-
Medical journals
- Career
Epidemiology of Cronobacter spp. isolates from patients admitted to the Olomouc University Hospital (Czech Republic)
Authors: O. Holý 1; J. Petrželová 2; V. Hanulík 2; M. Chromá 2; I. Matoušková 1; S. J. Forsythe 3
Authors‘ workplace: Department of Preventive Medicine, Faculty of Medicine and Dentistry, Palacký University Olomouc, Olomouc, Czech Republic 1; Department of Microbiology, Faculty of Medicine and Dentistry, Palacký University Olomouc, Olomouc, Czech Republic 2; School of Science and Technology, Nottingham Trent University, Nottingham, United Kingdom 3
Published in: Epidemiol. Mikrobiol. Imunol. 63, 2014, č. 1, s. 69-72
Category: Short Communication
Overview
The data on the incidence of Cronobacter spp. was collated from hospital records for the seven-year period 2005–2011. The majority of Cronobacter spp. isolates (n = 91) were from throat swabs (61), followed by urine (5), tracheal aspirates (5), bronchoalveolar lavage (4), cannulae (4), and sputum (3) samples. This is the first study which profiles the carriage of Cronobacter spp. according to patient age, based on seven years of clinical data from 2005–2011. It reveals a high recovery (63.7% of strains, n = 91) of the organism from children, 1–14 years in age.
Keywords:
Cronobacter spp. – meningitis – nosocomial infectionINTRODUCTION
In recent years Cronobacter spp. has attracted considerable attention due to severe, though rare, neonatal infections [1–3]. Many of these cases have been attributed to contaminated reconstituted infant formula [4]. Subsequently, the FAO/WHO held three risk assessments (2004, 2006, 2008) to consider control measures for neonatal exposure to Cronobacter through formula ingestion. In addition, the FAO/WHO reported that the majority of Cronobacter spp. infections (ie. bacteraemia) were in the adult population [5]. This statement was based on data from the Health Protection Agency for England and Wales submitted to the FAO/WHO following their ‘call for data’ [5]. However no comparable surveillance data for the bacterium has been published. This communication is the first to report an age profile for Cronobacter spp.
METHODS
The University Hospital Olomouc (Czech Republic) receives 45,831 patients per year in average and 776,000 outpatients per year from a catchment population size of 1,000,000. Department of Microbiology conducts microbiological examination for University Hospital Olomouc. Biological samples for bacteriological monitoring, depending on the patient’s condition, are sent once or twice a week. Microbiological samples are processed by accredited methods CIA (Czech Accreditation Institute). The first sample, identified as Cronobacter was included.
The incidence of Cronobacter spp. isolation was collated from hospital records for the seven year period 2005–2011 (Table 1). The isolates had been identified using the BD PhoenixTM automated microbiological system which uses 45 phenotypic characters to identify bacterial cultures. Substrates for biochemical tests: sorbitol, methyl-beta-glucoside and esculin were positive, while adonitol was negative. The above are main biochemical tests distinguishing E. sakazakii strain from E. cloacae strain [6]. Antibiotic sensitivity of the strains was determined according to minimal inhibitory concentrations (MIC). The antibiotics tested were for Gram-negative bacteria (ATB I and II line) as given by Trios (Czech Republic). The standard microdiluting method was used [7]; the susceptibility was assessed in Mueller-Hinton broth. To establish antibiotic sensitivity, a set for determining minimal inhibitory concentrations (MIC) by means of a standard micro method in a microtiter plate was used. The majority of Cronobacter isolates (n = 91) were from throat swabs (61), followed by urine (5), tracheal aspirates (5), bronchoalveolar lavage (4), cannulae (4), and sputum (3) samples, as shown in the Table 2. These samples had been taken for general screening purposes, and not specifically according to the patient clinical presentation. Nevertheless, isolates taken from immune-deficient paediatric patients, the isolation of any microorganism particularly from sputum, bronchoalveolar lavage and indwelling cannulae could be of high clinical significance. Such additional strains had been isolated from clinical investigations (see Tables 1 and 2).
1. Isolation of Cronobacter spp. from throat swabs of patients according to age group Tabulka 1. Izolace Cronobacter spp. z výtěru z krku u pacientů podle věkových skupin 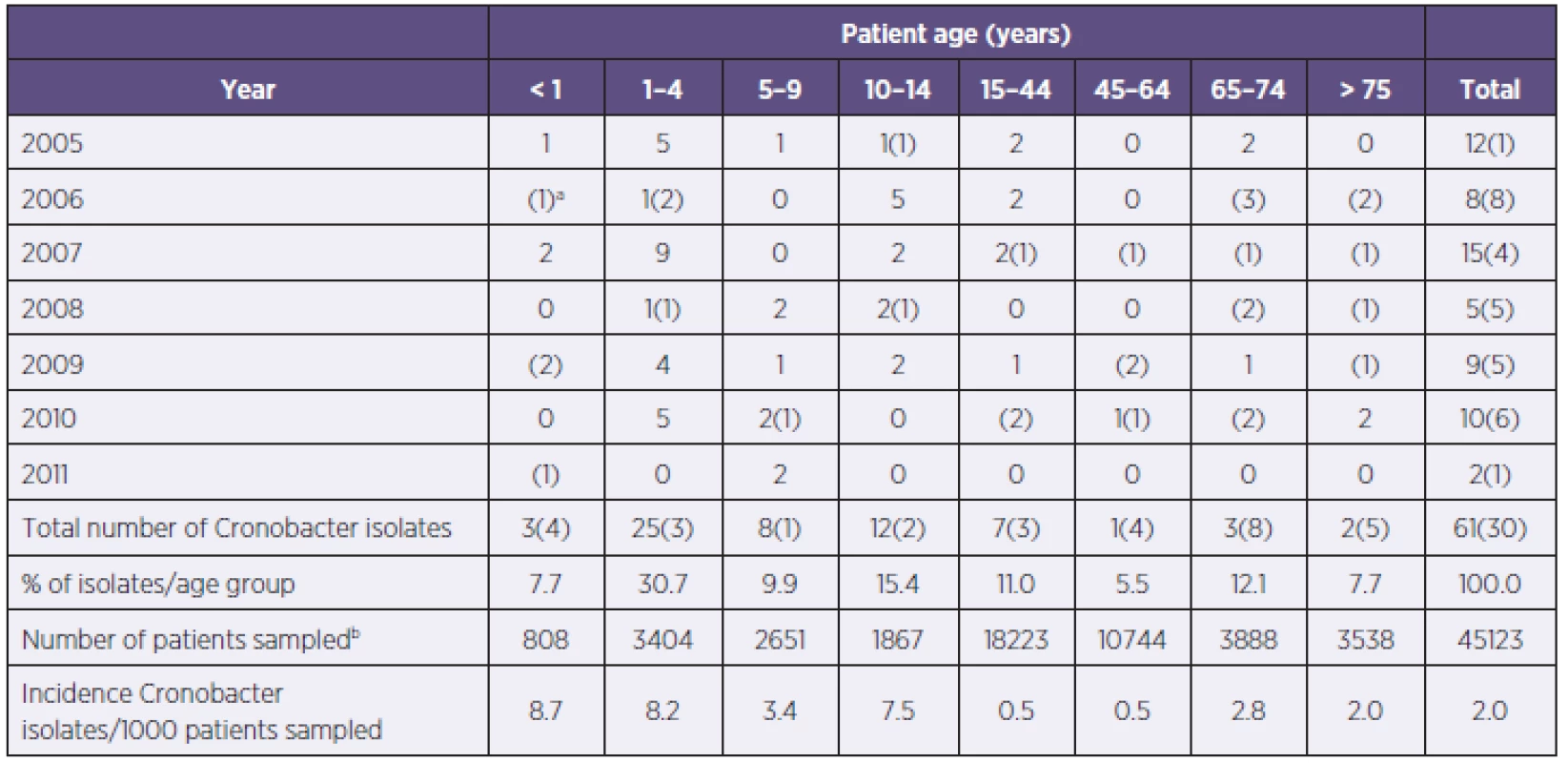
aNumbers in parenthesis indicate additional isolates from normally sterile sites, sampled according to the clinical presentation of the patient. bTotal number during period 2005–2011. aČísla v závorkách představují další izoláty z míst, která jsou normálně sterilní a z nichž byly vzorky odebrány na základě klinického stavu pacienta. bCelkový počet za období 2005–2011. 2. Numbers of clinical material examinations (2005–2011) and number of isolates positive for Cronobacter spp. for the period 2005-2011 Tabulka 2. Počty vyšetření klinického materiálu (2005–2011) a počty pozitivních izolátů Cronobacter spp. v období 2005–2011 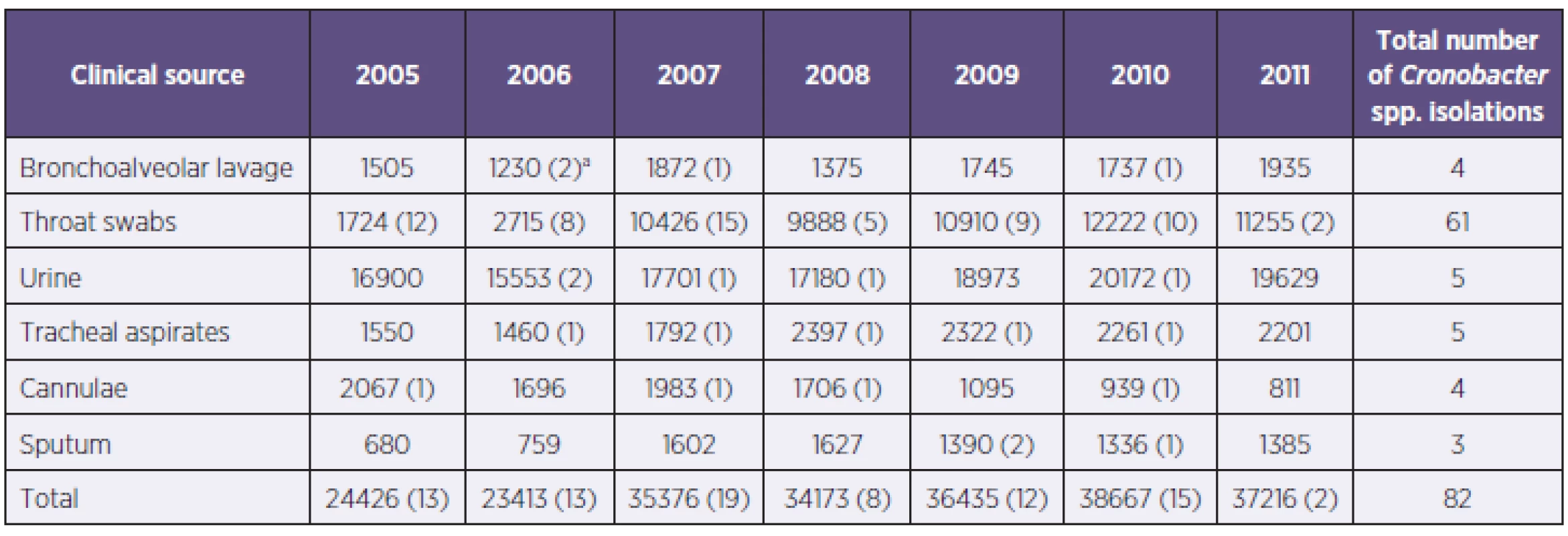
Clinical material from which the Cronobacter spp. was most frequently isolated. aNumber of Cronobacter spp. isolates Klinický materiál, z něhož byly kmeny Cronobacter spp. nejčastěji izolovány. aPočet izolátů Cronobacter spp. RESULTS
In the seven year period there had been a total of 61 Cronobacter spp. isolations from the general screening of patients, and an additional 30 isolates from normally sterile sites such as blood, which had been sampled as part of a clinical investigation. The strains were identified as Cronobacter spp. at the 99% confidence level. All strains were sensitive to therapeutic antibiotics. There was considerable yearly variation (2–15/y) in the number of Cronobacter isolations from screened patiens (see Table 1). There were nearly an equal number of Cronobacter spp. isolates from patients on the general wards (49.5%), as there were from intensive care units (45.1%), the remainder were from outpatients (4.4%) and from pathological investigations (1.1%). As shown in Table 1, Cronobacter spp. was isolated from all age groups. The highest incidences was in the age groups < 1 year (8.7/1000 patients) and 1–4 years (8.2/1000 patients) (see Table 1), and the majority of the strains were isolated in the age group below 14 years of age (63.7%). This corresponds with the majority of isolates being from the Department of Pediatrics (57), followed by Department of Neonatology (6), Department of Internal Medicine (5) and Department of Respiratory Medicine (5). Most of the (44%) Cronobacter spp. strains were from patients with immunodeficiency (i.e. acute lymphoblastic leukaemia, acute myeloblastic leukaemia, lymphoid leukaemia).
DISCUSSION
Surprisingly despite the raised awareness of Cronobacter spp. since 2002, there is no comparable data on its carriage by different age groups. The only age profiled data, known to the authors, is for 819 Cronobacter spp. bacteraemia cases reported for England and Wales between 1992 and 2007, as given by FAO/WHO [5]. In their report, the majority (91%) of bacteraemia cases were patients >15 years in age. The wide age range for the recovery of Cronobacter spp. is not surprising given the numerous possible routes of exposure. Cronobacter spp. is present in water, soil, households, flies, fresh and processed foods [8–11]. The severity of Cronobacter spp. infections in premature neonates is probably due to their immune-compromised status, whereas older children and adults may carry the organism as part of their normal flora. Routes of infections (ie. bacteraemia) in non-infants are uncertain, but are probably following a lowering of their immune status, and puncture wounds. Although the bacterium is isolated from many foods, no foodborne infections have not been reported to date. The data compiled here demonstrates that further research is needed to clarify the asymptomatic carriage of Cronobacter spp. This is of particular concern, as it is plausible that inadequate hygienic practices in the preparation of infant feed may lead to infant infection. The recent recognition of Cronobacter infections in the healthcare setting has shown that C. malonaticus is more associated with adult infections, as opposed to C. sakazakii which predominates infant infections [12]. Subsequently the strains collected over the 2005–2011 period from known age groups, are currently undergoing further microbiological analysis.
CONCLUSIONS
The majority of Cronobacter spp. isolates (n = 91) were from throat swabs (61), followed by urine (5), tracheal aspirates (5), bronchoalveolar lavage (4), cannulae (4), and sputum (3) samples. These had been taken for general screening purposes, and not specifically according to the patient clinical presentation. This is the first study which profiles the carriage of Cronobacter spp. according to patient age, based on seven years of clinical data from 2005–2011. It reveals a high recovery (63.7% strains, n = 91) of the organism from children, 1–14 years in age.
Acknowledgement: This project was supported by Research Support Foundation, Vaduz, grant project [801100021/39].
Do redakce došlo dne 29. 7. 2013.
Address for correspondence:
Stephen Forsythe
School of Science and Technology
Nottingham Trent University
Clifton Lane, Nottingham
NG11 8NS, UK
email: stephen.forsythe@ntu.ac.uk
Address for correspondence:
Ondřej Holý
Department of Preventive Medicine
Faculty of Medicine and Dentistry
Palacký University Olomouc
Olomouc
Czech Republic
email: holy.ondrej@seznam.cz
Sources
1. Hariri S, Joseph S, Forsythe SJ. Predominance of Cronobacter sakazakii ST4 clonal complex strains in Cronobacter neonatal meningitis infections in US 2011. Emerg Infect Dis, 2013;19 : 175–177.
2. Bowen AB, Braden CR. Invasive Enterobacter sakazakii disease in infants. Emerg Infect Dis, 2006;12 : 1185–1189.
3. Joseph S, Forsythe SJ. Insights into the emergent bacterial pathogen Cronobacter spp., generated by multilocus sequence typing and analysis. Front Microbiol, 2012;3 : 397.
4. Himelright I, Harris E, Lorch V. Enterobacter sakazakii infections associated with the use of powdered infant formula-Tennessee. 2001, JAMA, 2002;287 : 2204–2205.
5. FAO/WHO [Food and Agriculture Organization of the United Nations/World Health Organization], 2008. Enterobacter sakazakii (Cronobacter spp.) in powdered follow-up formulae. Microbiological Risk Assessment Series No. 15, Rome. 90 pp.
6. Abbott S. 37. Klebsiella, Enterobacter, Citrobacter, Serratia, Plesiomonas, and Other Enterobacteriaceae. In: Versalovic J, Carroll KC, Funke G, Jorgensen JH, et al. Manual of Clinical Microbiology. 10th edition, ASM Press; 639–657.
7. CLSI (Clinical and Laboratory Standards Institute): performance standards for antimicrobial susceptibility testing, 19th informational supplement, M100-S19. Clinical and Laboratory Standards Institute, Wayne (PA, USA), 2009.
8. Joseph S, Cetinkaya E, Drahovska H, Levican A, et al. Cronobacter condimenti, sp. nov., isolated from spiced meat and Cronobacter universalis sp. nov., a novel species designation for Cronobacter sp. genomospecies 1, recovered from a leg infection, water, and food ingredients. Int J Syst Evol Microbiol, 2012;62 : 1277–1283.
9. Friedemann M. Enterobacter sakazakii in food and beverages (other than infant formula and milk powder). Int J Food Microbiol, 2007;116 : 1–10.
10. Iversen C, Forsythe SJ. Isolation of Enterobacter sakazakii and other Enterobacteriaceae from powdered infant formula milk and related products. Food Microbiol, 2004;21 : 771–776.
11. Turcovský I, Kuniková K, Drahovská H, Kaclíková E. Biochemical and molecular characterization of Cronobacter spp. (formerly Enterobacter sakazakii) isolated from foods. Antonie van Leeuwenhoek, 2011;99 : 257–269.
12. Holý O, Forsythe SJ. Cronobacter species as emerging causes of healthcare-associated infection. J Hospt Infection [Epub ahead of print].
Labels
Hygiene and epidemiology Medical virology Clinical microbiology
Article was published inEpidemiology, Microbiology, Immunology

2014 Issue 1-
All articles in this issue
- Clinical and epidemiological characteristics of patients hospitalized with severe influenza in the season 2012–2013
- Bacteria of the Burkholderia cepacia complex: epidemiology and diagnosis of infection in patients with cystic fibrosis
- Genotyping results, laboratory diagnosis, and epidemiology of the mumps virus circulating in the Czech Republic in 2012
- Legionella infection – a neglected problem
- Evaluation of prophylactic precautions after exposure to biological materials
- Epidemiological investigation in five dental offices of the Clinic of Dentistry, Faculty of Medicine, Palacký University, Olomouc and of the Olomouc University Hospital
- Sequencing analysis of the antigens included in the four-component vaccine against serogroup B meningococcus in Czech isolates of Neisseria meningitidis from 2007–2013
- Influenza in seasons 2009-2013 in the Faculty Hospital Hradec Kralove, East Bohemia
- Epidemiological factors influencing the development of relapsing and severe Clostridium difficile infection
- Epidemiology of Cronobacter spp. isolates from patients admitted to the Olomouc University Hospital (Czech Republic)
- Epidemiology, Microbiology, Immunology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Legionella infection – a neglected problem
- Bacteria of the Burkholderia cepacia complex: epidemiology and diagnosis of infection in patients with cystic fibrosis
- Epidemiological investigation in five dental offices of the Clinic of Dentistry, Faculty of Medicine, Palacký University, Olomouc and of the Olomouc University Hospital
- Clinical and epidemiological characteristics of patients hospitalized with severe influenza in the season 2012–2013
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

