-
Medical journals
- Career
A study of a co-processed dry binder composed of microcrystalline cellulose and glycerol monostearate
Authors: Jitka Mužíková; Sandra Muchová
Authors‘ workplace: Department of Pharmaceutical Technology Charles University in Prague, Faculty of Pharmacy in Hradec Králové, Czech Republic
Published in: Čes. slov. Farm., 2012; 61, 229-233
Category: Original Articles
Přišlo dne 23. srpna 2012 / Přijato 18. září 2012
Overview
The paper studies the co-processed dry binder LubriToseTM MCC from the viewpoint of energy evaluation of the compression process, strength and disintegration time of tablets. The results were compared with the identical evaluation of physical mixtures of microcrystalline cellulose with several types of lubricants. LubriTose MCC showed the lowest value of energy for friction, the highest value of energy accumulated by the tablet, and the highest plasticity of all tableting materials under study. There were no marked differences in the values of the energy of decompression. The tensile strength of tablets from LubriTose MCC was lower than in those from the mixture of Vivapur® 12 and glycerol monostearate, in the compression forces of 4 and 5 kN it was comparable with the tensile strength of tablets from Vivapur 12 with Poloxamer 407. Disintegration time of tablets from LubriTose MCC was shorter than that of those from Vivapur 12 with glycerol monostearate at the compression force of 3 kN, in the case of the compression forces of 4 and 5 kN no statistically significant difference was found between the values of these tableting materials.
Keywords:
LubriTose MCC • Vivapur 12 • lubricants • tensile strength of tablets • disintegration time of tablets • energy profile of compressionIntroduction
Co-processed dry binders are becoming a trend in direct compression of tablets. They are auxiliary substances of multifunctional character and are composed of a number of auxiliary substances which have different functions, or the substances which complement each other in their functions. They may contain two dry binders, a dry binder and a glidant, a dry binder and a lubricant, a dry binder and a disintegrating substance, or a greater number of auxiliary substances. The most frequently employed method of preparation of these substances is spray drying, precipitation or crystallization1). One substance is incorporated into the particle structure of another substance, thus causing physical changes without a chemical transformation2). One of the latest substances of this type is also LubriToseTM MCC, which contains 98% of the dry binder microcrystalline cellulose and 2% of the lubricant glycerol monostearate. Microcrystalline cellulose is a very frequently employed dry binder due to its excellent compressibility. It also possesses lubricating effects, in combination with active ingredients; nevertheless it requires addition of a lubricant3). In this co-processed dry binder both auxiliary substances are contained. The aim of the paper was to test the strength and disintegration time of tablets made from this substance in dependence on compression force, to formulate the energy profile of compression, and to compare the results with physical mixtures of microcrystalline cellulose with glycerol monostearate as well as other lubricants in the same concentration.
Experimental part
Materials
LubriToseTM MCC – co-processed dry binder with microcrystalline cellulose (98%) and glycerol monostearate (2%) (Kerry Bio - Science, USA);
Vivapur®12 – microcrystalline cellulose (JRS Pharma GmbH & Co. KG, Germany);
Glycerol monostearate (ERCA, Italy);
Magnesium stearate (Acros Organics, USA);
Lutrol® F 127 – Poloxamer 407 (BASF, Germany);
Lutrol® F 68 – Poloxamer 188 (BASF, Germany);
Pruv – sodium stearyl fumarate (JRS Pharma GmbH & Co. KG, Germany).
Preparation of tableting compositions
Altogether 7 tableting materials were examined, out of which two were the pure dry binders Vivapur 12 and LubriTose MCC, the remaining 5 materials being the mixtures of Vivapur 12 with the above-mentioned lubricants in the concentration of 2%. These mixtures were prepared by mixing in a mixing cube KB 15S (Erweka GmbH, Hausenstamm, Germany) for a period of 2.5 minutes. The cube rotated at the speed of 17 rotations per minute, the weight of the prepared tableting compositions was 30 g.
Preparation of tablets and energy evaluation of compression process
Tablets were compressed using a material testing equipment T1-FRO 50 TH.A1K Zwick/Roell (Zwick GmbH & Co, Germany) by means of a special matrix with a lower and an upper punch. Compression forces were 3, 4 and 5 kN. In each compression force 16 tablets were prepared. Compression rate was 40 mm/min, pre-loading was 2 N and pre-loading rate was 2 mm/s. Tablets possessed a cylindrical shape without facets, a diameter of 13 mm, the weight being 0.5 ± 0.0010 g. The computer programme testXpert V 9.01 recorded in 10 tablets of each tableting material the compression process by means of the record “force-displacement” and numerically evaluated the energy balance of compression, i.e. the individual types of energy. It was energy E1, i.e. the energy consumed for friction, and E2, which is the energy accumulated by the tablet after compression, and energy E3, i.e. the energy released in the course of decompression4). The other calculated parameters were the total energy Emax, which is the sum total of all energies, and plasticity, which is calculated from the above-mentioned energies according to the equation [1]4):
Measurement of tensile strength of tablets and calculation of the LSR value
Tensile strength of tablets was always measured in 10 tablets no sooner than 24 hours after compression. Measurements were performed using a Schleuniger apparatus, which measures the diameter and height of tablets with a precision of 0.01 mm and destruction force in N. Tensile strength of tablets was subsequently calculated according to the equation [2]5):
where P is tensile strength of tablets in MPa, F is destruction force in N, d is the diameter of tablets in mm, h is the height of tablets in mm.
The average values of tensile strength of tablets for pure Vivapur 12 and its mixtures with lubricants served to calculate the LSR values, which quantify the sensitivity of the dry binder to the addition of the lubricant, according to the following equation [3]6):
where Csu is the crushing strength of tablets without an added lubricant and Csl is the crushing strength with a lubricant. The more this value approaches 1, the more the dry binder is sensitive to an added lubricant from the viewpoint of decreased strength of compacts6). In the present paper, the values of tensile strength, not those of crushing strength, are used in the equation.
Measurement of the disintegration time of tablets
Disintegration time was always measured in 6 tablets at each compression force at least 24 hours after compression. The measurements were made on a device for testing the disintegration time of tablets Erweka ZT 301 (Erweka GmbH, Hausenstamm, Germany) using the method described in the Czech Pharmacopoeia 2009 – Supplement 20107). The test was carried out without discs in the medium of purified water tempered for 37 °C ± 1 °C. The tablets were considered disintegrated at the moment when on the net of the tube there was no remainder.
Statistical processing of results
Experimentally obtained values of tensile strength of tablets and disintegration time were statistically processed using the computer programmes Excel and QC.Expert 3.1. The values of energies and plasticity were statistically processed by the computer programme testXpert V 9.01 directly during processing. The average values with standard deviations of strength and disintegration time of tablets were processed graphically; the average values with the standard deviations of energies and plasticity were tabulated. In the case of unclear differences in the values the unpaired t-test at the level of significance of 0.05 was employed.
Results and discussion
The paper aimed to evaluate the strength and disintegration time of tablets made from the co-processed dry binder LubriTose MCC in dependence on compression force and to carry out an energy evaluation of the compression process. Another aim was to compare the results with the identical evaluation in the physical mixtures of microcrystalline cellulose with selected lubricants in a concentration of 2%. They included glycerol monostearate, which is also contained in the dry binder under study, magnesium stearate, sodium stearyl fumarate, Poloxamer 407, and Poloxamer 188. The microcrystalline cellulose employed was Vivapur 12, because its distribution of particle sizes was closest to the substance LubriTose MCC. The evaluation of the obtained results of strength included also the sensitivity of Vivapur 12 to the addition of lubricants by means of the LSR values. The compression forces of 3, 4 and 5 kN were selected in such a way to make the strength of tablets made from the majority of the tableting materials under study to range as close as possible to the optimum range of strength, which is 0.56–1.12 MPa8). The results of the paper are recorded in three Figures and one Table.
The results of the energy evaluation of the compression process are shown in Table 1. It enumerates the total energy of compression Emax, energy for friction E1, energy accumulated by the tablet after compression E2, energy of decompression E3, and plasticity. For energy evaluation of the compression process, the compression force of 4kN was selected because at this compression force the values of tensile strength of tablets made from nearly all of the tableting materials were within the optimal range, which is 0.56–1.12 MPa. The total energy of compression is higher in LubriTose MCC than in the mixture of Vivapur 12 with glycerol monostearate due to a higher value of energy accumulated by the tablet after compression, because the energy for friction is, on the other hand, markedly lower and there is no marked difference between the values of the energy of decompression. The total energy is higher only in pure Vivapur 12. The value of energy for friction is the lowest of all tableting materials under study in LubriTose MCC, the second lowest value is found in Vivapur 12 with magnesium stearate. On the other hand, the energy accumulated by the tablet is the highest of all tableting materials under study in LubriTose MCC, the mixture of Vivapur 12 with magnesium stearate possesses the lowest value. There is no marked difference between the values of the energy of decompression in the tableting materials under study. The value of plasticity is again highest in LubriTose MCC, thus exceeding even the plasticity of the physical mixture of Vivapur 12 with glycerol monostearate. The lowest plasticity was observed in the mixture of Vivapur 12 with magnesium stearate.
1. Values of energies of compression and the value of plasticity at the compression force of 4 kN 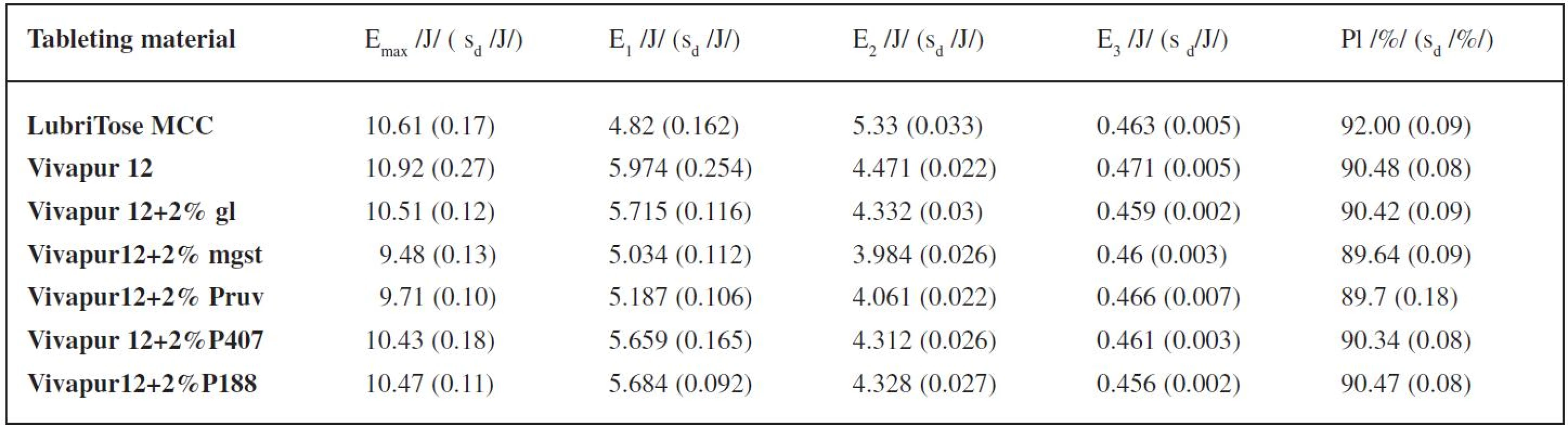
Explanations: Emax – total energy (E1 + E2 + E3); E1 – energy of friction; E2 – energy accumulated by the tablet; E3 – energy of decompression; Pl – plasticity; sd – standard deviation; gl – glycerol monostearate; mgst – magnesium stearate; P407 – Poloxamer 407; P188 – Poloxamer 188 Figure 1 represents the dependence of the tensile strength of tablets on the compression force for the tableting materials under study. The highest strength is achieved by the tablets made from pure Vivapur 12, an addition of lubricants decreases the strength, least in the case of added Poloxamer 407 and glycerol monostearate, most in the case of magnesium stearate. Tablets made from LubriTose MCC show lower strength than those made from the physical mixture of Vivapur 12 and glycerol monostearate, the values at the compression forces 4 and 5 kN are comparable with those for the mixture of Vivapur 12 with Poloxamer 407.
Fig. 1. Tensile strength of tablets in function of compression force Explanations: gl – glycerol monostearate; mgst – magnesium stearate; P407 – Poloxamer 407; P188 – Poloxamer 188 
Figure 2 presents the LSR values for Vivapur 12 which quantify the sensitivity of this substance to an addition of the lubricants tested. The higher the LSR value, the higher the sensitivity of the dry binder, the highest achievable values being 16). The Figure clearly shows a decrease in the LSR value in dependence on the compression force excepting Poloxamer 407. The lowest sensitivity was demonstrated for glycerol monostearate; the highest one, on the other hand, for magnesium stearate and sodium stearyl fumarate.
Fig. 2. <i>Values of LSR in function of compression force for Vivapur 12</i> 
Figure 3 represents the disintegration time of tablets in dependence on compression force. The disintegration time of tablets increases with the compression force excepting pure Vivapur 12 and the mixture of Vivapur 12 with glycerol monostearate, in which there is no statistically significant difference in the values for the compression forces of 3 and 4 kN. At the compression force of 3 kN, the longest disintegration time is observed in the tablets made from Vivapur 12, then from Vivapur 12 with glycerol monostearate and from LubriTose MCC. At the compression forces of 4 and 5 kN, there is no statistically significant difference between the values of disintegration time of tablets made from these tableting materials. The shortest disintegration time is found in the tablets from the mixture of Vivapur 12 with sodium stearyl fumarate, excepting the compression force of 3 kN, where there is no statistically significant difference between these tablets and the tablets with magnesium stearate. The short disintegration time of these tablets corresponds with a significant decrease in the strength of tablets. A comparison of the effects of Poloxamers yields a longer disintegration time for the tablets with Poloxamer 407 excepting the compression force of 5 kN, at which there is no statistically significant difference in the values of the tablets with Poloxamers.
Fig.3. Disintegration time in function of compression force Explanations: gl – glycerol monostearate; mgst – magnesium stearate; P407 – Poloxamer 407; P188 – Poloxamer 188 
Conflict of interest: none.
The study was supported by the firm Kerry Bio-Science and JRS Pharma GmbH & Co. KG, which supplied the samples of the dry binders tested.
Adress:
PharmDr. Jitka Mužíková, Ph.D.
Department of Pharmaceutical Technology Charles University in Prague
Faculty of Pharmacy in Hradec Králové, Czech Republic
Heyrovského 1203, 500 05 Hradec Králové
e-mail: muzikova@faf.cuni.cz
Sources
1. Saha S., Shahiwala A. F. Multifunctional coprocessed excipients for improved tabletting performance. Expert Opin. Drug Deliv. 2009; 6, 197–208.
2. Nachaegari S. K. Bansal A.K. Coprocessed excipients for solid dosage forms. Pharm. Tech. 2004; 28, 52–64.
3. Carlin B. A. C. Direct compression and the role of filler - binders. In: Augsburger, L. L., Hoag S. W. (eds.) Pharmaceutical dosage forms: Tablets, Vol. 2, 3th ed. New York: Informa Healthcare USA, Inc. 2008; 173–216.
4. Ragnarsson G. Force-displacement and network measurements. In Alderborn G., Nystrőm Ch. (eds.) Pharmaceutical Powder Compaction Technology New York: Marcel Dekker, Inc. 1996; 77–97.
5. Fell J. T., Newton J. M. Determination of tablet strength by diametral-compression test. J. Pharm. Sci. 1970; 59, 688–691.
6. Bos C. E., Bolhuis H., van Doorne Lerk C. F. Native starch in tablet formulations: properties on compaction. Pharm. Weekbl. Sci. Ed.1987; 9, 274–282.
7. Czech Pharmacopoeia 2009 – Supplement 2010 Praha: Grada Publishing 2010; 4082–4085.
8. Belousov V. A. K voprosu o vybore optimalnikh davlenii pressovania pri tabletirovanii lekarstvennykh poroshkov. Khim. Farm. Zh. 1976; 10, 105–111.
Labels
Pharmacy Clinical pharmacology
Article was published inCzech and Slovak Pharmacy

2012 Issue 5-
All articles in this issue
- Bergenia genus – content matters and biological activity
- Tacrine and its derivatives in the therapy of Alzheimer’s disease
- Important toxic and undesirable effects of medicaments in animals from the pharmacist’s viewpoint
- Methods of pharmaceutical technology in preparation of pellets for detection of acetylcholinesterase inhibitors
- A contribution to the development of advertising in pharmacy II Historical development of regulation of advertising of medicinal products
- On the origin of the Czechoslovak Pharmacopoeia
- 76th Prague Meeting on Macromolecules
- A study of a co-processed dry binder composed of microcrystalline cellulose and glycerol monostearate
- Czech and Slovak Pharmacy
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Important toxic and undesirable effects of medicaments in animals from the pharmacist’s viewpoint
- Tacrine and its derivatives in the therapy of Alzheimer’s disease
- Methods of pharmaceutical technology in preparation of pellets for detection of acetylcholinesterase inhibitors
- Bergenia genus – content matters and biological activity
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career




