-
Medical journals
- Career
Fatty acid composition of lipids of Iris sibirica
Authors: Olga Mykhailenko; Vladimir Kovalyov; Sergey Kovalyov; Erika Toryanik; Tatyana Osolodchenko; Yurii Buidin
Published in: Čes. slov. Farm., 2017; 66, 220-226
Category: Original Articles
Overview
Different Iris species are a rich source of secondary metabolites and they are widely used due to their medicinal properties, i.e. such as antibacterial, cytotoxic, hepatoprotective, antiplasmodial, and immunomodulatory effects. Determination of the fatty acid composition is necessary to create complex phytopreparations on their basis, and also, it is important for understanding of the adaptive capabilities of the plant. In our study, a comparative analysis of fatty acids composition of total lipids of the leaves and rhizomes of Iris sibirica was carried out by the gas chromatography-mass spectrometry method. The degree of unsaturated fatty acids and the activity of acyl-lipid desaturases were determined by the Lyons method. 14 fatty acids were identified in the leaves of I. sibirica, their content being 6.1 mg/g; 18 acids were found in the rhizomes, their content being 6.9 mg/g. Among the saturated fatty acids palmitic acid dominates (C16 : 0), and among the unsaturated ones, it is linoleic (C18 : 2ω6) and α-linolenic (C18 : 3ω3) acids. The content of the unsaturated fatty acids in the leaves is higher (45%) due to a high content of the polyunsaturated fatty acids, as well as the reduced share of the saturated and the monounsaturated acids, compared to the rhizomes (the content of the unsaturated fatty acids is 40%), which causes a higher value of the double bonds index (1.10), and the coefficient of unsaturation (0.81), which indicate the relative cold resistance of the plants. By contrast, in the rhizomes the concentration of the saturated fatty acids increases and the level of the unsaturated fatty acids reduces. The fatty acids composition of the leaves and rhizomes of Iris sibirica was established for the first time.
Key words:
Iris sibirica • fatty acids • acyl-lipid desaturases • gas chromatography-mass spectrometryIntroduction
Analysis of the fatty acid composition of lipids is one of the components of the determination of the medicinal quality of plants. Fatty acids are important for the normal functioning of the human body. Polyunsaturated fatty acids are the structural elements of phospholipids and lipoproteins of cell membranes, they are part of the connective tissue and the membranes of nerve cells, they take part in the transportation and oxidation of cholesterol, provide the elasticity of blood vessels and regulate many processes in the body1).
The unsaturated fatty acids of oleic acid (ω-9) can be synthesized by combining the reactions of elongation (extension of the chain) and desaturation (formation of the extra double unsaturated bonds), due to the presence of Δ9-desaturase system. However, the human body cannot synthesize linoleic (ω-6) and α-linolenic (ω-3) acids due to a lack of Δ12 and Δ15 desaturases2, 3). These acids are essential or indispensable and must come from food because they are necessary for the synthesis of other polyunsaturated fatty acids of ω-6 and ω-3 type.
Acyl lipids play an important role in plants life having bactericidal and fungicidal properties, they perform the protective function; stimulate the growth of plants; they are the storage compounds, providing the host with energy. Lipids actively change the metabolism during the autumn period and increase the resistance of plants to low temperatures4, 5).
In the study of plant lipids, growing under the conditions of abiotic stress, it is important to identify the qualitative and quantitative composition of fatty acids, with the aim of creation the complex plant-based preparation, as well as for understanding their role in metabolism and plant adaptation.
The Iridaceae family contains approximately 1,800 species in 80 genera6, 7). The genus Iris is the most extensive and comprises about 200 species, distributed in the most of the Northern Hemisphere, 16 species grow in Ukraine8), and about 40 species grow in the territory of the neighbouring states9). Species of the genus Iris were introduced into the culture as ornamental plants and they take one of the first places in the world among the flower crops in the amount of varieties. All irises are perennial grasses with annual inflorescence and perennial shortened vegetative shoots, which are rhizogenic and which are immersed in the soil or crawling on the surface. For research we have chosen the Iris sibirica (series Sibiricae (Diels) Lawrence), section Limniris (Tausch) Spach em. Rodion) of the Iridaceae Juss. Family6). The plant is unpretentious and easily breeds by seeds.
Analysis of the literature showed that the plants of the Iris genus contained various biologically active substances: flavonoids10, 11), isoflavones12, 13), xanthones14, 15), carboxylic acids16), terpenoids, ether oil, and tannins; however, the fatty acid composition has not been almost examined. Information about the use of the polyunsaturated fatty acids as pharmacological agents is rare in the literature. It is known that the oil extract of the rhizomes of I. pseudacorus is used in the treatment of non-healing wounds, burns, cuts, erosions, as analgetic, haemostatic and anti-burn agents17). The antibacterial activity of lipophilic extracts from the rhizomes and leaves of I. pseudacorus18), I. hungarica and I. sibirica19) by diffusion in agar and serial dilutions are also established. The antibacterial, antifungal activity of the chloroform and ethyl acetate extracts of the rhizomes of I. germanica has been found, and the petroleum extract showed antibacterial, antifungal and insecticidal activity12, 20). The antioxidant and anticholinesterase activities of the chloroform extract from the rhizomes of I. albicans have been established21).
The fatty acids composition of I. pseudacorus, I. pseudacorus f. alba22), I. hungarica23) was previously studied by us. For the productive cultivation of species and varieties of irises, it is necessary to examine the composition of the fatty acid as one of the indicators that is responsible for the biochemical processes in the cells.
The aim of this study was a comparative analysis of the fatty acid composition of total lipids, the determination of the degree of unsaturation of fatty acids and the activity of acyl-lipid desaturases of the leaves and rhizomes of I. sibirica.
Experimental part
Plant material
The objects of the examination were the leaves and rhizomes of I. sibirica, which were prepared in N. N. Gryshko National Botanical Garden of the National Academy of Sciences of Ukraine, Kiev, in the latter half of September 2015. I. sibirica from the collection of nursery of the Botanical Garden is the introduced view from the seeds that were received by hortus botanicus and collected in the phase of mass flowering. The systematic belonging of plant was established by one of the authors. Analysis and estimation of the results were carried out with the air-dry raw materials. Voucher specimens have been deposited in the Herbarium of the Pharmacognosy Department and Botany Department, National University of Pharmacy, Kharkiv, Ukraine.
Analysis of methyl esters of fatty acids
Analysis of methyl esters of fatty acids was carried out by gas chromatography with mass spectrometric detection (GC/MS)24, 25). The internal standard (solution 50.0 µg of tridecane in hexane) and 1.0 ml methylating agent (14% of BCl3 in methanol, Supelco 3-3033) were added to the weight of the raw materials (50.0 mg) contained in a 2.0 ml vial. This mixture was kept in a hermetically closed vial for 8 hours at 65 ºC. At this time fatty oil was fully extracted and hydrolyzed into its constituent of the fatty acids and their methylation was done. The reaction mixture was drained off from a precipitation of the plant material and was diluted with 1.0 ml of distilled water. Methyl esters were extracted with 0.2 ml of methylene chloride, carefully shacked up for several times within an hour, then the obtained extract was chromatographed.
Chromatographic conditions
A sample injection (2.0 µl) was carried out into the chromatographic column in the splitless mode within 0.2 minutes. The contents of methyl esters of the fatty acids were determined on an Agilent Technologies 6890 chromatograph with a mass spectrometric detector 5973. A capillary column HP-INNOWAX was used for splitting (30 m × 0.25 mm × 0.50 µm). Mobile phase: helium, gas flow rate is 1.2 ml/min. Temperature of a heater of the sample injection is 250 ºC. Temperature of a furnace is programmable from 50 to 320 ºC with the rate of 4 degree/min.
Identification of the components
Individual components were identified by comparison of their mass spectra using both “NIST-MS Library 05” and “Wiley GC-MS Library 2007” 26,27). The substance content was estimated relating to the internal standard. Calculation of the components content С (mg/kg) was carried out by the formula:
where P1 – the peak area of the tested substance; P2 – the peak area of the standard; 50 – the mass of the internal standard (μg), injected into the sample; m – the sample mass (g).
The relative component content was defined in per cent from their total amount.
Determination of the degree of unsaturation of fatty acids and the activity of acyl-lipid desaturases
The double bonds index (DBI) for the assessment of unsaturation of fatty acids (FA) was estimated by the method of Lyons3):
where – Pj is the content of each unsaturated FA (%) and nj is the number of double bonds in its molecule. The unsaturated coefficient was estimated as the total content of unsaturated (UFA) – to – saturated acids (SFA) ratio:
The activity of acyl-lipid -, ω6 and ω-3 membrane desaturases2, 29), catalyzing the introduction of double bonds into the carbon chains of oleic (C18 : 1), linoleic (C18 : 2) and linolenic (C18 : 3) acids, were determined as steroyl - (SDR), oleyl - (ODR) and linoleyl - (LDR) desaturase ratio, which were estimated as the content (% from total FA) of the components C18, according to the equations:
where C18 : 0, C18 : 1, C18 : 2 and C18 : 3 is the percentage of the total acids of stearic, oleic, linoleic and linolenic acids, respectively.
Statistical analysis
The results were the mean ± SD of three parallel measurements. All statistical comparisons were made by means of Student’s t-test, values of p < 0.05 were regarded as significant. Statistical analysis of the results obtained was carried out by the method of the smallest squares according to the SPhU monograph “5.3.N.1. Statistical analysis of the results of chemical experiment” (2015). For the calculations and statistical analysis of the obtained data Microsoft Office Excel was used.
Results
The GC/MS analysis of the fatty acid composition of total lipids has showed that in the leaves of I. sibirica there were 14 fatty acids (FA), their total content being 6.1 ± 0.11 mg/g; in the rhizomes 18 FA have been identified, their content being 6.9 ± 0.12 mg/g. Lengths of carbon chains were from 12 to 24 atoms (Figs. 1–2, Table 1).
1. GC-MS chromatogram of fatty acids of I. sibirica rhizomes 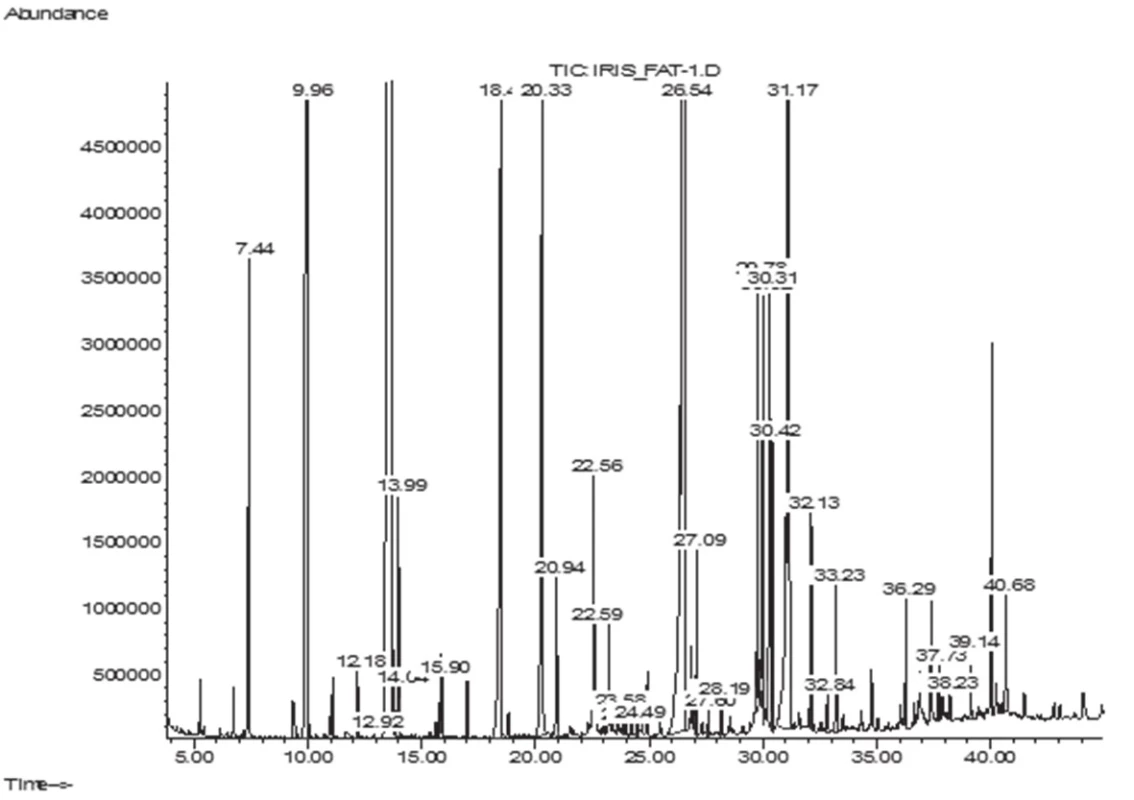
2. GC-MS chromatogram of fatty acids of I. sibirica leaves 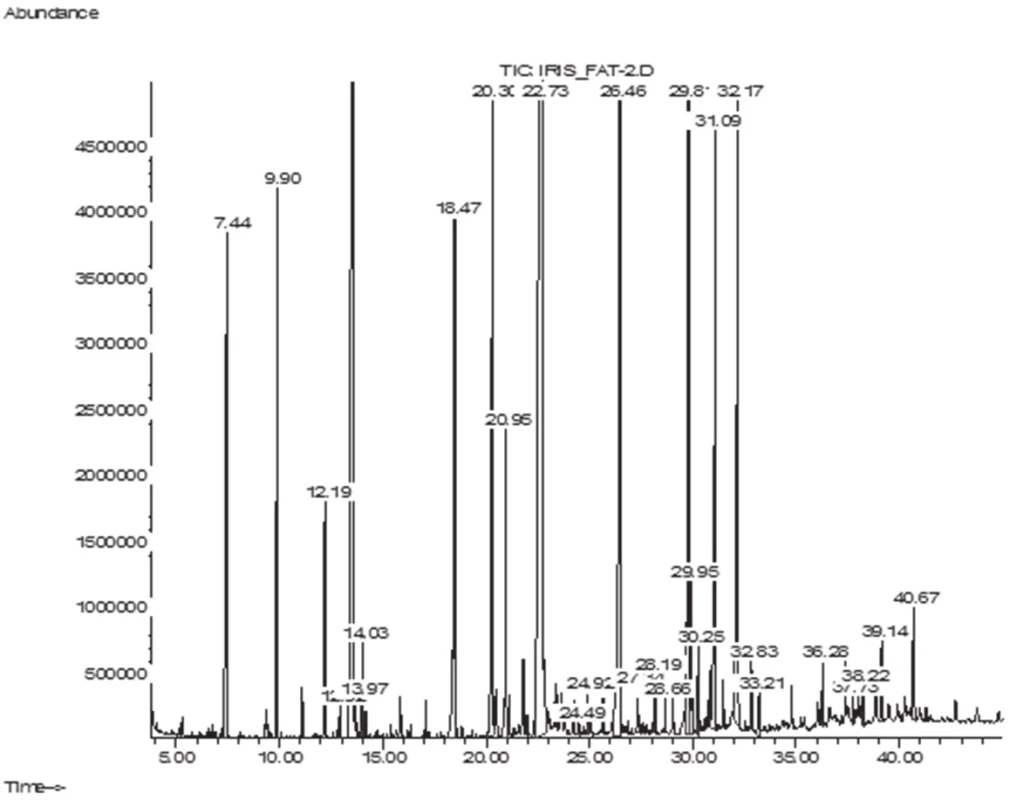
1. Fatty acid content (% of total FA), double bond indexes, coefficients of unsaturation, an index reflecting activity of ω9 (SDR), ω6 (ODR), ω3 (LDR) desaturases in the leaves and rhizomes of Iris sibirica L.а 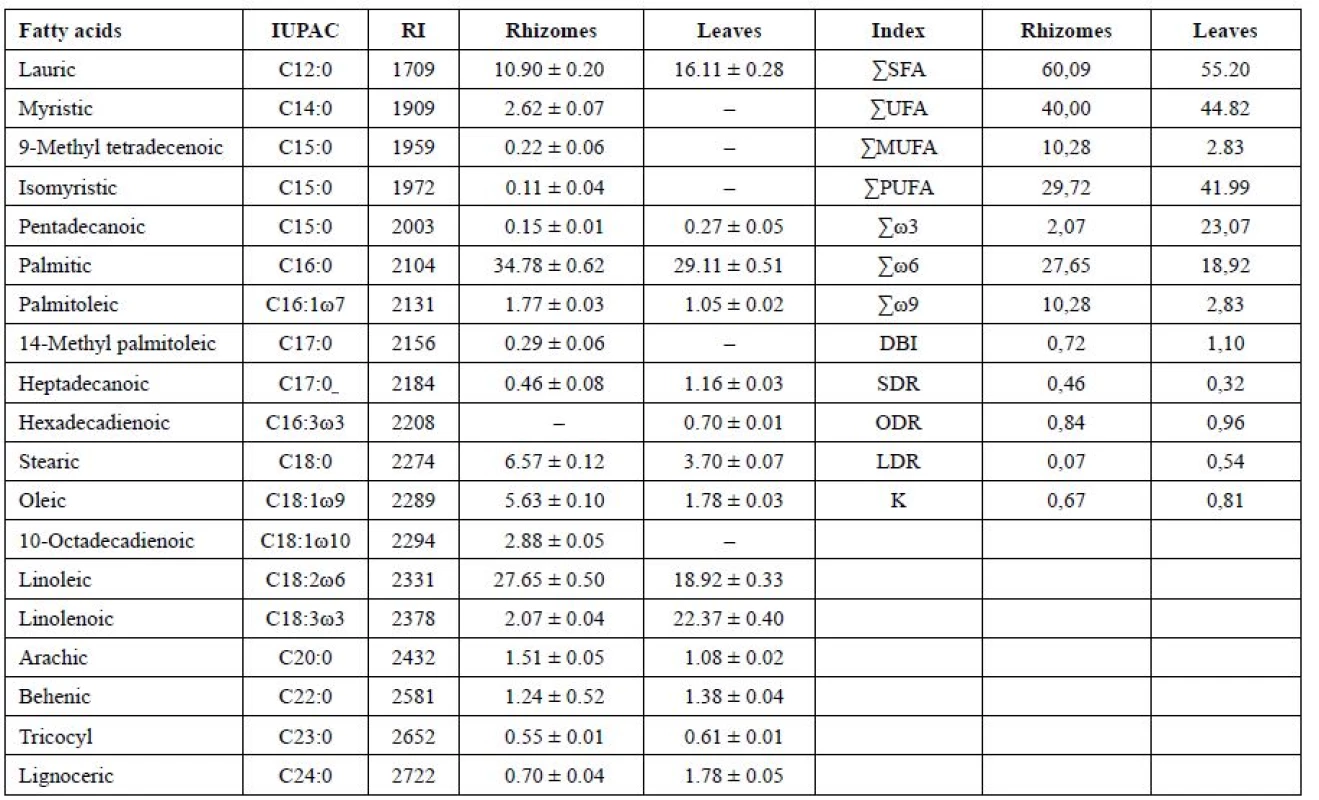
аValues expressed are mean ± SD of three parallel measurements (p < 0.05), ΣSFA – Total saturated fatty acids, ΣUFA – Total unsaturated fatty acids, ΣMUFA – Total monounsaturated fatty acids, ΣPUFA – Total polyunsaturated fatty acids, DBI – Double bond index, K – coefficients of unsaturation, SDR – Stearoyl-CoA desaturase attitude, ODR – oleoyl CoA desaturase attitude, LDR – linoleyl-CoA desaturase attitude. The symbol “–“ means that the compound was not identified. The submitted data have shown that in the leaves and rhizomes of I. sibirica the dominant saturated acids were palmitic (C16 : 0) (in the leaves, 29.11%; in the rhizomes, 34.78%) and lauric (C12 : 0) acids (16.11% and 10.90%, respectively). In addition to these acids, saturated fatty acids with 14, 15, 17, 20, 22, 23, 24 carbon atoms have been identified. The content was not more than 1–2% of each of them. An exception was stearic acid (C18 : 0), its content being 3.70% of the total acids in the leaf and 6.57% in the rhizomes. The content of the unsaturated acids in the leaf was 45%, which was by 5% higher than in the rhizomes, by reducing the content of palmitic, lauric acids and myristic (C14 : 0) it was absent in the leaf.
Among the unsaturated fatty acids of lipids, monoenoic, dienoic, trienoic acids were identified, forming ω9, 2ω6, 3ω3 of the biosynthetic families of the fatty acids with cis-orientation of double bonds in the chain. The highest percentage of ω6 dienoic linoleic (C18 : 2ω6) acid was obtained in the rhizomes (27.65%) and a smaller content was detected in the leaves (18.92%). The highest content of ω3 trienoic α-linolenic (C18 : 3ω3) acid (22.37%) was determined in the leaves of I. sibirica, at the same time, in the rhizomes α-linolenic acid (2.07%) was in the least amount.
Discussion
The aim of this study was a comparative analysis of the component composition of fatty acids of the leaves and rhizomes of I. sibirica by the chromatography-mass spectrometry method. Besides, analysis of composition of unsaturated acids is of scientific interest for the determination of the adaptive capacities of the plant4).
In the leaves of I. sibirica in the unsaturated fraction the content of monoenoic acids was decreased almost by 3 times by oleic acid (C18 : 1ω9), because of their active conversion into polyenoic acids, their content being increased from 30% in the rhizomes to 42% in the leaves. The content of monoenoic acids has been 2.83% and 10.28% for the leaves and for the rhizomes, respectively. Among monoenoic ω9 acids in the rhizomes, oleic (5.63%), palmitooleic (C16 : 1ω7; 1.77%) and 10-octadecenoic (C18 : 1ω10; of 2.88%) acids have been identified. In the leaves the content of oleic acid has been 1.78%, and palmitooleic acid, 1.05%.
From dienoic fatty acids, where in the synthesis ω6 desaturase takes a part, linoleic (C18 : 2ω6) acid similarly has been identified, its content in the leaves and the rhizomes of Iris being 18.92% and 27.65%, respectively. It is known28) that the low content of oleic acid with a high level of linoleic and linolenic acids can further the activation of R-genes (resistance genes) and the development of hypersensitivity reactions. Stark comparison in the content of these acids can be seen in the leaves.
Trienoic acids in the rhizomes have been represented only by α-linolenic (C18 : 3ω3) acid in a small amount of 2.07%. At that time, in the leaves the content of α-linolenic acid has been increased by 10 times (22.37%). It should be noted that in the leaves hexadecadienoic acid (C16 : 3ω3; 0.7%) appears, which plays a large part in the determination of the cold resistance of plants, and promotes the process of photosynthesis at low temperatures29). The biosynthesis of dienoic and trienoic acids 18 : 2ω6, 18 : 3ω3 and 16 : 3ω3 plays a defining role in the adaptation of plants to hypothermia. These polyunsaturated fatty acids regulate the “fluidity” of cell membranes in a wide temperature range, influence the resistance of plants to difficult environments4, 28).
The unsaturated coefficient characterizes the change in membrane fluidity due to the changes in the saturated – to – unsaturated fatty acids ratio in the membrane lipids. An increase in the unsaturated coefficient in the leaves was provided by increasing the fraction of the polyunsaturated fatty acids, which was very important for the maintenance by membranes of the liquid-crystalline state and increasing the resistance of leaves to hypothermia30). In the rhizomes the unsaturated coefficient was lower than in the leaves, it was probably connected with reducing their physiological activity and the cell membrane reacted to gel and they almost were not in effect.
It is known31) that a level C>20 of fatty acids in the vegetative organs of the higher plants is usually less than 1–2%. However, according to the literature data32), an increased content of the level C20 of fatty acids in the vegetative organs of the plants indicates that the plant has adapted to adverse environmental conditions. The fraction C20-24 of fatty acids in the rhizomes was 4% and in the leaves, 4.85%. This confirms that I. sibirica has adapted to the conditions of our latitude6).
The degree of unsaturation of lipids is best characterized by the double bonds index (DBI)3). As you can see, the DBI in the leaves of Iris (1.10) is higher than in the rhizomes (0.72), by all appearances, this is due to the mechanisms of survival of the leaves and their adaptation to the action of the negative temperatures as of autumn-winter period. Irises belong to the long term vegetative plants that do not have pronounced period of winter dormancy, because the foliage in the central part of foliate fascicle remains green6).
When the temperature reduces, the fluidity of membranes decreases and as the result, there is an enhancement of the synthesis of enzymes of desaturases in the cell and also the quantity of polyunsaturated fatty acids is increased. As the result, the membranes assume the liquid-crystalline state and their fluidity restores1, 30, 33). Therefore, the activity of desaturases is the determinant in the adaptation of plants to low temperatures. For Iris sibirica, the activity of steroyl - (SDR), oleyl - (ODR) and linoleyl - (LDR) desaturases is different. The value of desaturase ω9 (SDR) in the leaf is smaller, perhaps it is due to the fact that in the rhizomes the content of short-chain fatty acids is higher, and they contribute to the maintenance of membrane fluidity at the required level, i.e. the reaction of the underground organs to the adaptation were faster2, 29). The value of ODR for the leaf has been determined as high – 0.96, and it has revealed that oleic acid was actively converted by acyl-lipid ω6-desaturases into linoleic acid. On the other hand, the reaction of ω-3 desaturase (LDR) that causes the introduction of a third double bond is different: in the rhizomes it was only 0.07, while in the leaf this value was 0.54, due to the appearances of hexadecatrienoic acid and the content of α-linolenic acid increased.
The differences in the fatty acid composition in the leaves and rhizomes of Iris show different regulatory mechanisms in the plant. By the adaptation of the leaf of Iris to cold snap the main role belongs to ω-3 desaturase as the increase of its activity leads to the transformation of linoleic acid to linolenic. Adaptation of the rhizomes is achieved by increasing the activity of ω6 desaturase that is responsible for the biosynthesis of linoleic acid and of ω-3 desaturase in the less degree.
In addition, it should be noted that fatty acids are known for their antioxidant, antifungal, anti-inflammatory and immunomodulatory properties34), they are involved in metabolism, they have a positive effect in digestion, and create favourable conditions for the life activity of beneficial intestinal microorganisms1, 5). In recent years, the research investigated fatty acids and the results obtained showed that they have significant sedative and hypnotic effects34), as well as antibacterial, antitumour, antimycobacterial and antiviral activities35, 36). In our previous papers19), we reported the results of the antimicrobial activity screening of the dry extracts of the rhizomes and leaves of I. sibirica which at the concentration of 1% inhibited the growth of gram-positive and gram-negative bacteria and fungi. So, the pharmacological activity of Iris plants is due to the composition of their biologically active compounds.
Conclusions
As a result of the experiment, it has been established that the leaves and rhizomes of I. sibirica have a different fatty acid composition. The content of the saturated fatty acids in the rhizomes is prevalent, and in the leaves the fraction of unsaturated (in particular the polyenoic) fatty acids is larger due to polyenoic ones; it confirms the value of the coefficient of unsaturation and the double bonds index. When the temperatures decrease, the membrane of the rhizomes become gel state and the biochemical processes slow down. The aboveground part adapts to weather conditions due to the increased content of polyunsaturated fatty acids. In addition, the experimental data show that for a harvest of the raw materials it is better to select the period of early autumn, when the biologically active substances in the organs of the plant accumulate. This is the first report on the determination of the fatty acids from the leaves and rhizomes of I. sibirica by GC/MS. The composition of fatty acids also gives the prerequisites to use I. sibirica for medical purposes.
Conflicts of interest: none.
Received September 19, 2017
Accepted November 6, 2017
Olga Mykhailenko, PhD (Pharmacy)
V. Kovalyov
S. Kovalyov
E. Toryanik
National University of Pharmacy
4-Valentunivska St., 61168 Kharkiv, Ukraine
e-mail: zolya85@gmail.com
T. Osolodchenko
Head of the Laboratory of Biochemistry of Microorganisms and Nutrient Media
Institute of Microbiology and Immunology named after I. I. Mechnikov of the National Academy of Medical Sciences of Ukraine, Kharkiv, Ukraine
Y. Buidin
Head of Department of the Ornamental plants, Senior Researcher National Botanical Garden n.a. M.M. Gryshko of NAS of Ukraine, Kyiv, Ukraine
Sources
1. Gunstone F. D. Fatty acids and lipid chemistry. London: Blackie Academic and Professional 1996.
2. Los D. A. Fatty acid desaturase. Мoscow: Nauchnuy mir 2014.
3. Lyons J. M., Weaton T. A., Pratt H. K. Relationship between the physical nature of mitochondrial membranes and chilling sensitivity in plants. Plant Physiol. 1964; 39, 262–270.
4. Zheng G., Tian B., Zhang F., Tao F., Li W. Plant adaptation to frequent alternations between high and low temperatures remodeling of membrane lipids and maintenance of unsaturation levels. Plant Cell Env. 2011; 34, 1431–1442.
5. Murphy D. J. Plant lipids: biology, utilization and manipulation. Wiley-Blackwell 2005.
6. Rodionenko G. I. The genus Iris L.: questions of morphology, biology, evolution, and systematics. London: British Iris Society 1987.
7. Goldblatt P., Manning J. C. The Iris Family: natural history and classification. Portland: Timber Press 2008.
8. Mosyakin S. L., Fedoranchuk M. M. Vascular plants of Ukraine. A nomenclatural Checklist. Kiev 1999.
9. Alexeyeva N. Genus Iris (Iridaceae) in the Russia. Turczaninowia 2008; 11(2), 5–68.
10. Mykchailenko O. O., Kovalyov V. M. Phenolic compounds of the genus Iris plants (Iridaceae). Čes. slov. Farm. 2016; 65(2), 70–77.
11. Wang H., Cui Y., Zhao C. Flavonoids of the genus Iris (Iridaceae). Mini Rev. Med. Chem. 2010; 10(7), 643–661.
12. Singab A. N. B, Ayoub I. M., El-Shazly M., Korinek M., Wu T. Y., Cheng Y. B., Chang F. R., Wu Y. C. Shedding the light on Iridaceae: ethnobotany, phytochemistry and biological activity. Industrial Crops and Products 2016; 92, 308–335.
13. Mykchailenko O. O., Kovalyov V. M., Kovalyov S. V., Krechun A. V. Biologically active compounds from the rhizomes of Iris hungarica. J. of organic and pharmaceutical chemistry 2016; 14(4), 63–66.
14. Tsukasa I., Ootani S. Flavonoids of the genus Iris; structures, distribution and function: review. Ann. Tsukuba. Bot. Gard. 1998; 17, 147–183.
15. Mykhailenko O., Kovalyov V., Kovalyov S., Krechun A. Isoflavonoids from the rhizomes of Iris hungarica and antibacterial activity of the dry rhizomes extract. Ars Pharmaceutica 2017; 58(1), 39–45.
16. Isaev J. I., Mykchailenko О. O., Kovalyov V. N., Gurbanov G. M., Suleymanov M. Y. Gas chromatography-mass spectrometry studies of the component composition of carboxylic acids of the rhizomes of Iris medwedewii and Iris carthaliniae (Iridaceae). Čes. slov. Farm. 2017; 66(1), 9–14.
17. Vasileva S. A., Vasilev V. V., Sedova O. U. Remedy for burns and method for its preparation. Patent of the Russian Federation, 2195307(13)С1; Chelyabinsk CSTI, А61К35/78, 2001126478/14; 2002.
18. Zatylnikova O. A, Osolodchenko T. P, Kovalev V. N. Antimicrobial activity of extracts of Iris pseudacorus L. Scientific J. An. Mechnikov’s Inst. 2010; 4, 43–47.
19. Kovalev V. M., Mykhailenko O. O., Krechun A. V., Osolodchenko T. P. Antimicrobial activity of extracts of Iris hungarica and Iris sibirica. Annals of Mechnikov Institute, 2017; 2, 57–64.
20. Orhan I., Nasim S., Sener B., Ayanoglu F., Özgüven M., Choudhary M. I., Atta-ur-Rahman. Two isoflavones and bioactivity spectrum of the crude extracts of Iris germanica rhizomes. Phytother. Res. 2003; 17, 575–577.
21. Hacıbekiroğlu I., Kolak U. Screening antioxidant and anticholinesterase potential of Iris albicans extracts. Arabian J. Chem. 2015; 8(2), 264–268.
22. Mykchailenko O. A., Kovalyov V. N., Kovalyov S. V. Chromatography-mass spectrometric study of bioactive substances of rhizomes with roots of Iris pseudacorus f. alba. Farmaciya Kazachstana 2015; 3(166), 38–41.
23. Kovalyov V. N., Mykchailenko O. A., Krechun A. V. Investigation of lipid composition of rhizomes with roots of Iris hungarica (Iridaceae). Rastitelnye resursy 2015; 3, 406–415.
24. Bicchi C., Brunelli C., Cordero C., Rubiolo P. Direct resistively heated column gas chromatography (Ultrafast module-GC) for high-speed analysis of essential oils of differing complexities. J. Chromatogr. A 2004; 1024(1–2), 195–207.
25. Carrapiso A. I., García C. Development in lipid analysis: some new extraction techniques and in situ transesterifi cation. Lipids 2000; 35(11), 1167–1177.
26. NIST Mass Spec Data Center SES. Mass Spectra, 6th edn. National Institute of Standards and Technology: Gaithersburg MD 2005a.
27. NIST Mass Spec Data Center SES. Retention Indices, 6th edn. National Institute of Standards and Technology: Gaithersburg MD 2005b.
28. Upchurch R. G. Fatty acid unsaturation, mobilization, and regulation in the response of plants to stress. Biotechnol. Lett. 2008; 30, 967 – 977.
29. Sysoeva M. I., Matveeva E. M., Lavrova V. V., Sherudilo E. G. Potato plant responses to temperature drop and phytonematode infestation under continuous lighting. Acta Horticulturae 2012; 956, 621–628.
30. Macartney A. I, Maresca B., Cossins A. R. Acyl-CoA desaturases and the adaptive regulation of membrane lipid composition. AR. Cossins, ed. Temperature adaptation of biological membranes. Portland: London 1999, 129–139.
31. Cassagne C., Lessire R., Bessoule J. J., Moreau P., Creach A., Schneider F., Sturbois B. Biosynthesis of very long chain fatty acids in higher plants. Prog. Lipid Res. 1994; 33, 55–69.
32. Badea C., Basu S. K. The effect of low temperature on metabolism of membrane lipids in plants and associated gene expression. Plant Omics 2009; 2, 78–84.
33. Zhang Q. X., Wang Q. L., Han J. H. Effect of fatty oil in Periploca sepium on neural system in mice. Xi’an. Med. Univ. 1995; 16, 43–44.
34. Almaarri K., Zedan T. A., Albatal N. Chemical analysis of essential oils of some Syrian wild Iris species. Am. J. Biochem. Mol. Biol. 2013; 3, 38–49.
35. Wren R. C. Potter’s new encyclopedia of botanical drugs and preparations. Publ. C. W. Daniel Co. Ltd. 1988.
36. Innis S. M. Essential fatty acids in growth and development. Prog. Lipid Res. 1991; 30(1), 39–103.
Labels
Pharmacy Clinical pharmacology
Article was published inCzech and Slovak Pharmacy

2017 Issue 5-
All articles in this issue
- Idiopathic thrombocytopenia refractery to therapy of cyclosporine A in clinical practice – case report
- Active substances from marine organisms in clinical trials and practice
- Herbs for increasing breast-milk production
- Fatty acid composition of lipids of Iris sibirica
- Development of the composition of intramammary combined preparation based on silver citrate for veterinary use
- Czech and Slovak Pharmacy
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Herbs for increasing breast-milk production
- Idiopathic thrombocytopenia refractery to therapy of cyclosporine A in clinical practice – case report
- Active substances from marine organisms in clinical trials and practice
- Development of the composition of intramammary combined preparation based on silver citrate for veterinary use
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career





