-
Medical journals
- Career
Burkitt lymphoma (BL): reclassification of 39 lymphomas diagnosed as BL or Burkitt-like lymphoma in the past based on immunohistochemistry and fluorescence in situ hybridization
Authors: R. Kodet 1; M. Mrhalová 1; E. Stejskalová 2; E. Kabíčková 2
Authors‘ workplace: Department of Pathology and Molecular Medicine, Charles University 1; nd Faculty of Medicine and Faculty Hospital Motol, Prague, Czech Republic 2; Department of Pediatric Hematology and Oncology, Charles University 2; nd Faculty of Medicine and Faculty Hospital Motol, Prague, Czech Republic 2
Published in: Čes.-slov. Patol., 47, 2011, No. 3, p. 106-114
Category: Original Article
Overview
Burkitt lymphoma (BL) is a well characterized entity. For atypical findings a term Burkitt-like lymphoma (B-LL) was applied in the past, but the interpretation of the morphological appearances was subjective and poorly reproducible. We used a combined approach (morphology using classical histological staining; immunohistochemistry – IHC; fluorescence in situ hybridization – FISH on interphase nuclei; cytogenetics) to perform a retrospective study on 39 patients diagnosed as BL and B-LL at our department in the years 1982 to 2002. By FISH we demonstrated t(8;14)(q24;q32) in 31 patients; in further two we found a break at 8q24, suggestive of a variant translocation. In three patients with the cytogenetic investigation available we confirmed the findings of FISH – two lymphomas had the t(8;14)(q24;q32), one had t(2;8)(p12;q24). IHC showed CD20, CD10, BCL-6, p53 expression, and Ki-67 antigen in >95 % of the tumor cell population in a majority of the patients. There was a group of 4 patients in whom the t(8;14)(q24;q32) or a break at 8q24 were not found (FISH). These cases were reclassified within the WHO defined grey zone subgroup of B-cell lymphoma unclassifiable with features intermediate between diffuse large cell lymphoma (DLBCL) and Burkitt lymphoma – I-DLBCL/BL. Two further cases were reclassified as DLBCL based on a combined IHC and FISH findings. A lymphoma of one of these patients had breaks at 3q27 (BCL6) and at 14q32 (IGH) suggestive of t(3;14)(q27;q32).
The overall survival estimate of 33 patients with the diagnosis of BL was 54 %. Most of deaths occurred within 6 months after the tumor diagnosis. The unfavorable clinical outcome appears to be associated with a strong expression of the p53 protein in the tumor cell population.
Individually utilized methods in the diagnosis of BL may lead to false diagnostic conclusions. A combined approach helps to establish a more reliable diagnosis of BL and to separate grey zone lymphomas I-DLBCL/BL and DLBCL with morphological mimics of BL to start adequate treatment. I-DLBCL/BL is a non-homogenous group of lymphomas necessitating further analysis in a prospective study.Keywords:
Burkitt lymphoma – diagnosis – immunohistochemistry – interphase FISH – cytogeneticsThe diagnosis of Burkitt lymphoma (BL) is based on well defined morphological criteria. The tumor is formed by relatively uniform medium sized tightly packed cells with nuclei of the same size or smaller than those seen in the adjacent macrophages, and with three to five small nucleoli. This simple definition has been used for a long time but it has some drawbacks as some BL may differ in details of the morphological appearance. In the past and even until recently, a group of lymphomas with some variations of the tumor cell morphology distinguishing these lesions from the prototypic BL were classified separately as Burkitt-like lymphomas (B-LL) or atypical BL (1,2). The concept of B-LL has been based on the notion that some cases resembling otherwise to typical BL have single large nucleoli, variously sized nuclei and generally a greater degree of polymorphism. In practical diagnostics these tumors were difficult to separate from BL on one side and sometimes from diffuse large B cell lymphomas (DLBCL) on the other (3,4). From this reason we, as many others, kept using the term B-LL in some cases which we felt that they did not fit entirely into the category of BL. In recent years the B-LL category was repeatedly questioned. First, if we go back to the early BL descriptions they do not stress a cellular uniformity as a definition feature. The descriptions based on a simple histological staining point out that there is a certain degree of polymorphism in the size and shape of the tumor cell nuclei (5). Second, it became apparent that both BL and B-LL have common features as detected by molecular profiling (6) including characteristic, though not entirely specific, rearrangement of the gene MYC. A fragment of lymphomas imposing as DLBCL morphologically may reveal features typical of BL if analyzed using gene expression profiling thus showing the importance of a complex diagnostic approach (7). In the current version of the WHO classification the B-LL are grouped with a general category of BL and its morphological variants (e.g. also plasmacytoid (8) or BL with granulomatous reaction (9)). However, a new group of lymphomas was defined with some preliminary cautions which separate a group of difficult to categorize lymphomas of varying features between classical BL and DLBCL, so called “B-cell lymphoma unclassifiable with features intermediate between diffuse large cell lymphoma and Burkitt lymphoma” (10). To shorten the long title we use a simplifying abbreviation I-DLBCL/BL in the following text.
From the current pediatric oncology practice the distinction and correct diagnosis of BL is important. Clinical partners consider the occurrence of BL in a patient as an acute oncological event necessitating an immediate therapeutic intervention as soon as the diagnosis is clarified.
Because of a specific therapeutic approach to patients with BL, the diagnosis of the tumor should be established with a certainty and as quickly as possible. In some cases this task may appear treacherously easy with the aid of standard hematoxylin and eosin stained sections but a precise final characterization always requires a support of immunohistochemistry (IHC), and, if possible, of other methods suitable to demonstrate characteristic chromosomal translocations. The translocations typically involve MYC oncogene at 8q24, and either the immunoreceptor gene coding for immunoglobulin heavy chain (IGH) at 14q32 or one of the genes coding for the immunoglobulin light chains (IGK and IGL at 2p12 and 22q11, respectively). Although these translocations are not exclusively present only in BL, their identification in the context with the results of histopathology and immunophenotyping is diagnostically helpful (11). Until recently, the chromosomal changes could be demonstrated by classical cytogenetics using metaphase chromosomes. This procedure requires sterile and fresh tissue samples, which are available in a small proportion of cases. Laboratory procedures using PCR to recognize the MYC/IGH (MYC/IGK, MYC/IGL) gene rearrangements are available but not routinely employed; the breakpoints are variable and widely separated so that so called long distance PCR should be used preferably (12,13). Long distance PCR requires high quality DNA isolates. Such a prerequisite is seldom achieved because patients with abdominal BL are frequently operated on because of the symptoms of an acute abdominal episode in outside hospitals, at nights or at weekends. Therefore, the material is rarely submitted fresh or frozen for the DNA/RNA isolation. Instead, the specimens are routinely formalin fixed. To circumvent these problems a mixture of fluorescence in situ hybridization (FISH) probes designed to cover the areas of most common breakpoints at 8q24 and 14q32 may be used on interphase nuclei (14). A complementary experiment utilizes probes visualizing a break at 8q24 to screen for potential variant translocations if the classical one is not found. Using both types of probes increases the likelihood of identification the IG/MYC translocations (15).
In this study we characterize a group of 39 patients with lymphomas diagnosed as BL or B-LL as defined in the past by a morphological evaluation only, and are reevaluated using a complex IHC investigation, and FISH on interphase nuclei. Cytogenetic analysis was performed in three of these patients.
MATERIALS AND METHODS
Patients and Tissue Processing
As a first step we retrieved a group 68 patients with the diagnosis of BL and B-LL made between 1982 and 2002 from the Morphologic Pediatric and Lymphoma Tumor Registry (Department of Pathology and Molecular Medicine). Thirty nine patients had paraffin blocks with a sufficient tissue available. On this subgroup a morphological review, IHC and FISH were performed as well as available clinical data on the patients’ survival were retrieved. The data on follow-up of the patients were evaluated using Kaplan-Meier survival estimate. Cases diagnosed at our department since 2003 to date will be analyzed separately with an aspect that the treatment approach has changed significantly so that the outcome of the disease has improved significantly in the past decade, and the clinical data analyzing contemporary results would be uncomparable with the results of the past (16,17).
Fluorescence in situ Hybridization (FISH)
FISH was performed on formalin fixed, paraffin embedded tissue sections. Directly labeled probes (Vysis) purchased from Abbott Molecular (Abbott Laboratories, Abbott Park, IL, USA) were used: a) LSI IGH (Spectrum Green) / MYC (Spectrum Orange), CEP8 (Spectrum Aqua, chromosome enumeration probe) Tri-color, Dual Fusion Translocation Probe was used as a key system for the study. b) To screen for variant translocations – t(2;8)(p12;q24) and t(8;22)(q24;q11), in the absence of t(8;14)(q24;q32), we utilized an indirect procedure consisting of a demonstration of the MYC locus break – LSI MYC Dual Color, Break Apart Rearrangement Probe. In case of failure to detect either the classical translocation or break at 8q24, we utilized other probes to characterize the tumors. c) LSI IGH Dual Color, Break Apart Rearrangement Probe to reveal breaks at 14q32; d) LSI BCL6 Dual Color, Break Apart Rearrangement Probe to detect breaks at 3q27; e) LSI IGH/BCL2 Dual Color, Dual Fusion Translocation Probe to make an attempt to identity the t(14;18)(q32;q21), characteristic of most follicular lymphomas and some DLBCL; f) LSI MYC Probe to detect the number of the MYC gene copies.
The tissue sections 3 μm thick were deparaffinized and dehydrated. After pretreatment (HCl, NaSCN, trypsin), post-fixation in 4% paraformaldehyde and denaturation of the DNA in 70% formamide were performed. The slides were dehydrated and hybridized overnight with a mixture of the denaturated probes. After washing (SSC, NP-40) the nuclei were counterstained with DAPI in antifade solution. A fluorescence microscope BX51 (Olympus) with appropriate filters was used for the evaluation of fluorescence signals.
Evaluation of FISH results. MYC/IGH, BCL2/IGH probes: translocation absent – two red (8q24, 18q21), two green (14q32) and two blue (CEP8) signals; translocation present – two yellow (co-hybridized red and green) signals of the translocated loci and one red (MYC, BLC2) and green (IGH) signal from the uninvolved loci and two blue signals of chromosome 8. MYC, BCL6, IGH probes - marking the breakpoints from centromeric and telomeric sides by a different fluorescence dye; break absent - two yellow signals, break present - one red and green signal from the broken locus and one yellow signal from the uninvolved locus. MYC was considered amplified if more than five copies of the locus per a nucleus were present.
Cytogenetics
Fresh tissue containing tumor cells was collected from three patients out of the whole group (two patients – infiltrated bone marrow; one case – ascitic cells). After half an hour of culture in RPMI 1640 medium (Gibco, USA) with fetal calf serum (FCS) and Colcemid (Gibco, USA) the samples were harvested (in case of ascites Colcemid was added without FCS). After incubation, standard cytogenetic harvesting procedures followed. After methanol / acetic acid fixation (Merck, ČR) the cold suspension was dropped onto slides. Following a period of ageing the slides were trypsin-Giemsa stained (Difco, USA) and analyzed under the microscope (Olympus BX40). Ten to twenty cells were counted and three to five were fully karyotyped. An automated system for chromosome analysis (MetaSystems) was used.
Immunohistochemistry (IHC)
Formalin fixed and paraffin embedded tissue sections were used. The primary antibodies included CD20 (clone L26), BCL-6 (clone PG-B6p), p53 (clone DO-7), CD3 (clone F7.2.38), Bcl-2 (clone 124), Epstein-Barr virus latent membrane protein (EBV LMP, clone CS.1-4), Ki-67 (clone MIB-1) (Dako, Denmark); CD10 (clone 56C6), myc (clone 9E11) (Novocastra Laboratories Ltd., UK). LSAB detection kit with diaminobenzidine (Dako, Denmark) or Super Sensitive detection kit with a biotinylated secondary antibody and a complex of streptavidine/alkaline phosphatase with Fast Red (BioGenex, USA) were used. The results of IHC were interpreted in the following way (the intensity and an estimate of a percentage of positive cells were evaluated): the intensity of the IHC reaction was evaluated as weak – light brown color (1+), as a strong positivity – dark brown precipitate (3+), and a moderate positivity defined per exclusion of the two previous possibilities (2+).
The positive result was considered in a case of any type of the intensity of the IHC reaction provided the percentage of positive tumor cells exceeded 60 %. In case of Ki-67 protein we evaluated the cases individually.
RESULTS
Morphology
A review of the original hematoxylin and eosin / Giemsa diagnoses (and a restricted IHC to CD20 and CD3 mostly) revealed that the group is morphologically relatively homogenous with some variations of the tumor cell size and shape in eight cases which were originally classified as B-LL. Using a combined newly applied and broadened immunohistochemical panel supplemented by FISH analysis the patients were separated into three groups. The term B-LL was not used any longer in keeping with the last WHO classification (10), and some cases called B-LL previously fell into the category of BL. As a result of this study the diagnosis of BL was established in 33 patients (four of them were originally called B-LL). According to the contemporary WHO classification four cases fitted to the grey zone category of I-DLBCL/BL and two further cases were reclassified as DLBCL. A detailed description will explain the reasoning for the new categorization.
On hematoxylin and eosin / Giemsa staining 31 lymphomas were originally classified as BL (Fig. 1a). Eight cases were previously diagnosed as B-LL: the nuclei were irregular in size and shape the nucleoli were single, large, and centrally placed in the nuclei (Fig. 1b). In one of the latter cases the diagnosis of B-LL was preferred, and the patient was treated accordingly, but DLBCL was also considered in the differential diagnosis.
Figure 1. Histology findings. BL: the tumor cells grow in sheets, they are of intermediate size with round nuclei; there are several nucleoli; macrophages are scattered to form a starry sky phenomenon; hematoxylin and eosin, x600 (A). Original diagnosis of B-LL: the tumor cells vary in size, the nucleoli are single, large and centrally placed; hematoxylin and eosin, x600 (B). 
Reclassification of the cases using a combined analysis
Burkitt Lymphoma (BL)FISH. In 31 patients, using the IGH/MYC/CEP8 probe mixture, we found a signal pattern typical of t(8;14)(q24;q32) (Fig. 2a). In two patients the signals did not co-hybridize but we observed three signals for the 8q24 locus. Such a finding was suggestive of a variant translocation. Application of MYC break apart probe for the 8q24 region revealed its breakage supporting the concept of a variant translocation in these patients (Fig. 2b). All these cases (n = 33) were finally categorized as BL (originally BL n = 29 and B-LL n = 4) (Table 1).
Figure 2. FISH on interphase nuclei and cytogenetic investigation in the group of BL. The reciprocal translocation t(8;14)(q24;q32) involving MYC and IGH loci is visualized as two co-hybridized (red-yellow-green) signals; the uninvolved alleles 8q24 and 14q32 are seen as red and green signals respectively; the CEP8 shows two blue signals of the chromosome 8; LSI IGH/MYC/CEP8 Tri-color, Dual Fusion Translocation Probe (A). The LSI MYC Dual Color, Break Apart Rearrangement Probe shows one unbroken allele of the 8q24 locus (yellow signal); separate red and green signals indicate the break at the 8q24 locus of the allelic chromosome, suggesting a variant translocation involving the MYC gene (B). Cytogenetic investigation demonstrates the t(8;14)(q24;q32); trypsin-Giemsa stain (C). 
1. The results of FISH analysis of cases with the final diagnosis of BL, B-cell lymphoma unclassifiable with features intermediate between DLBCL and BL (I-DLBCL/BL), and DLBCL 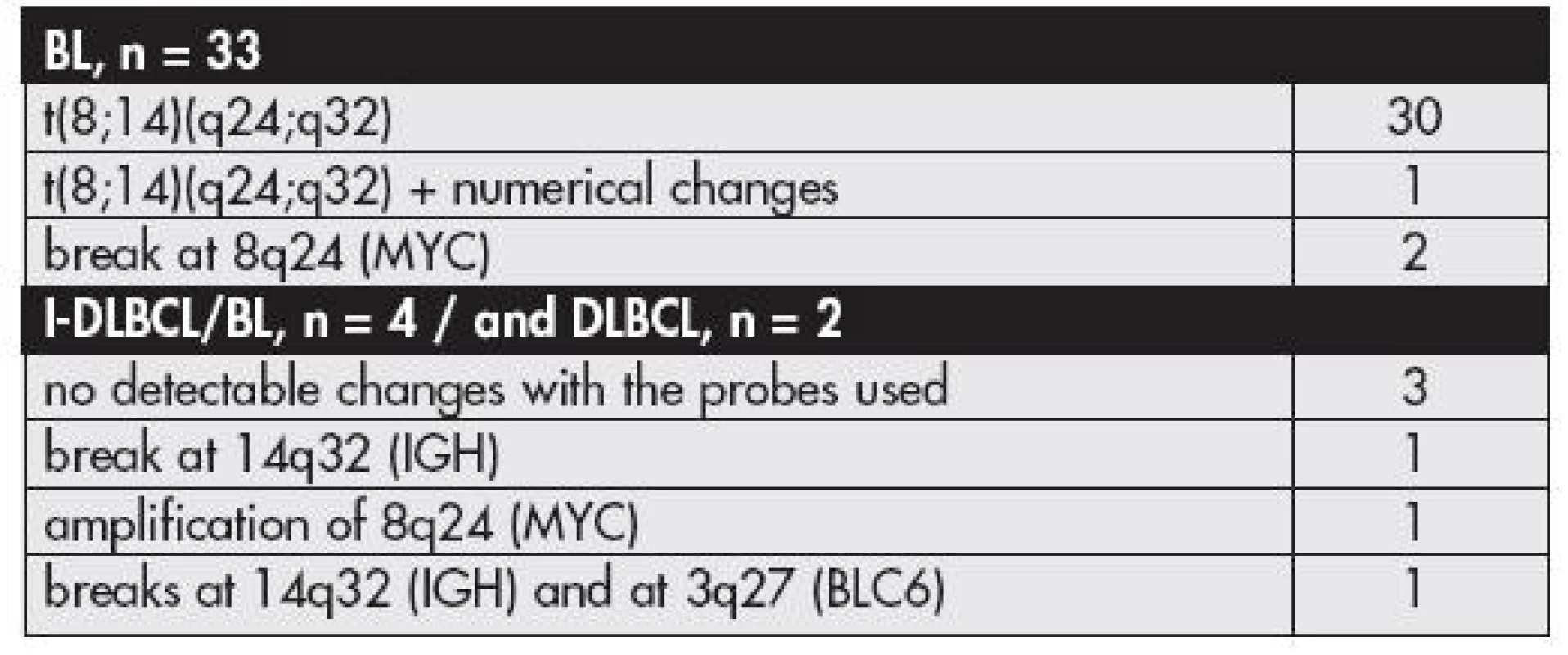
In one patient of the group we found also numerical changes in the tumor cells: three co-hybridized signals of the translocated alleles, three CEP8 signals, and three signals for the uninvolved alleles; the case was morphologically classified as BL with no detected differences from the classical pattern of morphology.
Cytogenetics. A reproducible result was obtained from three patients with a fresh tissue available. In two we found the t(8;14) (q24;q32). In one of them the translocation was the only pathological finding (Fig. 2c). In another we detected two clones, each characterized by the t(8;14), but these two clones differed in other cytogenetic aberrations:
Clone I: 46,XY,del(6)(q21),t(8;14)(q24;q32),dupl(13)(q?32-34)
Clone II: 46,XY,t(3;5)(p21;q31),t(4;7)(q31;q36),t(8;14)(q24;q32)
In the third patient with the tumor of the abdominal cavity ascitic fluid revealed tumor cells characterized by a variant translocation t(8;22)(q24;q11). This finding was in agreement with the break at 8q24 and with the absence of the t(8;14) found by interphase FISH in this case.
IHC. Summarized results of immunohistochemical investigations are given in Table 2. All 33 cases with the original diagnosis of BL (n=29) and B-LL (n=4) were characterized by a strong CD20 positivity. CD10 molecule was expressed in 31 cases, being most prominent in 15 (Fig. 3a). BCL-6 protein was strongly expressed in more than 60 % of the tumor cell population in two thirds of the tumors in this group (22 cases) – a positive result; scattered positive cells were present in further 9 cases but such a result was below the limit of a positivity set for this study (in some instances we were unable to separate residual non neoplastic BCL-6 positive cells from the tumor cells) (Fig. 3b,c). A combined CD10 and BCL-6 protein tumor cell positivity was found in 21 cases. A strong cytoplasmatic expression of the myc protein was found in 23 cases (Fig. 4a). In seven cases the reaction was less pronounced and the intensity was comparable with that observed in stromal cells such as vascular endothelia, fibroblasts, and in some epithelial cells of the tissues infiltrated by the lymphoma. In three patients the myc protein was negative due to technical problems. Protein p53 was strongly positive in most of the tumor cell nuclei in 19 cases. Latent membrane protein (LMP) of the Epstein-Barr virus (EBV) was detected in one case of BL involving the jaw. A Vietnamese origin of this patient, LMP positivity and the primary tumor of the bone were suggestive of the endemic type of BL. Consistently negative results were obtained for CD3 and Bcl-2 protein in all patients of this group. Ki-67 antigen was positive in 27 cases. Virtually all tumor cells (>95 %) were positive, in 21 lymphomas strongly, in 6 the staining was of a moderate intensity (Fig. 4b). Of the remaining six cases the investigation was limited by a technical failure in five, and in one by a presence of massive apoptosis / tumor cell necrosis, though viable tumor cells were positive (the percentage of tumor cell positivity was, however, difficult to estimate).
2. The IHC results of the group finally classified as BL, n= 33 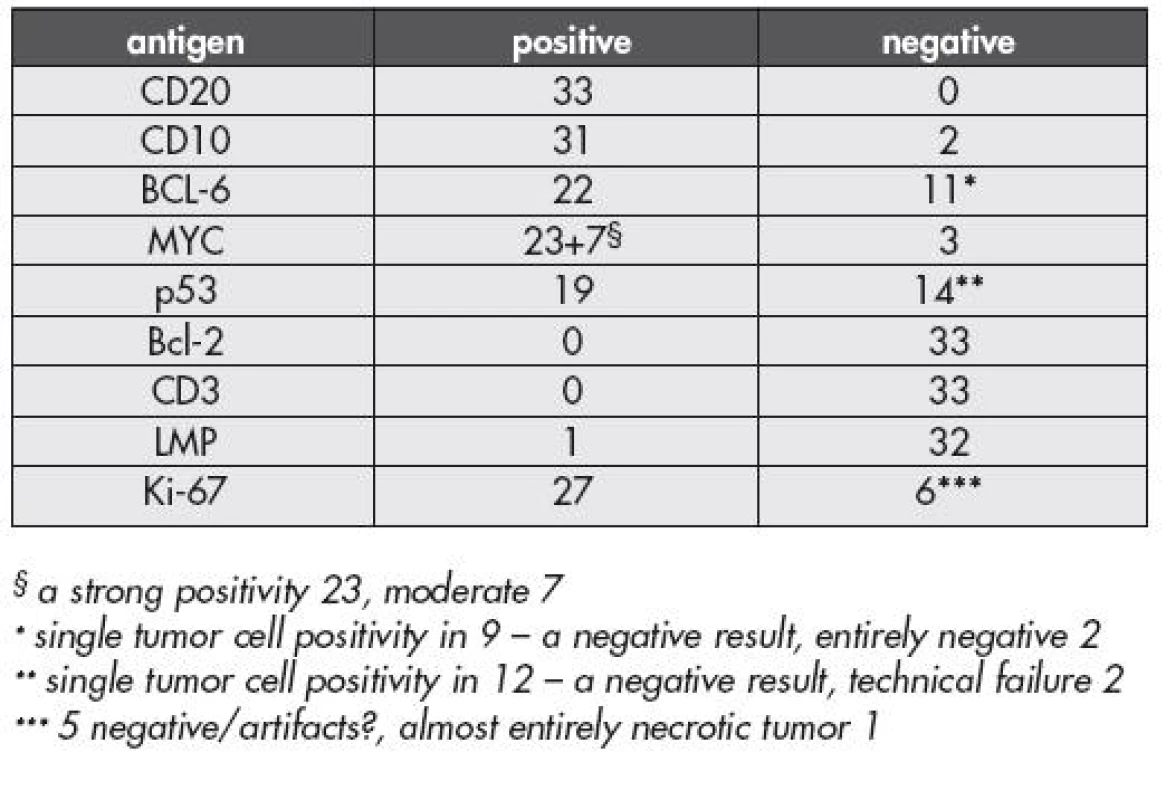
Figure 3. IHC results in the group of BL. CD10 positivity of the tumor cell membranes, x400 (A). A strong BCL-6 protein positivity in a majority of the tumor cell nuclei (B). A moderate BCL-6 protein expression of scattered tumor cell nuclei (C). Super Sensitive detection kit: biotinylated secondary antibody, complex of streptavidine / alkaline phosphatase, Fast Red, x400. 
Figure 4. IHC results in a group of BL. A strong and overall myc protein expression detected in the cytoplasm of the tumor cells; some of the stromal cells are also moderately positive; LSAB detection kit, diaminobenzidine, x400 (A). Ki-67 antigen reveals uniform and strong tumor cell positivity (B). Super Sensitive detection kit: biotinylated secondary antibody, complex of streptavidine / alkaline phosphatase, Fast Red, 400x. 
When the results of IHC in BL were compared with those obtained in the four cases classified as B-LL in this group we did not find any consistent data to separate the cases on the basis of the IHC typing. The reason to call these cases B-LL in the past was based on the tumor cell cytology only and such a diagnosis was consistent with recommendations of the previous edition of the WHO (1). Curiously but not entirely surprisingly, the p53 protein was strongly expressed in a majority of the tumor cells in all four cases diagnosed previously as B-LL.
Clinico-pathological correlation. There were 28 males aged at the time of diagnosis 1 to 18 years (median 9 years) and 5 females aged 4 to 15 years (median 8 years). The male-to-female ratio was 5.6. The primary tumor was localized in the abdominal cavity or in the retroperitoneum in nearly two thirds of the patients (Graph 1). In nearly half of these cases (43 %) we were unable to specify the primary abdominal / retroperitoneal site due to a large tumor mass involving most of the abdominal cavity (mesentery, omentum). The tumors were clinically characterized by a rapid progression, but in most patients they also responded well to the initial chemotherapy. Of the patients with the follow-up available, 11 died of the disease progression and three of a complication. One patient died few hours after the admission. The overall survival estimate of patients with BL was 54 % (Graph 2). It was apparent that all deaths occurred within eight months after the diagnosis, and those who survived the first six months or more had a good chance of an overall survival.
Graph 1. Primary tumor site of the patients enrolled in the BL group (n=33) 
Graph 2. Kaplan-Meier survival estimate for the patients reclassified as BL 
The analysis of the survival versus the results of IHC revealed that the p53 protein positivity only may have some impact. Of 16 patients with follow-up data available and with a strong overall tumor cell p53 protein expression 9 died of the disease progression. Of further nine patients with p53 protein expressed in scattered individual tumor cells (up to 10 % of the tumor cell population) none died of the disease progression (Logrank test, P=0.0068; Chi square 7.328). Two patients were lost to follow-up. The patient who died after the admission and three patients who died of complications were excluded from the analysis. In two patients the expression of the p53 protein was not evaluated due to unyielding results (one died of the disease progression in 4.5 months, one is alive and well 149 months).
B-cell lymphoma unclassifiable with features intermediate between DLBCL and BL (I-DLBCL/BL)
Four patients fell “de novo” into the category of I-DLBCL/BL. Two had the morphology with a monomorphic cellular composition thus fulfilling former criteria of what we called BL, two others were initially categorized as B-LL.
FISH. Fluorescence in situ hybridization revealed interpretable results in all four patients. The t(8;14) or a break at 8q24 were not found (Tables 1,3). In two of these cases we did not observe any abnormality with the probes utilized. In one patient, a 10-year old boy with a tumor in the orbit originally classified as a “clear cut” BL, we found a break at 14q32 which implied that this region was a potential partner for a translocation but we were unable to specify any translocation partner using FISH. One patient had a tumor characterized by two signals of CEP8 and by amplification of the MYC locus (as found by LSI MYC/IGH/CEP8 probe and confirmed by LSI MYC probe) (Fig. 5a). The patient was an 18-year old boy with the tumor in the ileocecal region.
3. Data on patients with lymphomas reclassified as I-DLBCL/BL and DLBCL 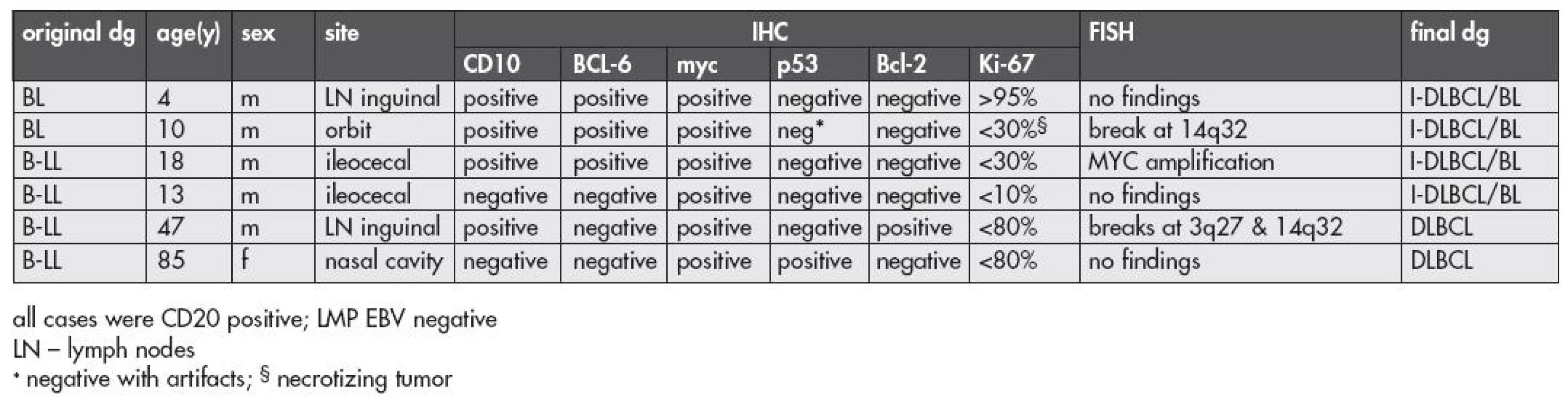
Figure 5. Interphase FISH results as exemplified by two cases excluded from the BL group. (A) An 18-year old boy, ileocecal tumor; the tumor cell nucleus shows numerous red signals of the 8q24 with the MYC gene, indicating its amplification; the red spot on the left is a cluster of the locus copies in another cell under focus. LSI MYC Probe. 5b-d A 47-year old man, peripheral lymphadenopathy. No co-hybridized signals are seen using the LSI IGH/MYC/CEP8 Tri-color, Dual fusion Translocation Probe. Three green signals indicate a probable break at 14q32 (IGH locus) (B). The LSI IGH Dual Color, Break Apart Rearrangement Probe shows one normal allele (yellow additive fluorescence) and one allele split (separated red and green signals), thus confirming the break at 14q32 (C). The LSI BCL6 Dual Color, Break Apart Rearrangement Probe shows one normal allele (yellow) and two split signals red and green indicating a break of the BCL6 gene (D). 
IHC. The results of immunohistochemical investigations are summarized in Table 3. CD20 and CD10 were positive in all 4 cases, BCL-6 protein revealed a positive result (3+ in more than 60 % of the tumor cells) in 3 cases. Interestingly, myc protein expression was positive in all four tumors of this group but the intensity of the reaction was generally weaker than in the group of BL. Such a finding was, however, a semiquantitative estimate and difficult to objectify. A marked difference was found for the p53 protein, which was moderately positive in one case only in contrast with its frequent positivity in the BL group. The proliferation marker, Ki-67 antigen, varied in this group of patients and in only one patient the tumor cell positivity achieved nearly 100% percentage as in BL. The estimate of positive cells in the remaining cases varied from 30 % to 80 % of the tumor cell population.
Clinico-pathological data, a final morphology correlation. The primary tumor localization, the gender and age of the patients are shown in Table 3. The morphology review of this subgroup revealed that the findings were heterogeneous both morphologically and with a combined IHC an FISH findings. The two cases with lymphomas originally diagnosed as a typical BL fit to the category of I-DLBCL/BL due to lack of evidence of the BL characteristic translocations. Undoubtedly, these lymphomas were of high grade, with CD10 and BCL-6 protein positivity, and Bcl-2 negativity. One of them, a 4-year old boy, had also a high proliferation rate as estimated by Ki-67. Two cases with a lymphoma in the ileocecal region differ from BL in both IHC and FISH findings. IHC had the profile showing a low proliferation fraction as compared to typical BL in both, and in one these cases CD10 was negative. FISH in one revealed MYC locus amplified, and in the other we did not disclose any changes with the probes used in this study. From this reason these cases also were also reclassified as I-DLBCL/BL.
Diffuse large B cell lymphoma (DLBCL)
FISH. In one patient originally diagnosed with B-LL, a 47-year old man with inguinal lymphadenopathy, the investigation using the IGH/MYC/CEP8 probe revealed three signals for 14q32 region thus suggesting a breakpoint at the locus (Fig. 5b). Subsequently, we applied the translocation probe IGH/BCL2 in an attempt to identify a transformed follicular lymphoma but, again, we found three signals for the IGH locus, and the t(14;18) was not disclosed. The finding of a break at 14q32 was confirmed by using a break apart probe for the IGH locus (Fig. 5c). Finally, we were able to find a break at 3q27 / BCL6 (Fig. 5d). Such findings were highly suggestive of t(3;14). Immunohistochemistry revealed a following profile of this tumor: CD10 positive, BCL-6 negative, Bcl-2 positive, Ki-67 protein below 80 % – features different from a typical BL profile. This result further supported a modification of the original diagnosis of B-LL to the final diagnosis of DLBCL.
The last patient was an 85-year old woman, with a B lymphoma of the nasal cavity classified originally as B-LL. FISH did not reveal any chromosomal changes, and the IHC result - CD10 and BCL-6 negative (BCL-6 positive in a small fraction of the tumor cells), and a relatively low proliferation fraction did not fit to the group of BL. This tumor was also reclassified as DLBCL.
DISCUSSION
The morphological definition of Burkitt lymphoma is concise and generally accepted (10). However, increasing experience led us to be more cautious with the diagnosis because some lymphoma categories may impose as BL morphologically but they lack either some of the immunophenotypical markers or defining chromosomal / molecular changes or both. This applies for lymphomas affecting children and even more so for lymphomas in adults. A group of experienced hematopathologists reached a poor concordance (55 %) in interpreting the morphology findings when considering diagnosis of BL and DLBCL with features of BL (18). The definition of B-LL used in the past was relatively vague, and due to this fact identification of B-LL was poorly reproducible (19). Moreover, using various names such as atypical Burkitt lymphoma or small non-cleaved non-Burkitt lymphoma was confusing (20).
Is has been known for a long period of time that there is a morphological overlap of BL with a group of diffuse large B cell lymphomas (DLBCL). However, DLBCL are tumors of a different category at the molecular level as well as with different clinicopathological characteristics, and, importantly, different treatment strategy. They should be separated form BL. Prototypic cases of BL and DLBCL are relatively easy to recognize, but tumors with overlapping morphology and sometimes with other characteristics (a subgroup of DLBLC may reveal MYC gene rearrangements) pose difficulties even nowadays. They represent a small fraction of patients but with new therapy modalities the exact classification may become increasingly more important for each patient.
The IHC investigation on paraffin embedded tissue sections reveals that BL, and in our study also lymphomas designed in the original description as B-LL, have a common profile in most cases which is helpful in the diagnostic evaluation. Beside a strong CD20 positivity, markers of germinal center B cells, such as CD10 and BCL-6 protein were expressed in a majority of cases, whereas Bcl-2 protein was consistently negative in all but one case. The latter case was, however, reclassified as DLBCL considering not only the Bcl-2 positivity but also findings of breaks of chromosomal loci 3q27 and 14q32 suggestive of t(3;14), a finding typical in a proportion of DLBCL.
A high proliferative rate of BL is reflected by an extremely strong and uniform positivity of the Ki-67 antigen (21). The tumors we reclassified as grey zone cases I-DLBCL/BL, and DLBCL were also positive with the Ki-67 antigen but the percentage of the tumor cells was variable and the positivity did not exceed the estimate of 80 %, except for one of these cases. Thus, though not an absolute discrimination marker, the Ki-67 antigen is utilized as a diagnostic aid in BL and its diagnostic mimics as was also recommended more than ten years ago by the WHO hematopoietic and lymphoid tissues classification Committee, and by other studies (22,23).
In this study we tried an antibody identifying the myc protein (clone 9E11) to evaluate its expression at the morphological level. Using this antibody the positivity was cytoplasmatic only. The protein was present in 30 out of 33 cases of the BL group, and in all 6 cases reclassified as I-DLBCL/BL and DLBCL. Though it seemed that the positivity of the myc protein in the group separated from the BL was generally weaker the finding is not consistent enough to be utilized in discriminating these lymphomas. Quantitative mRNA study may be a possible approach to answer this question as the mRNA was already found to be elevated in cases of non-immunoreceptor gene/MYC translocations (24). The transcription of the MYC gene is generally greater in BL, being activated by the translocations, but this phenomenon is not present in all cases; moreover, some reactive B cells express the same level of the myc protein as the tumor (25). There seems that using a different but not yet fully available antibody against the myc protein (monoclonal rabbit antihuman c-myc antibody no. 1472-1; Epitomics, Inc, Burlingame, CA) may be more useful as most BL and other lymphomas with MYC gene translocations show nuclear or a combined nuclear and cytoplasmatic positivity whereas lymphomas without MYC gene translocations show cytoplasmatic positivity only (26). The mechanisms of the MYC gene activation contributing to the tumor pathogenesis are complex and they are extensively reviewed in several papers (11,25). It has been shown that miRNAs (namely hsa-let-7c and hsa-mir-34b) have a significant role in the regulation of the gene MYC and its transcription (27).
The p53 protein was strongly expressed in an overwhelming majority of the tumor cell nuclei in 19 patients with diagnosis of BL. This phenomenon reflects an involvement of the gene TP53 in the tumor pathogenesis. Approximately one third of BL were shown to have the TP53 gene mutated (28,29); the mutation is considered a late event in the tumor development (30). A strong expression of the p53 as detected by IHC was suggestive of an unfavorable clinical behavior in some studies (31,32) which is also supported by our results.
Though the morphological findings and the IHC profile are diagnostically valuable they are not entirely specific of BL. According to our experience with the analyzed group of patients and in newer cases diagnosed from 2003 to day (data not shown) FISH identifying specific changes at the chromosomal / gene level should be employed whenever possible though it is clear that a majority of cases may be successfully diagnosed with the aid of IHC only (33). However, FISH has been proven to be an efficient tool in diagnosis of tumor specific translocations in various lymphomas, such as mantle cell lymphomas (34,35) and follicular lymphomas (36), and may be performed using archival paraffin embedded tissues. In BL there were several successful attempts to introduce diagnostic interphase FISH in the past, reviewed by Siebert et al. (14). The latter group used a two-color probe utilizing identification of a breakpoint at 8q24 with three red signals (one of the non-translocated allele, two of the split allele with one of the signals co-localized with a probe marking 14q32). We preferred using commercially available probes designed to reveal the reciprocal translocation by identification of two co-localized signals. Application of interphase fluorescence in situ hybridization revealed a characteristic translocation t(8;14)(q24;q32) with a fusion of the MYC gene with the IGH locus in 31 patients; in further 2 cases we found a break of the 8q24 locus suggesting one of the variant translocations known in BL. In three patients with BL the FISH findings were complemented by a cytogenetic study. In two cases with t(8;14)(q24;q32) detected by interphase FISH the cytogenetic findings concurred with the result. The third case without the t(8;14) but with a break at 8q24 identified by FISH was cytogenetically specified to have a variant translocation t(8;22)(q24;q11).
In six patients of the original group of 39 cases (15 %) the diagnosis of BL and B-LL was reevaluated, based on the reviewed morphology and a more complex IHC than it was performed at the time of the tumor diagnosis, and, notably, on the absence of the MYC oncogene rearrangement by translocations. In this group we found pathology of the MYC locus as detected by the interphase FISH in one case only. The MYC gene was amplified, a finding observed in some B cell lymphomas, including DLBCL (37). Because of the morphology indistinguishable from the BL and IHC characteristics these cases fit into a relatively broad category of I-DLBCL/BL. Two of these patients had the primary tumor localized at the terminal ileum, a site typical of sporadic BL in young individuals, which may, in the light of morphology indistinguishable from BL, lead to an incorrect diagnosis if based on an insufficient back up of IHC and FISH investigations. It seems, therefore, that a generally held view considering most high grade lymphomas involving the ileocecal region in young patients to be BL is not entirely correct. Some cases may represent other subtypes of lymphomas, such as DLBCL or grade 3 follicular lymphomas, as well as they may fit into a category now classified as I-DLBCL/BL (38).
Two further patients of the group separated from the BL and B-LL were reclassified as DLBCL with the aid of IHC and FISH findings compatible with the diagnosis of DLBCL (39).
The relatively high number of reclassified cases lead us to employ a complex IHC, FISH and cytogenetic study (if there is a fresh material available) in each case to be able to classify the tumor with a greater certainty. A panel of antibodies including anti Ki-67, CD10 and Bcl-2 in combination with FISH analysis of MYC as well as BCL2 and BCL6 breakpoints represents a minimum panel for the diagnosis of BL in adults (18). It has to be stressed, however, that none of the methods bring results entirely specific of BL. The morphology mimicking BL may not be accompanied by the BL characteristic chromosomal changes as we observed in this study. As opposed to that, translocations involved in the pathogenesis of BL may be observed in other types of lymphomas such as DLBCL (23,37), follicular lymphoma (40), chronic B lymphocytic leukemia (41), mantle cell lymphoma (42), lymphoblastic leukemias (43), and multiple myeloma (44). A comprehensive study by Au at al. showed also some additional hematological entities with secondary 8q24 changes, such as postransplant lymphoproliferative disease and other lymphomas than the listed above (2).
In most of these lymphomas the translocations or other chromosomal changes involving the MYC gene are considered secondary. In one case of childhood BL with complex genetic changes as defined by cytogenetics in this study we found a typical t(8;14) translocation (as also seen in FISH) but also two other chromosomal translocations beside other chromosomal changes. We were unable to analyze them closely. It is probable, however, that they represent complex chromosomal changes as a parallel to some DLBCL with double or triple translocations affecting MYC gene, BCL2 gene and / or BCL6 gene (3,37). In fact, BL are characterized either by simple chromosomal changes with typical sites of MYC translocations but in many cases a complex karyotype reveals that a plentitude of other genetic changes, usually non-repetitive, exists. Curiously, there were more frequent in BL with atypical morphological features, formerly B-LL (2). The reported child with complex changes of his tumor karyotype described in this study died of the disease progression but it is difficult to attribute the aggressive clinical course to the complex genetic changes in an individual case. However, it is a generally held view that patients with lymphomas such as DLBCL, follicular lymphomas and mantle cell lymphomas with double translocations – e.g. BCL2 and MYC gene or other changes at 8q24 fare poorly (2,6). Furthermore, it appears that patients with BL having simple karyotypic changes and BCL-6 protein expression have a more favorable outcome than BL with complex karyotype findings and BCL-6 protein negativity (45).
From the observations reported in this study as well as from many previous reports it is clear that there is a grey zone between BL and DLBCL and that there is a need of a complex diagnostic approach to separate such cases, including a carefully performed morphological evaluation. Definitions may be modified in future regarding that primary translocational deregulation of MYC gene may not be the only possible pathogenetic moment in BL and the molecular switch may be lightened by miRNAs or by other deregulation pathways (27).
In the group of 33 cases classified in this study as BL the overall survival estimate was 54 %. It has to be stressed, however, that about 70 % of patients of the analyzed group were diagnosed and treated prior to the introduction of newer therapy regimens and prior to the peripheral blood stem cell transplantation era. The data on a treatment efficacy of more recently diagnosed patients with BL seem more promising (46, 47). At present, there is no reason to separate cases with morphological variances within the group of BL as the patients with these tumors share a similar clinicopathological setting, response to the treatment and the general outcome, and differ with a higher risk of early death from DLBCL (4,48).
Genetic features involving the MYC gene and immunophenotypic features are similar in BL and B-LL and unlike in most DLBCLs. This is a reason why we consider BL and B-LL with the same chromosomal aberrations as morphological variants of one category of high grade B cell lymphomas. In cases in which we did not observe genetic changes involving the MYC gene translocation we were more cautious and classified them as I-DLBCL/BL or as DLBCL. Because current non-Hodgkin’s lymphoma therapy is tailored to specific histological subtypes, a correct diagnosis is essential.
ACKNOWLEDGEMENT
Supported by a grant of the Research Project of the Ministry of Health of the Czech Republic MZ0FNM2005/6704 and by the Research Project of The Ministry of Education, Youth and Sports of the Czech Republic MSM0021620813.
Correspondence address:
Prof. Roman Kodet, MD, Ph.D.
Department of Pathology and Molecular Medicine
Charles University, 2nd Faculty of Medicine and Faculty Hospital in Motol
V Uvalu 84, 150 06 Prague 5, Czech Republic
tel.: +420 224 435 600, fax: +420 224 435 620
e-mail: roman.kodet@lfmotol.cuni.cz
Sources
1. Jaffe ES, Harris NL, Stein H, Vardiman JW. World Health Organization classification of tumours (1st edn). IARC Press: Lyon; 2001 : 181–184.
2. Au WY, Horsman DE, Gascoyne RD, et al. The spectrum of lymphoma with 8q24 abberations: Clinical, pathological and cytogenetic study of 87 consecutive cases. Leuk Lymphoma 2004; 45(3): 519–528.
3. Macpherson N, Lesack D, Klasa R, et al. Small noncleaved, non-Burkitt’s (Burkit-Like) lymphoma: cytogenetics predict outcome and reflect clinical presentation. J Clin Oncol 1999; 17(5): 1558–1567.
4. Braziel RM, Arber DA, Slovak ML, et al. The Burkitt-like lymphomas: a Southwest Oncology Group study delineating phenotypic, genotypic, and clinical features. Blood 2001; 97(12): 3713–3720.
5. Ziegler JL, Wright DH, Kyalwazi SK. Differential diagnosis of Burkitt’s lymphoma of the face and jaws. Cancer 1971; 27(3): 503–514.
6. Hummel M, Bentink S, Berger H, et al. A biologic definition of Burkitt’s lymphoma from transcriptional and genomic profiling. N Engl J Med 2006; 354(23): 2419–2430.
7. Dave SS, Fu K, Wright GW, et al. Molecular diagnosis of Burkitt’s lymphoma. N Engl J Med 2006; 354(23): 2431–2442.
8. Guedez L, Martinez A, Zhao S, et al. Tissue inhibitor of metalloproteinase 1 (TIMP-1) promotes plasmablastic differentiation of a Burkitt lymphoma cell line: implications in the pathogenesis of plasmacytic/plasmablastic tumors. Blood 2005; 105(4): 1660–1668.
9. Janegová A, Janega P, Ilenčíková D, Babál P. Burkitt lymphoma with unusual granulomatous reaction. A case report. Cesk Patol 2011; 56(1): 19–22.
10. Swerdlow SH, Campo E, Harris NL, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues (2nd edn). IARC Press: Lyon; 2008 : 262–266.
11. Hecht JL, Aster JC. Molecular biology of Burkitt’s lymphoma. J Clin Oncol 2000; 18(21): 3707–3721.
12. zur Stadt U, Hoser G, Reiter A, Welte K, Sykora KW. Application of long PCR to detect t(8;14)(q24;q32) translocations in childhood Burkitt’s lymphoma and B-ALL. Ann Oncol 1997; 8(Suppl 1): 31–35.
13. Akasaka T, Akasaka H, Ohno H. Polymerase chain reaction amplification of long DNA targets: application to analysis of chromosomal translocations in human B-cell tumors (review). Int J Oncol 1998; 12(1): 113–121.
14. Siebert R, Matthiesen P, Harder S, et al. Application of interphase fluorescence in situ hybridization for the detection of the Burkitt translocation t(8,14)(q24,q32) in B-cell lymphomas. Blood 1998; 91(3): 984–990.
15. May PC, Foot N, Dunn R, Geoghegan H, Neat MJ. Detection of cryptic and variant IGH-MYC rearrangements in high-grade non-Hodgkin’s lymphoma by fluorescence in situ hybridization: implications for cytogenetic testing. Cancer Genet Cytogenet 2010; 198(1): 71–75.
16. Kavan P, Kabickova E, Gajdos P, et al. Treatment of pediatric B-cell non-Hodgkin’s lymphomas at the Motol Hospital in Prague, Czech Republic: results based on the NHL BFM 90 protocols. Pediatr Hematol Oncol 1999; 16(3): 201–212.
17. Kabíčková E, Kavan P, Koutecký J, et al. Vysokodávkovaná chemoterapie v léčbě dětí s maligním lymfomem (High-dose chemotherapy in the treatment of children with malignant lymphoma). Čas Lék čes 2000; 139(20): 623–629.
18. Haralambieva E, Boerma EJ, van Imhoff GW, et al. Clinical, immunophenotypic, and genetic analysis of adult lymphomas with morphologic features of Burkitt lymphoma. Am J Surg Pathol 2005; 29(8): 1086–1094.
19. The NHL. A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin’s lymphoma. The Non-Hodgkin’s Lymphoma Classification Project. Blood 1997; 89(11): 3909–3918.
20. Yano T, van Krieken JH, Magrath IT, et al. Histogenetic correlations between subcategories of small noncleaved cell lymphomas. Blood 1992; 79(5): 1282–1290.
21. Brown DC, Gatter KC. Ki67 protein: the immaculate deception? Histopathol 2002; 40(1): 2–11.
22. Harris NL, Jaffe ES, Diebold J, et al. World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues: report of the Clinical Advisory Committee meeting-Airlie House, Virginia, November 1997. J Clin Oncol 1999; 17(12): 3835–3849.
23. Nakamura N, Nakamine H, Tamaru J, et al. The distinction between Burkitt lymphoma and diffuse large B-Cell lymphoma with c-myc rearrangement. Mod Pathol 2002; 15(7): 771–776.
24. Bertrand P, Bastard C, Maigonnat C, et al. Mapping of MYC breakpoints in 8q24 rearrangements involving non-immunoglobulin partners in B-cell lymphomas. Leukemia 2007; 21(3): 515–523.
25. Boxer LM, Dang CV. Translocations involving c-myc and c-myc function. Oncogene 2001; 20(40): 5595–5610.
26. Ruzinova MB, Caron T, Rodig JS. Altered subcellular localization of c-MYC protein identifyies aggressive B -cell lymphomas harboring a c-MYC translocation. Am J Surg Pathol 2010; 34(6): 882–891.
27. Leucci E, Cocco M, Onnis A, et al. MYC translocation-negative classical Burkitt lymphoma cases: an alternative pathogenetic mechanism involving miRNA deregulation. J Pathol 2008; 216(4): 440–450.
28. Gaidano G, Ballerini P, Gong JZ, et al. p53 mutations in human lymphoid malignancies: association with Burkitt lymphoma and chronic lymphocytic leukemia. Proc Natl Acad Sci U S A 1991; 88(12): 5413–5417.
29. Ichikawa A, Hotta T, Saito H. Mutations of the p53 gene in B-cell lymphoma. Leuk Lymphoma 1993; 11(1–2): 21–25.
30. Gutierrez MI, Bhatia K, Cherney B, et al. Intraclonal molecular heterogeneity suggests a hierarchy of pathogenetic events in Burkitt’s lymphoma. Ann Oncol 1997; 8(10): 987–994.
31. Kaneko H, Sugita K, Kiyokawa N, et al. Lack of CD54 expression and mutation of p53 gene relate to the prognosis of childhood Burkitt’s lymphoma. Leuk Lymphoma 1996; 21(5–6): 449–455.
32. Nagai J, Kigasawa H, Koga N, et al. Clinical significance of detecting p53 protein in Burkitt lymphoma and B-cell acute lymphoblastic leukemia using immunocytochemistry. Leuk Lymphoma 1998; 28(5–6): 591–597.
33. Chuang SS, Ye H, Du MQ, et al. Histopathology and immunohistochemistry in distinguishing Burkitt lymphoma from diffuse large B-cell lymphoma with very high proliferation index and with or without a starry-sky pattern: a comparative study with EBER and FISH. Am J Clin Pathol 2007; 128(4): 558–564.
34. Remstein ED, Kurtin PJ, Buno I, et al. Diagnostic utility of fluorescence in situ hybridization in mantle-cell lymphoma. Br J Haematol 2000; 110(4): 856–862.
35. Kodet R, Mrhalova M, Krskova L, et al. Mantle cell lymphoma: improved diagnostics using a combined approach of immunohistochemistry and identification of t(11;14)(q13;q32) by polymerase chain reaction and fluorescence in situ hybridization. Virchows Arch 2003; 442(6): 538–547.
36. Poetsch M, Weber-Matthiesen K, Plendl J-J, Grote W, Schlegelberger B. Detection of the t(14;18) chromosomal translocation by interphase cytogenetics with yeast-artificial-chromosome probes in follicular lymphoma and nonneoplastic lymphoproliferation. J Clin Oncol 1996; 14(3): 963–969.
37. Akasaka T, Akasaka H, Ueda C, et al. Molecular and clinical features of non-Burkitt’s, diffuse large-cell lymphoma of B-cell type associated with the c-MYC/immunoglobulin heavy - chain fusion gene. J Clin Oncol 2000; 18(3): 510–518.
38. Jain D, Agrawal S, Chopra P. B cell lymphoma unclassifiable with features intermediate between diffuse large B cell and Burkitt lymphoma-presented with multiple lymphomatous polyposis of gastrointestinal tract. J Gastrointest Cancer 2011; 42(1): ahead of print.
39. Ye BH, Rao PH, Chaganti RS, Dalla-Favera R. Cloning of bcl-6, the locus involved in chromosome translocations affecting band 3q27 in B-cell lymphoma. Cancer Res 1993; 53(12): 2732–2735.
40. Karsan A, Gascoyne RD, Coupland RW, et al. Combination of t(14;18) and a Burkitt’s type translocation in B-cell malignancies. Leuk Lymphoma 1993; 10(6): 433–441.
41. Reddy K, Satyadev R, Bouman D, et al. Burkitt t(8;14)(q24;q32) and cryptic deletion in a CLL patient: report of a case and review of literature. Cancer Genet Cytogenet 2006; 166(1): 12–21.
42. Vaishampayan UN, Mohamed AN, Dugan MC, Bloom RE, Palutke M. Blastic mantle cell lymphoma associated with Burkitt-type translocation and hypodiploidy. Br J Haematol 2001; 115(1): 66–68.
43. Masauzi N, Kasai M, Suzuki G, et al. A translocation t(8;14) and c-myc gene rearrangement associated with the histological transformation of B-cell acute lymphocytic leukemia (FAB-L2) into Burkitt’s type (FAB-L3) leukemia. Leuk Lymphoma 1997; 27(3–4): 357–363.
44. Shou Y, Martelli ML, Gabrea A, et al. Diverse karyotypic abnormalities of the c-myc locus associated with c - myc dysregulation and tumor progression in multiple myeloma. Proc Natl Acad Sci U S A 2000; 97(1): 228–233.
45. Seegmiller AC, Garcia R, Huang R, et al. Simple karyotype and bcl-6 expression predict a diagnosis of Burkitt lymphoma and better survival in IG-MYC rearranged high-grade B-cell lymphomas. Mod Pathol 2010; 23(7): 909–920.
46. Nomura Y, Karube K, Suzuki R, et al. High-grade mature B-cell lymphoma with Burkitt-like morphology: Results of a clinicopathological study of 72 Japanese patients. Cancer Sci 2008; 99(2): 246–252.
47. Meinhardt A, Burkhardt B, Zimmermann M, et al. Phase II window study on rituximab in newly diagnosed pediatric mature B-cell non-Hodgkin’s lymphoma and Burkitt leukemia. J Clin Oncol 2010; 28(19): 3115–3121.
48. Miller TP, Grogan TM, Dahlberg S, et al. Prognostic significance of the Ki-67-associated proliferative antigen in aggressive non-Hodgkin’s lymphomas: a prospective Southwest Oncology Group trial. Blood 1994; 83(6): 1460–1466.
Labels
Anatomical pathology Forensic medical examiner Toxicology
Article was published inCzecho-Slovak Pathology

2011 Issue 3-
All articles in this issue
- Histological diagnosis of Ph-negative myeloproliferative neoplasia. An overview.
- Malignant lymphomas, or what do clinicians expect from pathologists?
- Importance of cyclin D1 (and CD5) detection in the diagnosis of malignant lymphomas other than mantle cell lymphoma
- Our experience with detection of JAK2 mutations in paraffin-embedded trephine bone marrow biopsies of patients with chronic myeloproliferative disorders
- Quantitative molecular analysis in mantle cell lymphoma
- Burkitt lymphoma (BL): reclassification of 39 lymphomas diagnosed as BL or Burkitt-like lymphoma in the past based on immunohistochemistry and fluorescence in situ hybridization
- Coincidence of chronic lymphocytic leukaemia with Merkel cell carcinoma: deletion of the RB1 gene in both tumors
- Uterine leiomyoma with amianthoid-like fibers
- Glomus tumor of the stomach: A case report and review of the literature
- Mucosal changes after a polyethylene glycol bowel preparation for colonoscopy are less than those after sodium phosphate
- Czecho-Slovak Pathology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Our experience with detection of JAK2 mutations in paraffin-embedded trephine bone marrow biopsies of patients with chronic myeloproliferative disorders
- Histological diagnosis of Ph-negative myeloproliferative neoplasia. An overview.
- Importance of cyclin D1 (and CD5) detection in the diagnosis of malignant lymphomas other than mantle cell lymphoma
- Glomus tumor of the stomach: A case report and review of the literature
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

