel) 2020; 9(6), 1–11.
- Yakovleva L. V., et al. Eksperymental’ne vyvchennya novykh anabolichnykh zasobiv. Metodychni rekomendatsiyi. [Experimental study of new anabolic drugs. Guidelines]. Kyiv 2007; 8–16 (in Ukrainian).
- Kerimova G. F., Korol V. V., Rybak V. A. Osoblyvosti mekhanizmu diyi ta zastosuvannya fitopreparativ-anabolikiv z metoyu stvorennya likars’kykh preparativ na osnovi sukhoho ekstraktu Iris hungarica [Special aspects of the mechanism of action and use of anabolic phytopreparations for the purpose of creation of drugs on the basis of dry extract of Iris hungarica]. Perspectives of world science and education: materials of the II international scientific practice. conf. in Osaka, Japan, 30–31 Oct. 2019. Osaka 2019; 50–56.
- Mykhailenko O., Ivanauskas L., BezrukI., Lesyk R., Georgiyants V. Comparative investigation of amino acids content in the dry extracts of Juno bucharica, Gladiolus Hybrid Zefir, Iris Hungarica, Iris Variegata and Crocus Sativus raw materials of ukrainian flora. Sci. Pharm. 2020; 88(1), 8. https://doi.org/10.3390/scipharm88010008
- Kerimova G. F., Rybak V. A., Krechun A. V., Kovalev V. M. Vyvchennya anabolichnoyi aktyvnosti sukhykh ekstraktiv lystya i korenevyshch Iris hungarica v intaktnykh tvaryn [Study of anabolic activity of dry extracts of leaves and rhizomes of Iris hungarica in intact animals]. Fitoterapiya. Chasopys. 2020; 2, 50–55 (in Ukrainian).
- Mironova A. N. Guidelines for conducting preclinical studies of medicinal substances. Part 1. M.: Grif i K, 2012; 944 (in Ukrainian).
- Siddiqui H., Sami F., Bajguz A., Hayat S. Glucose escalates PSII activity, dynamics between anabolic and catabolic pathways, redox and elemental status to promote the growth of Brassica juncea. S. Afr. J. Bot. 2021; 137, 68–84.
- Pierre M., Yollande M. F. G., Emmanuel A. A, et al. Anabolic activity of alcoholic extract of Piper guineense in immature castrated rat. Pharmacologyonline 2007; 1, 99–107.
- Redmile-Gordon M. A, et al. A comparison of two colorimetric assays, based upon Lowry and Bradford techniques, to estimate total protein in soil extracts. Soil Biology & Biochemistry. 2013; 67(100), 166–173.
- Sharma V., Thakur M., Chauhan N. S., et al. Evaluation of the anabolic, aphrodisiac and reproductive activity of Anacyclus pyrethrum DC in male rats. Sci. Pharm. 2009; 77(1), 97–110.
- Copetta A., Laura M. The double-layer method to the genesis of androgenic plants in Anemone coronaria. Methods Mol. Biol. 2021; 2264, 187–196.
- Stefanov O. V. Doklinichni doslidzhennya likars’kykh zasobiv: metodychni rekomendatsiyi [Preclinical studies of drugs: guidelines]. K.: Avicena 2001; 528 (in Ukrainian).
- Pope H. G. Jr, Wood R. I., Rogol A., et al. Adverse health consequences of performance-enhancing drugs: an endocrine society scientific statement. Endocr. Rev. 2014; 35(3), 341–375.
Štítky
Farmacie Farmakologie
Článek vyšel v časopiseČeská a slovenská farmacie
Nejčtenější tento týden
2021 Číslo 2- Psilocybin je v Česku od 1. ledna 2026 schválený. Co to znamená v praxi?
- Ukažte mi, jak kašlete, a já vám řeknu, co vám je
- Přerušovaný půst může mít významná zdravotní rizika
-
Všechny články tohoto čísla
- Causes and interventions of medication errors in healthcare facilities
- Synthesis and HPLC enantioseparation of novel derivatives of 3-alkoxy-4-hydroxyphenylalkanones of a potential α/β-blocker type
- Investigation of the anabolic activity of dry extracts of Iris hungarica leaves and rhizomes on the model of hydrocortisone-induced protein catabolism
- Formulation development of anti-stress compressed lozenges using a fractional factorial Latin cube design and ANOVA approach
- Česká a slovenská farmacie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Causes and interventions of medication errors in healthcare facilities
- Formulation development of anti-stress compressed lozenges using a fractional factorial Latin cube design and ANOVA approach
- Synthesis and HPLC enantioseparation of novel derivatives of 3-alkoxy-4-hydroxyphenylalkanones of a potential α/β-blocker type
- Investigation of the anabolic activity of dry extracts of Iris hungarica leaves and rhizomes on the model of hydrocortisone-induced protein catabolism
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



