-
Medical journals
- Career
Infection in patients hospitalised with advanced chronic liver disease (cirrhosis) – single-centre experience
Authors: Bystrianska N. 1; Skladaný Ľ. 1; Adamcova-Selcanova S. 1; Vnencakova J. 1; Jancekova D. 1; Koller T. 2
Authors‘ workplace: HEGITO – Hepatology, Gastroenterology and Liver Transplantation Division of the Department of Internal Medicine II, Faculty of Medicine, Slovak Medical University, F. D. Roosevelt Hospital, Banska Bystrica, Slovakia 1; 5th Department of Internal Medicine, Comenius University Faculty of Medicine, University Hospital Bratislava Ruzinov, Bratislava, Slovakia 2
Published in: Gastroent Hepatol 2020; 74(2): 111-115
Category: Hepatology: original article
doi: https://doi.org/10.14735/amgh2020111Overview
Introduction: Advanced chronic liver disease (ACLD) is associated with immune deficiency, which increases the risk of bacterial infections and the consequences thereof, including mortality.
Aim: We aimed to characterise infections by the frequency, aetiology, type and associated prognosis in patients (pts) with ACLD hospitalised at the Liver Unit.
Methods: Retrospective study of consecutive adult pts hospitalised with ACLD, enrolled in the Registry HEGITO 7 (RH7). The data for RH7 were received from the Hospital Information System (CareCenter Copyright 2000, CGM version 3.19.1) for the interval between July 2014 and September 2016. We excluded pts with malignancies, those after liver transplantation and those treated for ascites by peritoneal catheter. We recorded the demographic parameters, type of infection, microbial culture, type of agent, antibiotic resistance and in-hospital mortality.
Results: We enrolled 400 pts, excluded 46 for pre-defined criteria and analysed 354 pts: 158 females (44.63%), aged 55 years, with MELD (Model for End-Stage Liver Disease) 16 points (p), and Child–Turcotte–Pugh 9 p. The most frequent aetiology was alcohol-associated liver disease in 214 pts (60.4%). We diagnosed infection in 95 pts (27%), 49% were healthcare-associated, 35% nosocomial and 15% community-associated. The most frequent infection sites were urinary tract infection, spontaneous bacterial peritonitis and respiratory infections. Microbial cultures were positive in 66% of the episodes. The prevalence of multidrug-resistant bacteria (MDR) was 33%. In-hospital mortality in infected as compared to non-infected pts was 18 vs. 2% (P = 0.0001).
Conclusion: The prevalence of bacterial infections in our study was 27%. Infections were associated with in-hospital mortality. One in three cultures yielded MDR strains. The results are similar to those from the GLOBAL Study. Stewardship has to be re-evaluated.
Keywords:
mortality – ACLD – bacterial infections
Introduction
According to the Global Burden of Disease Study, liver cirrhosis is responsible for more than million deaths a year. In liver-related mortality, Slovakia ranks 4th in Europe and liver disease has become the leading cause of death in people 35–55 years old [1–3]. One in three advanced chronic liver disease (ACLD)-related deaths is mediated by infection, operating either as the cause or the consequence of decompensation (dACLD) [4–6]. With a prevalence of approx. 30%, infections are more frequent in dACLD as compared to compensated ACLD; they play particularly important role in the acute decompensation (AD) of ACLD, and in the syndrome of acute-over chronic liver failure (ACLF) [5–10]. In ACLF, infections have been recognised as an important trigger of organ failures, such as acute kidney injury (in 46% of the cases), hepatic encephalopathy (35–47%) and bleeding (66%) [11–14]. The pivotal multinational GLOBAL Study provided important information on infections in patients with ACLD from 15 European centres; Slovakia was not included [15].
Aim
With the GLOBAL Study as the approximate template, we aimed to characterise infections in our Registry of Hospitalised Patients with ACLD (RH7) in terms of the frequency, aetiology, site and associated prognosis.
Patients and Methods
For this retrospective registry study, we used the Registry-HEGITO-7 (RH7) dataset, into which we have been enrolling consecutive adult patients (pts) hospitalised with ACLD, who provided informed consent. The RH7 dataset contains demographics, aetiology and basic clinical characteristics – such as those needed for the calculation of the model for the end-stage liver disease score (MELD), the Child-Turcotte-Pugh score (CTPS), the specific complications of ACLD etc. The data for RH7 have been retrieved from the Hospital Information System (CareCenter Copyright 2000, CGM version 3.19.1), and uploaded by the study nurse according to the principles of the General Data Protection Regulation. For this analysis, we used data from the interval between July 2014 and September 2016. We excluded pts with malignancies except for hepatocellular carcinoma in the Milan criteria, pts after liver transplantation and those treated for resistant ascites by an indwelling peritoneal catheter. We recorded the site of the infection, microbial culture positivity, type of the agent, its antibiotic resistance, and in-hospital mortality. Infections were diagnosed according to the conventional criteria [16,17]. This study has been conducted in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments.
Results
We enrolled 400 pts, excluded 46 pts for predefined criteria and analysed 354 pts: 158 females (44.63%), aged 55 years. The most frequent aetiology of ACLD was alcohol-associated liver disease (ALD) in 214 pts (60.4%), followed by non-alcoholic steatohepatitis (NASH) in 29 pts (8.2%), a group of miscellaneous etiologies in 26 pts (7.3%), dual ALD plus NASH in 22 pts (6.2 %), hepatitis C virus infection in 17 pts (4.8%), primary biliary cholangitis in 16 pts (4.5%), autoimmune hepatitis in 13 pts (3.7%), primary sclerosing cholangitis in 12 pts (3.4%) and secondary biliary cirrhosis in 5 pts (1.4%). The MELD and CTPS scores were 16 points (9.88–22.38) and 9 (6.99–11.51) points, resp. We diagnosed infection in 95 of our pts (27%), 56 males (58.95%), aged 52 years (NS, as compared with pts without infections). The main aetiology of ACLD in infected pts was ALD in 60 pts (63.15%) with MELD, and CTPS scores of 19.82 (± 6.79) and 10.57 (± 1.75), resp. (for the difference between non-infected pts, P = 0.0001). Of the 112 infectious episodes, 55 (49.11%) were healthcare-associated, 40 (35.1%) nosocomial and 17 (15.17%) community-associated, resp. (Graph 1). The most frequent infection site was urinary tract infection (UTI) in 31 pts (27%), followed by spontaneous bacterial peritonitis (SBP) in 27 pts (24%), respiratory tract infections (RTI) in 24 pts (21%), spontaneous bacteraemia in 20 pts (18%), clostridium difficile infection in 7 pts (6%) and soft-tissue infections in 3 pts (1.6%) (Graph 2). Microbial cultures were positive in 66% of infectious episodes, with gram-negative bacteria in 48% (mainly Enterococci, Escherichia coli, and Klebsiella pneumoniae), gram-positive in 42%, and fungi in 9.5% (Graph 3). The prevalence of MDR was 33%, with K. pneumoniae the most prevalent agent (39%). As concerns the prevalence of infections according to the type of dACLD, we have recorded infections in 49 of 252 pts with chronic decompensation (CD) 19.4%, in 24 of 69 pts with AD (19.5%), and in 22 of 33 pts with ACLF (66.7%) (P = 0.01). The most prevalent infection sites in the subcohorts with CD, AD and ACLF were UTI (7.5%), SBP (14.5%) and RTI (21.2%). In-hospital mortality in infected as compared to non-infected pts was 17.9% and 1.97%, resp. (P = 0.001) (Graph 4).
1. Inception of infections according to the setting in I-RH7 study (N = 112 infectious episodes).
Graf 1. Vznik infekcií podľa nastavenia v štúdii I-RH7 (n = 112 infekčných epizód).
2. Site of infections in I-RH7 study (N = 112 infectious episodes).
Graf 2. Miesto infekcie v štúdii I-RH7 (n = 112 infekčných epizód).
3. Pathogen distribution in I-RH7 study (N = 93).
Graf 3. Distribúcia patogénu v štúdii I-RH7 (n = 93).
4. Thirty-day survival in infected and non-infected patients in I-RH7 study (N = 354).
Graf 4. Tridsaťdňové prežitie infikovaných a neinfikovaných pacientov v štúdii I-RH7 (n = 354).
Discussion
Mortality in ACLD is strongly linked to infections [3,18]. They are frequent consequence of liver failure, or emerge as a cause thereof – either as the direct trigger (as in ACLF), or as an intermediating factor (e. g. of variceal bleeding); in any way, infections influence prognosis, increase readmissions to hospital and increase the cost of care [18,19]. We have decided to slice the RH7 and to analyse the first 2 years of the dataset, because infections are one of the fastest-changing specific complications of ACLD – especially in terms of aetiology and the proportion of MDR bacteria. The prevalence of infections in our cohort was 27%, which is well within the range of 25–47%, as seen in the literature [18–20]. We consider underestimation improbable, because the study was registry-based with due emphasis on the high index of suspicion for the possibility of infection in all the patients admitted with any complication of ACLD. Of the known risk factors for infections, we have found associations with MELD and CTPS, but not with the age, sex, and aetiology of ACLD [21]. In light of the published data showing increased risk of infection in patients with MELD over 15, and CTPS C, our cohort with MELD 19.8 and CTPS 10.6 can be considered high-risk [20].
The most common sites of infection in the whole cohort compared well with the data from the literature: UTI represented 27%, SBP 24% and RTI 21%, resp. [5,22]. When we subdivided the patients according to the type of decompensation, the highest prevalence (66.6%) was found in ACLF. There is a close relationship between infections and ACLF, which can be either a trigger (in 30%), or a complication (in 20–40%); moreover, this so-called iACLF can be associated with worse prognosis as compared to other types of ACLF [6–10]. In-hospital mortality in our infected patients was 18% as compared to 2% in non-infected patients. In the literature, in-hospital mortality has been around 15% in infected patients with ACLD, with infections accounting for 30–50% of all deaths [6]. When viewed according to the type of decompensation, mortality remained numerically higher in infected patients across the subtypes, but was statistically significant only in patients with CD (14 vs. 8%, P = 0.0002). The fact that the difference did not reach statistical significance in AD and ACLF patients, we explain by the low number of patients [23].
Our data should be perceived in the context of the GLOBAL Study (Tab. 1) [24]. This landmark endeavour was a prospective, multicentric, intercontinental study focused on infections in ACLD (study interval between October 2015 and September 2016). More than 1,300 patients from 46 centres in Asia (15), Europe (15), South America (11) and North America (5) were enrolled; Slovakia was not included. The demographics and CTPS were similar to RH7, the MELD was higher at 21 and there were 35% patients with ACLF. The major difference between the GLOBAL and the I-RH7 studies was seen in the proportion of infections incepted in community (48 vs. 15.17%), in association with healthcare (26 vs. 49.11%) or in hospital (nosocomial 26 vs. 35.71%) (Graph 1). The explanation could only be speculative and would lie in the characteristics of the healthcare and social systems of the Western as compared to the Central European regions. Cultures were positive in a similar proportion of patients in both studies, as was the representation of MDR strains (Tab. 1 and Graph 5). The high frequency of MDR bacteria (34%) is cause for concern and would lead to the further re-evaluation of our antibiotic stewardship strategy [24] . The majority of the strains (57%) were gram-negative, fungi represented 4% of the positive cultures. In-hospital mortality was high at 23% and increased in patients with infection as compared to those without.
1. Characteristics of the I-RH7 and the GLOBAL study.
Tab. 1. Charakteristika I-RH7 a GLOBAL štúdia.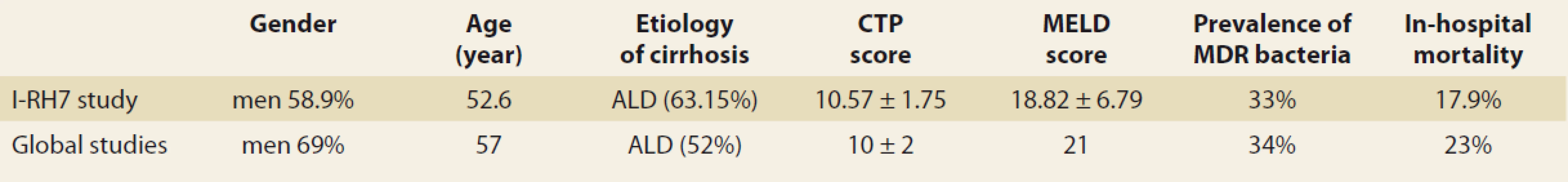
CTP – Child-Turcotte-Pugh score, MELD – model for the end-stage liver disease score, MDR – multidrug-resistant bacteria, ALD – alcohol-associated liver disease 5. The multidrug-resistant strains distribution (multi drug resistant bacteria represented 33% of all infections) (I-RH7 study, N = 354).
Graf 5. Distribúcia kmeňov odolných voči viacerým liečivám (baktérie rezistentné voči viacerým liečivám predstavovali 33% všetkých infekcií) (štúdia I-RH7, n = 354).
Conclusions
The prevalence of infections in patients hospitalised with ACLD in a tertiary referral centre was 27%, which compares well with the data from the literature. Infections were associated with CTPS, MELD and 30-day mortality. The proportions of nosocomial infections and MDR strains were alarming. This analysis, its interpretation in the context of the GLOBAL Study and comparison with the results from following years of RH7 would serve as the framework for modifications in site-specific stewardship.
Submitted/ Doručeno: 15. 3. 2020
Accepted/ Přijato: 31. 3. 2020
Natalia Bystrianska, MD
Department of Internal Medicine II
Faculty of Medicine
F. D. Roosevelt Hospital
Square L. Svobodu 1
975 17 Banska Bystrica
Slovak Republic
Conflict of Interest: The authors declare that the article/ manuscript complies with ethical standards, patient anonymity has been respected, and they state that they have no financial, advisory or other commercial interests in relation to the subject matter.
Publication Ethics: This article/ manuscript has not been published or is currently being submitted for another review. The authors agree to publish their name and e-mail in the published article/ manuscript.
Dedication: The article/ manuscript is not supported by a grant nor has it been created with the support of any company.
The Editorial Board declares that the manuscript met the ICMJE „uniform requirements“ for biomedical papers.
Konflikt záujmov: Autori deklarujú, že text článku zodpovedá etickým štandardom, bola dodržaná anonymita pacientov, a vyhlasujú, že v súvislosti s predmetom článku nemajú finančné, poradenské ani iné komerčné záujmy.
Publikačná etika: Príspevok nebol doteraz publikovaný ani nie je v súčasnosti zaslaný do iného časopisu na posúdenie. Autori súhlasí s uverejnením svojho mena a e-mailového kontaktu v publikovanom texte.
Dedikácia: Článok nie je podporený grantom ani nevznikol za podpory žiadnej spoločnosti.
Redakčná rada potvrdzuje, že rukopis práce splnil ICMJE kritériá pre publikácie zasielané do biomedicínskych časopisov.
Sources
1. Wang FS, Fang JG, Zhang Z et al. The global burden of liver disease: the major impact of China. Hepatology 2014; 60(6): 2099–2108. doi: 10.1002/ hep.27406.
2. Mokdad AA, Lopze AD, Shahraz S et al. Liver cirrhosis mortality in 187 countries between 1980 and 2010: a systematic analysis. BMC Med 2014; 18(12): 145. doi: 10.1186/ s12916-014-0145-y.
3. Blachier M, Leleu H, Peck-Radosavljevic M et al. The burden of liver disease in Europe: a review of available epidemiological data. J Hepatol 2013; 58(3): 593–608. doi: 10.1016/ j.jhep.2012.12.005.
4. Plequezuelo M, Benitez JM, Jurado J et al. Diagnosis and management of bacterial infections in decompensated cirrhosis. World J of Hepatol 2013; 5(1): 16–25. doi: 10.4254/ wjh.v5.i1.16.
5. Taneja SK, Dhiman RK. Prevention and management of bacterial infections in cirrhosis. Int J Hepatol 2011; 2011 : 784540. doi: 10.4061/ 2011/ 784540.
6. Arvaniti V, D’Amico G, Fede G et al. Infections in patients with cirrhosis increase mortality four-fold and should be used in determining prognosis. Gastroenterology 2010; 139(4): 1246–1256. doi: 10.1053/ j.gastro.2010.06.019.
7. Bajaj JS, O’Leary JG, Reddy KR et al. Survival in infection-related acute-on-chronic liver failure is defined by extrahepatic organ failures. Hepatology 2014; 60(1): 250–256. doi: 10.1002/ hep.27077.
8. Arroyo V, Moreau R, Jalan R et al. Acute-on-chronic liver failure: a new syndrome that will reclassify cirrhosis. J Hepatol 2015; 62 (Suppl 1): S131–S143. doi: 10.1016/ j.jhep.2014.11.045.
9. Levesque E, Hoti E, Azoulay D et al. Prospective evaluation of the prognostic scores for cirrhotic patients admitted to an intensive care unit. J Hepatol 2012; 56(1): 95–102. doi: 10.1016/ j.jhep.2011.06.024.
10. Marciano S, Mauro EM, Gadano AC. Acute-on-chronic liver failure: an update. OA Hepatology 2013; 1(1): 1–8.
11. Martín-Llahí M, Guevara M, Torre A et al. Prognostic importance of the cause of renal failure in patients with cirrhosis. Gastroenterology 2011; 140(2): 488–496. doi: 10.1053/ j.gastro.2010.07.043.
12. Mumtaz K, Ahmed U, Abid S et al. Precipitating factors and the outcome of hepatic encephalopathy in liver cirrhosis. J Coll Physicians Pak 2010; 20(8): 514–518. doi: 08.2010/ JCPSP.514518.
13. Tariq M, Iqbal S, Khan NU et al. Precipitating factors of hepatic encephalopathy. Rawal Med J 2009; 34 : 95–97.
14. Goulis J, Armonis A, Patch D et al. Bacterial infection is independently associated with failure to control bleeding in cirrhotic patients with gastrointestinal hemorrhage. Hepatology 1998; 27(5): 1207–1212. doi: 10.1002/ hep.510270‚504.
15. Piano S, Singh V, Caraceni P et al. Epidemiology and effects of bacterial infections in patients with cirrhosis worldwide. Gastroenterology 2019; 156(5): 1368–1380. doi: 10.1053/ j.gastro.2018.12.005.
16. Rimola A, García-Tsao G, Navasa M et al. Diagnosis, treatment and prophylaxis of spon-taneous bacterial peritonitis: a consensus document. International Ascites Club. J Hepatol 2000; 32(1): 142–153. doi: 10.1016/ s0168-8278(00)80201-9.
17. Horan TC, Andrus M, Dudeck MA. CDC/ NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control 2008; 36(5): 309–332. doi: 10.1016/ j.ajic.2008.03.002.
18. Dražilová S, Janíčko M. Infekcie pri cirhóze. Gastroenterol prax 2015; 14(1): 31–34.
19. Jalan R, Fernandez J, Wiest R et al. Bacterial infections in cirrhosis: a position statement based on the EASL Special Conference 2013. J Hepatol 2014; 60(6): 1310–1324. doi: 10.1016/ j.jhep.2014.01.024.
20. Fagiuoli S, Colli A, Bruno R et al. Management of infections in cirrhotic patients: report of a consensus conference. Dig Liver Dis 2014; 46(3): 204–212. doi: 10.1016/ j.dld.2013.07.015.
21. Mihaličová L, Bališová Z, Janičko M et al. Infekcie pri cirhóze pečene – naše skúsenosti. Gastroent Hepatol 2016; 70(2): 133–137. doi: 10.14735/ amgh2016133.
22. Wong F, Bernardi M, Balk R et al. Sepsis in cirrhosis: report on the 7th meeting of the International Ascites Club. Gut 2015; 54(5): 718–725. 10.1136/ gut.2004.038679.
23. Pop A, Andreica V. Infections and liver cirrhosis: a dangerous liaison. HVM Bioflux 2015; 7(4): 264–270.
24. Skladaný Ľ, Kásová S, Purgelová A et al. Spontánna baktériová peritonitída. Klin Mikrobiol Infekc Lek 2016; 22(4): 136–140.
Labels
Paediatric gastroenterology Gastroenterology and hepatology Surgery
Article was published inGastroenterology and Hepatology
2020 Issue 2-
All articles in this issue
- Covid-19 and the liver
- Editorial
- Guideline of the Czech Hepatology Society of the ČLS JEP for diagnosis and treatment of non-alcoholic fatty liver disease
- Doporučení pro léčbu idiopatických střevních zánětů v době pandemie covid-19
- Recommendations of the Slovak IBD Working Group on SGS for the Treatment of Biosimilar Anti-TNF Biologics in Adult and Pediatric Patients
- Endoscopic drainage of infected pancreatic necrosis with complicated course – case report
- Four-year experience of infliximab and adalimumab pharmacokinetics monitoring in patients with inflammatory bowel disease
- Position of vedolizumab in the current treatment of Crohn’s disease
- Laser lithotripsy as a solution of an obturating biliary stone in the colon
- Anderson-Fabry disease and gastrointestinal tract involvement
- Gastroenterology and gastrointestinal endoscopy under SARS-CoV-2 pandemic conditions
- 90th Associate Professor Milose Sedlackova, MD
- Anniversary of Ass. Hana Dvorakova, MD
- Comment on the article: Caha M, Politová P, Vlk R et al. The surprising cause of death of a patient with upper gastrointestinal bleeding. Gastroent Hepatol 2020; 74(1): 50–53. doi: 10.14735/ amgh202050.
- The selection from international journals
- Kreditovaný autodidaktický test
- Infection in patients hospitalised with advanced chronic liver disease (cirrhosis) – single-centre experience
- De novo non-alcoholic fatty liver disease after liver transplantation – as diagnosed by magnetic resonance
-
Novel Pancreatic Developmentsprof. Peter Layer – Gastro Update Europe 2019, Budapest
Nové poznatky o pankreatu -
Biliopancreatic endoscopy
prof. Marco Bruno – Gastro Update Europe 2019, Budapest - Novel Developments In Intestinal Endoscopy<br> prof. Oliver Pech – Gastro Update Europe 2019, Budapest
- Gastroenterology and Hepatology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Covid-19 and the liver
- Endoscopic drainage of infected pancreatic necrosis with complicated course – case report
- Guideline of the Czech Hepatology Society of the ČLS JEP for diagnosis and treatment of non-alcoholic fatty liver disease
- Doporučení pro léčbu idiopatických střevních zánětů v době pandemie covid-19
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

