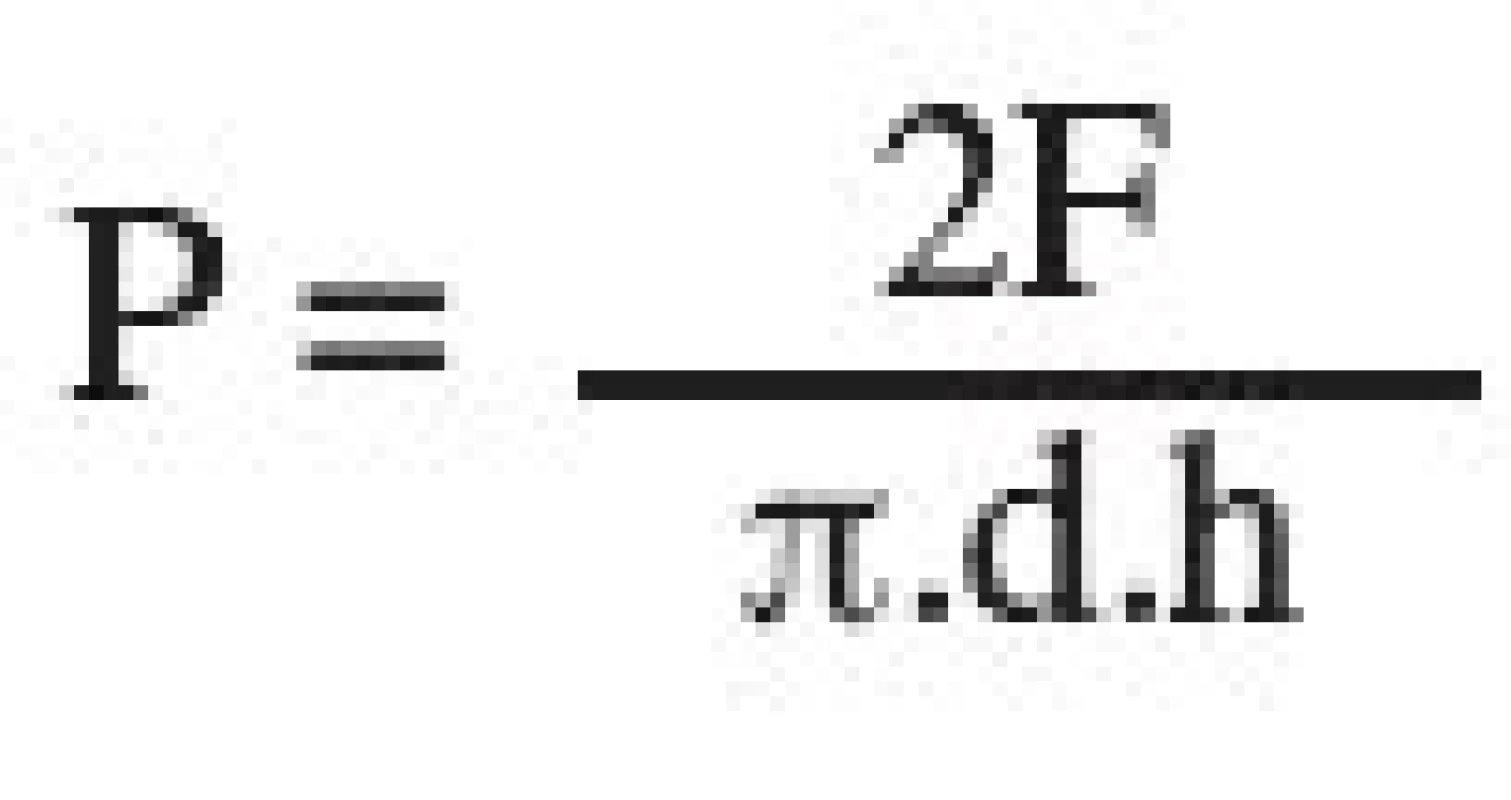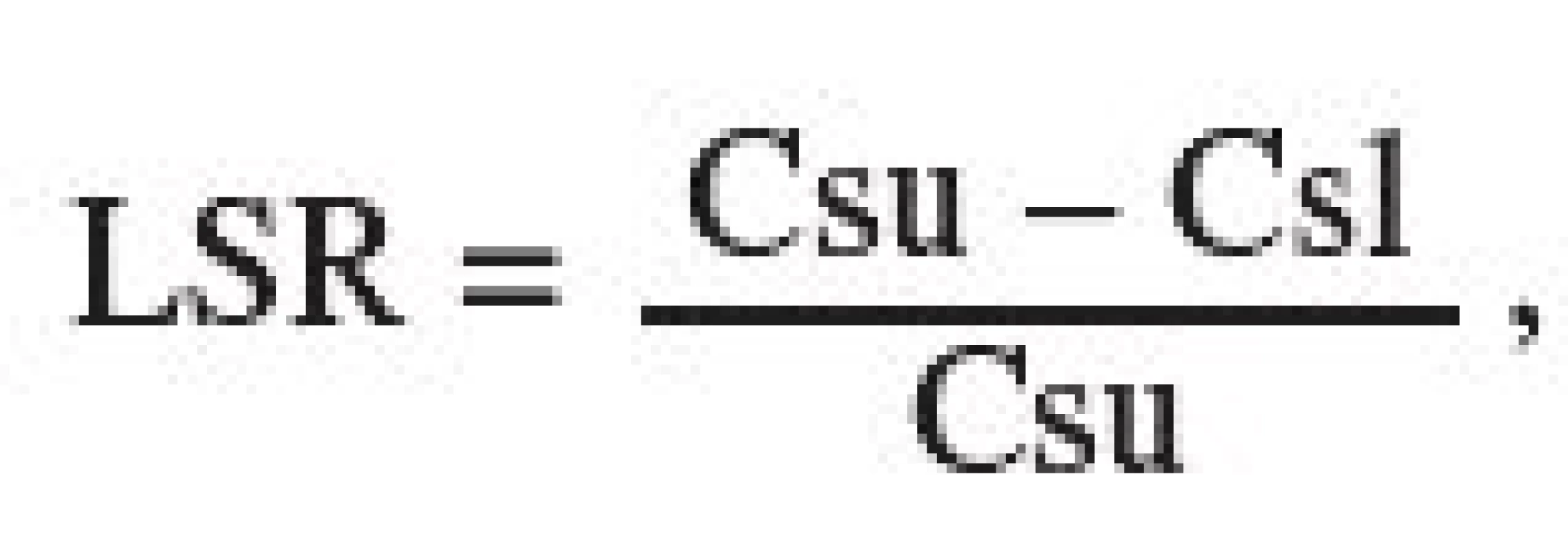-
Medical journals
- Career
A study of the properties of tablets from two types of directly compressible xylitol
Authors: J. Mužíková; H. Komínková
Authors‘ workplace: Charles University in Prague, Faculty of Pharmacy in Hradec Králové, Department of Pharmaceutical Technology
Published in: Čes. slov. Farm., 2008; 57, 170-176
Category: Original Articles
Overview
The paper deals with the study of tensile strength and disintegration time of tablets from two types of directly compressible xylitol, Xylitab 100 and Xylitab 200, in dependence on compression force, addition of two types of lubricants in one concentration, and two types of active ingredients. Tablets were compressed using the compression forces of 6, 8, and 10 kN, in the case of tableting materials with active ingredients, only 10 kN. The lubricants employed were magnesium stearate and sodium stearyl fumarate at the concentration of 1%, the active ingredients, ascorbic acid and acetylsalicylic acid at the concentration of 50%. The strength of tablets increased with compression force. Compacts from Xylitab 100 possessed a higher strength and it was more decreased by added lubricants than compacts from Xylitab 200, in which, in contrast to Xylitab 100, magnesium stearate decreased strength more. Disintegration time was longer in the case of compacts with Xylitab 200; it increased with compression force, except in those with Xylitab 200 with magnesium stearate and it was prolonged in the case of the presence of lubricants, more in the case of magnesium stearate . Compacts with both active ingredients were stronger and possessed a shorter disintegration time in the case of Xylitab 100. A significantly higher strength and a longer disintegration time was observed in the tablets with acetylsalicylic acid.
Key words:
Xylitab 100 – Xylitab 200 – lubricants – tensile strength of tablets – time of disintegrationIntroduction
Xylitol is a sugar alcohol, commonly occurring, e.g. in fruit and vegetables. It is the sweetest of all polyols and there is no aftertaste in the mouth, otherwise typical of artificial sweeteners. Most frequently it is obtained by hydrolysis of xylan from various sources of cellulose. It is used as a substitute for sucrose in foodstuffs and as a filler in classic as well as chewing tablets, where it exerts its sweet taste and cooling effect, originating due to high negative heat of dissolution. Xylitol is slightly hygroscopic. Its great advantages include decreased formation of dental plaque and no tooth decay, in contrast to, e.g. sucrose, which are used by many microorganisms for fermenting processes resulting in the production of acid products negatively acting in the oral cavity1).
Garr and Rubinstein 2) studied the principal properties of directly compressible powdered xylitol of an average particle size of 170 μm. The study resulted in the finding that radial strength and elastic renewal of xylitol tablets are independent of compression velocity and that the dominant mechanism of compression is the mechanism of fragmentation of particles. Tensile strength of tablets was increasing up to the compression force of 22 kN and then it began to decrease due to elastic relaxation.
Granulated form of compressible xylitol is known under the trade name Xylitab®. There are three principal types of Xylitab, Xylitab 100, 200, and 300. Xylitab 100 is granulated by polydextrose, Xylitab 200 by the carmellose sodium, and Xylitab 300 by the solution of xylitol alone. Xylitab 200 possesses a larger average particle size and bulk as well tap density than Xylitab 100 3). Both substances possess excellent flowability 4). On compression, it is necessary to use lubricants, in which the combination of 0.5% of magnesium stearate and 0.5% stearic acid proved to be most suitable 5).
The paper aimed to study the tensile strength and disintegration time of compacts from Xylitab 100 and 200 in dependence on compression force, addition of two types of lubricants at one concentration, and two types of active ingredients.
EXPERIMENTAL PART
Materials
Xylitab® 100 – agglomerated xylitol (96.8% xylitol + 3% polydextrose, 0.2% reducing sugars)
(Danisco, Finland);
Xylitab® 200 – agglomerated xylitol (98.5% xylitol + 1.5% carmellose sodium)
(Danisco, Finland);
magnesium stearate (Acros Organics, New Jersey, USA);
Pruv® – sodium stearyl fumarate (J. Rettenmaier & Söhne GmbH+Co, Rosenberg, Germany)
Acetylsalicylic acid (Schütz and Co. GmbH, Hamburg, Germany);
ascorbic acid (Northeast General Pharmaceutical Factory, Shenyang, China).
Preparation of tableting materials and tablets
A list of tableting materials evaluated in the study:
- Xylitab 100
- Xylitab 100 with 1% magnesium stearate
- Xylitab 100 with 1% Pruv
- Xylitab 100 with 50% acetylsalicylic acid and with 1% magnesiim stearate
- Xylitab 100 with 50% acetylsalicylic acid and with 1% Pruv
- Xylitab 100 with 50% ascorbic acid and with 1% magnesium stearate
- Xylitab 100 with 50% ascorbic acid and with 1% Pruv
- Xylitab 200
- Xylitab 200 with 1% magnesium stearate
- Xylitab 200 with 1% Pruv
- Xylitab 200 with 50% acetylsalicylic acid and with 1% magnesium stearate
- Xylitab 200 with 50% acetylsalicylic acid and with 1% Pruv
- Xylitab 200 with 50% ascorbic acid and with 1% magnesium stearate
- Xylitab 200 with 50% ascorbic acid and with 1% Pruv
The dry binder was mixed with the lubricant always for 5 minutes in a stainless steel cube KB 15S (Erweka GmbH, Hausenstamm, Germany). In the mixtures with an active ingredient, the dry binder was mixed with the active ingredient for 5 minutes first and then the lubricants sodium stearyl fumarate or magnesium stearate were added for 5 minutes.
All tableting materials were used to produce 16 tablets compressed with the use of a special die with an upper and a lower punch on a material testing equipment T1-FRO 50 TH.A1K Zwick/Roell (Zwick GmbH&Co, Ulm, Germany). The tablets were of a cylindrical shape without facets with a diameter of 13 mm and weight of 0.5 Ī 0.0010 g. Compression velocity was 40 mm/min and compression forces of 6, 8, and 10 kN. Mixtures including active ingredients were compressed using only a compression force of 10 kN.
Measurement of tensile strength of tablets and evaluation of the lubricant sensitivity of tableting materials
Tensile strength was always evaluated in 10 tablets, first no sooner than 24 hours after compaction. Measurements were performed on a Schleuniger apparatus (Dr. Schleuniger Pharmatron AG, Solothurn, Switzerland), which measured tablet sizes accurate to 0.01 mm and destruction force in N. Tensile strength of tablets was calculated according to Eq. [1]:
where P is tensile strength of tablets (MPa), F is destruction force (N), d is tablet diameter (mm), and h is thickness of the tablet (mm) 6).
LSR (lubricant sensitivity ratio) values, which make it possible to quantify and mutually compare the lubricant sensitivity of tableting materials, were calculated according to Eq. [2]:
where Csu is the crushing strength of tablets without an added lubricant and Csl is the crushing strength with a lubricant. The more this value approaches 1, the more the dry binder is sensitive to an added lubricant from the viewpoint of decreased strength of tablets 7). In the present paper, the values of tensile strength, not those of crushing strength, are used in the equation.
Measurements of the disintegration time of tablets
Disintegration times of tablets were evaluated earliest 24 hours after compaction always in 6 tablets. Measurements were performed on an apparatus for the determination of disintegration time following the method of Ph.B. MMV in the medium of purified water tempered to 37 °C Ī 1 °C. The tablet was considered disintegrated at the moment when there was no remainder on the net 8).
The results of strengths and disintegration times were statistically processed by means of the computer programmes Excel and Qcexpert. Elementary data analysis yielded the mean values with standard deviations, which were plotted into dependences on compression force. In the cases of unclear significance of differences in the values, unpaired t-test at a level of significance of 0.05 was employed.
RESULTS AND DISCUSSION
The paper aimed to study the properties of tablets from two sorts of directly compressible xylitol, Xylitab 100 and Xylitab 200. Tableting materials with xylitol contained two sorts of lubricants, magnesium stearate and sodium stearyl fumarate in 1% concentration. Selected compression forces of 6, 8, and 10 kN were selected in such a way as to make the tensile strength of tablets from xylitol with lubricants range about the upper limit of optimal tensile strength of tablets (1.11 MPa) 9). The compression force of 10 kN was employed to compress compacts with 50% concentration of ascorbic acid and acetylsalicylic acid, i.e. the active ingredients selected due to their different mechanisms of compression and different solubility in water.
Fig. 1 shows the dependence of tensile strength of tablets on the compression force for Xylitab 100 with lubricants. Excepting the compression force of 6 kN, the strength of tablets with an addition of Pruv is lower. At the compression force of 6 kN, the isolated point represents the strength of compacts from pure Xylitab 100; the result clearly shows that lubricants slightly decrease tensile strength of tablets. Pure Xylitab 100 was not compressed by means of higher compression forces, as it shows high friction and the compact could not be ejected from the matrix. An opposite result was observed in Xylitab 200, in which higher intervention of magnesium stearate was recorded (Fig. 2). At the compression force of 6 kN, a very slight decrease in tensile strength due to lubricants is seen again, though it is smaller than in the case of Xylitab 100. Fig. 3 represents tensile strengths of tablets from both Xylitabs in a column graph. At the compression force of 6 kN, tensile strengths from pure Xylitabs are shown. The strength from Xylitab 100 is higher, which is due to a smaller particle size of the substance. Further we can see a deeper intervention of stearate in the tensile strength of compacts from Xylitab 200 than the intervention of Pruv excepting the compression force of 6 kN, where the intervention of Pruv is comparable. The strength of tablets from all tableting materials rises with compression force.
1. Tensile strength of tablets in function of compression force: Xylitab 100 with lubricants 
2. Tensile strength of tablets in function of compression force: Xylitab 200 with lubricants 
3. Comparison of tensile strength of tablets: Xylitab 100 and Xylitab 200 without and with lubricants 
Fig. 4 shows the dependence of disintegration time of compacts on compression force for Xylitab 100. At the compression force of 6 kN it shows the disintegration time of compacts from pure substance and the values with lubricants are higher. At this compression force the longest disintegration time is observed in the compacts with stearate. With increasing compression force the difference in the effects of lubricants is decreased and at the compression force of 10 kN there is no statistically significant difference between the values. The same dependence is shown in Fig. 5 for Xylitab 200. There the differences in disintegration times are much more marked. Disintegration time is prolonged by lubricants and it is many times longer in the case of stearate, when it also decreases with increasing compression force. Fig. 6 shows disintegration times of both Xylitabs next to each other. Compacts from pure Xylitab 200 posses a longer disintegration time and in the case of added lubricants, above all stearate, this time is many times longer, though the strength of tablets from this substance is smaller. The long disintegration time in the case of tablets with lubricants is apparently due to the concentration of lubricants, which is probably too high for this larger particle size and thus a smaller specific surface.
4. Disintegration time in function of compression force: Xylitab 100 with lubricants 
5. Disintegration time in function of compression force: Xylitab 200 with lubricants 
6. Comparison of disintegration time of tablets: Xylitab 100 and Xylitab 200 without and with lubricants 
The compression force of 10 kN was employed to compress the compacts from the mixtures of Xylitabs with active ingredients in the ratio 1 : 1. The active ingredients employed were acetylsalicylic acid, which is compressed prevalently by plastic deformation and is badly soluble in water, and ascorbic acid, which is compressed by crushing the particles and is well soluble in water. The column graph in Fig. 7 shows the strengths of tablets with active ingredients. Tablets with acetylsalicylic acid are markedly stronger, their strength ranges within the optimal range of tensile strength of tablets (0.56–1.11 MPa) 9). Strength of compacts with ascorbic acid ranges only about 0.2 MPa. Compacts with Xylitab 100 are stronger than those with Xylitab 200 in the cases of both active ingredients, only in the case of ascorbic acid there is a statistically significant difference between the values for Xylitab 100 with 1% magnesium stearate and Xylitab 200 with 1% Pruv. It holds true within the framework of comparison of lubricants that smaller strength is found in the compacts with stearate. Fig. 8 shows the disintegration times of compacts with active ingredients. Due to bad solubility of acetylsalicylic acid and higher strength of compacts, the disintegration time in the case of compacts from this substance is markedly longer. In the case of ascorbic acid it holds true that a longer disintegration time is found in the compacts from Xylitab 200 and the compacts containing stearate. In the case of acetylsalicylic acid, disintegration time ranges approximately within 30–60 minutes, which is an extremely long disintegration time, which exceeds the pharmacopoeial limit of 15 minutes. In this active ingredient it holds true that the disintegration time is longer, in spite of a lower strength always in the case of Xylitab 200 and in the tablets containing Pruv.
7. Tensile strength of tablets at the compression force of 8 kN: Xylitab 100 and Xylitab 200 with active ingredients 
8. Disintegration time of tablets at the compression force of 8 kN: Xylitab 100 and Xylitab 200 with active ingredients Explanations of the abbreviations used in Figures: X 100 – Xylitab 100, X 200 – Xylitab 200, st – magnesium stearate, Ac. Acet. – Acetylsalicylic acid, Ac. Asc. – Ascorbic acid 
1. Values of LSR for the Xylitab 100 and Xylitab 200 at the compression force of 6 kN 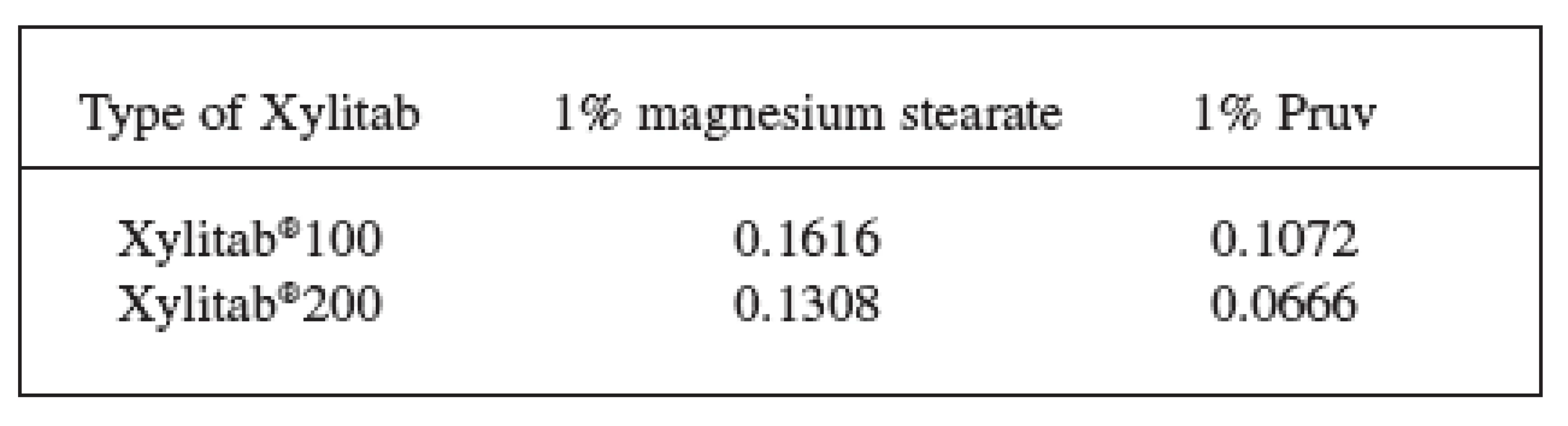
The study was supported by the grant MSM 0021620822 and by the firm Danisco, which supplied the samples of the dry binders tested.
Received 27 Juny / Accepted 2 July 2008
Sources
1. Kibbe, H. A.: Handbook of pharmaceutical excipients. Third ed., American Pharmaceutical Association and the Pharmaceutical Press, Washington, London, 2000, 602–605.
2. Garr, J. S. M., Rubinstein, M. H.: Int. J. Pharm., 1990; 64, 223–226.
3. Danisco: Excipients 2005, Firm lit., http://www.danisco. com/cms/connect/corporate/products%20and%20services/pharma%20and%20healtcare/dietary%20supplements/excipients/, cit. February 2008
4. Morris, L. E., Moore, J. C., Schwartz, J. B.: Drug Dev. Ind. Pharm., 1996; 22, 925–932.
5. Bolhuis, G. K., Armstrong, N. A.: Pharm. Dev. Technol., 2006; 11, 111–124.
6. Fell, J. T., Newton, J. M.: J. Pharm. Sci., 1970; 59, 688–691.
7. Bos, C. E., Bolhuis, H., Van Doorne, Lerk, C. F.: Pharm. Weekbl., 1987; Sci. Ed. 9, 274–282.
8. Ph.B. MMV, Grada Publishing, a.s., Prague, 2005, vol. I, s. 269.
9. Belousov, V. A.: Khim. Farm. Zh., 1976; 10, 105–111.
Labels
Pharmacy Clinical pharmacology
Article was published inCzech and Slovak Pharmacy

2008 Issue 4-
All articles in this issue
- Actual acidity of the environment and efficacy of auxiliary substances used for antimicrobial stabilization of medicinal preparations prepared in pharmacies
- Antiradical activity of substances with a potential effect on the cardiovascular system
- Monitoring of pharmacotherapy in seniors of rest homes in Brno region
- Synthesis, identification and physicochemical properties of novel potential ultrashort-acting beta-adrenergic blockers
- Characterization of microcrystalline celluloses by means of the parameters of a three-exponential compression equation
- A study of the properties of tablets from two types of directly compressible xylitol
- Czech and Slovak Pharmacy
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Actual acidity of the environment and efficacy of auxiliary substances used for antimicrobial stabilization of medicinal preparations prepared in pharmacies
- Monitoring of pharmacotherapy in seniors of rest homes in Brno region
- Antiradical activity of substances with a potential effect on the cardiovascular system
- A study of the properties of tablets from two types of directly compressible xylitol
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career