-
Medical journals
- Career
Can endarterectomy of the external carotid artery be beneficial? A critical overview
Authors: P. Dráč 1; D. Šaňák 2
Authors‘ workplace: Department of Vascular Surgery, University Hospital Olomouc, Czech Republic 1; Comprehensive Stroke Centre, Department of Neurology, University Hospital Olomouc, Czech Republic 2
Published in: Cesk Slov Neurol N 2019; 82(3): 285-288
Category: Review Article
doi: https://doi.org/10.14735/amcsnn2019285Overview
Endarterectomy of the external carotid artery (eCEA) with an occlusion of the internal carotid artery (ICA) stump is a less common treatment option for patients with neurological or ocular symptoms associated with a chronic ipsilateral ICA occlusion and stenosis of the ECA in the carotid bifurcation. The aim was to update the latest and largest published overviews concerning the outcomes of eCEA and critically evaluate the potential benefit of eCEA based on the available data. The results of several previous observational studies have showed that eCEA might be beneficial in selected symptomatic patients with a chronic ipsilateral ICA occlusion and ECA stenosis; however, a recent randomized study did not confirm this. The findings of previous observational studies are limited due to their great heterogeneity, retrospective design and small sample size (Grade 1, Level of evidence C). Thus, clear evidence for the benefits of eCEA is still missing.
颈外动脉内膜切除术是否有益?一个关键的概述
颈外动脉(eCEA)内膜切除术伴颈内动脉(ICA)残端闭塞是一种较少见的治疗方法,用于伴有神经或眼部症状的患者,这些症状与颈动脉分叉处的慢性同侧ICA闭塞和ECA狭窄有关。其目的是更新关于eCEA结果的最新和最大的已发表综述,并根据现有数据批判性地评估eCEA的潜在效益。以往的几项观察研究结果表明,eCEA可能对某些有症状的慢性同侧ICA闭塞和ECA狭窄患者有益;然而,最近的一项随机研究并没有证实这一点。以往的观察性研究由于异质性大、回顾性设计、样本量小(证据C级1级)等原因,研究结果有限,尚缺乏明确的eCEA益处的证据。
关键词:
颈外动脉内膜切除术-颈内动脉闭塞-颈动脉残端综合征
Keywords:
endarterktomie zevní krkavice – uzávěr vnitřní krkavice – syndrom karotického pahýlu
Introduction
The external carotid artery (ECA) is an important collateral pathway for cerebral perfusion in cases of internal carotid artery (ICA) occlusion and significant ECA stenosis can deteriorate supportive collateral cerebral or ocular blood flow. Moreover, unstable exulcerative ECA plaque may lead to cerebral or ocular embolization with clinical symptoms. Thus, endarterectomy of the ECA (eCEA) may improve collateral flow and decrease the risk of possible embolization from a source localized in the carotid bifurcation [1]. During the last 3 years, we have performed eCEAs in two carefully selected patients with symptomatic chronic ICA occlusion (transient ischaemic attack [TIA] in the first patient and recurrent amaurosis fugax in the second patient). No recurrent symptoms occurred during the follow-up exams (31 and 13 months) and this positive result inspired us to perform a critical overview.
This paper reviews the possible benefit of eCEA in patients with symptomatic chronic ICA occlusion based on perioperative and late clinical outcomes published in available literature.
Materials and methods
Search strategy
In 2013, Fokkema et al published what represents, to our knowledge, the largest overview of literature concerning the outcomes of eCEA [1]. The overview includes a series of patients published since 1982. A multiple electronic health database search was performed using Scopus, Medline (PubMed), Science Citation Index, Science Direct and Academic Search Ultimate.
Inclusion criteria
All articles irrespective of publication status published between January 1, 2012 and December 31, 2018 concerning eCEA in patients with a chronic ipsilateral ICA occlusion and ECA stenosis within the carotid bifurcation and/ or ICA stump with neurological or ocular symptoms were included in the search. The combined search strategy included the following phrase (containing the relevant key words): external carotid artery, external carotid artery AND stenosis, external carotid artery AND thrombus OR thrombosis, external carotid artery AND embolus OR embolism OR embolization, external carotid artery AND stroke OR transient ischaemic attack OR symptoms, symptomatic external carotid artery, external carotid artery AND treatment OR therapy OR management, external carotid endarterectomy, external endarterectomy, external CEA, internal carotid artery occlusion, occlusion of internal carotid artery AND treatment OR therapy OR management, carotid artery stump, carotid artery cul-de-sac, carotid stump syndrome, carotid artery stump AND stenosis OR thrombus OR thrombosis OR embolus OR embolism OR embolization OR stroke OR TIA OR symptoms, carotid artery cul-de-sac AND stenosis OR thrombus OR thrombosis OR embolus OR embolism OR embolization OR stroke OR TIA OR
symptoms.The extracted data were analyzed independently by two reviewers (P. D. and D. Š.) according to the heading title and abstract content. Duplicate records were removed. Any divergence was resolved using a critical discussion with final consensus. Full
articles were selected if the abstract suggested the presence of relevant data. The reference lists of all included papers were
hand-searched.Exclusion criteria
Papers in languages other than English, papers concerning eCEA in patients without related neurological or ocular symptoms, without ipsilateral ICA occlusion, with adjunctive subclavian or common carotid artery (CCA)-ECA bypass or with following extracranial-intracranial (EC-IC) bypass, for jaw claudications were excluded.
The main endpoints of the review were 30-day stroke/ death, recovery/ improvement of symptoms, late stroke/ death/ vascular death and restenosis/ occlusion. Recovery of symptoms was defined as no recurrence of related symptoms and improvement was defined as reduction of their frequency.
Results
Our selective searching found two eligible articles. A detailed flow diagramme of the searching process is presented in Fig. 1. The prospective randomized study found showed only one death due to myocardial infarction 6 months after surgery and one vascular event in the medically treated subgroup. Thus, an eCEA with surgical treatment of carotid stump syndrome was considered safe. Nevertheless, the low risk of stroke or TIA in both surgically and medically treated patients makes the benefit of surgery controversial [2]. Moreover, this study was not registered, did not provide any information about statistical analysis including pre-study power analysis and a small number of patients was included. Thus, the Level of Evidence for this randomized clinical trial is low. Additionally, this study did not monitor the occurrence of restenoses or occlusions during follow-up.
1. Flow diagramme representing searching process. ECA – external carotid artery; eCEA – endarterectomy of external carotid artery; ICA – internal carotid artery
Obr. 1. Vývojový diagram představující vyhledávací proces. ECA – zevní krkavice; eCEA – endarterktomie zevní krkavice; ICA – vnitřní krkavice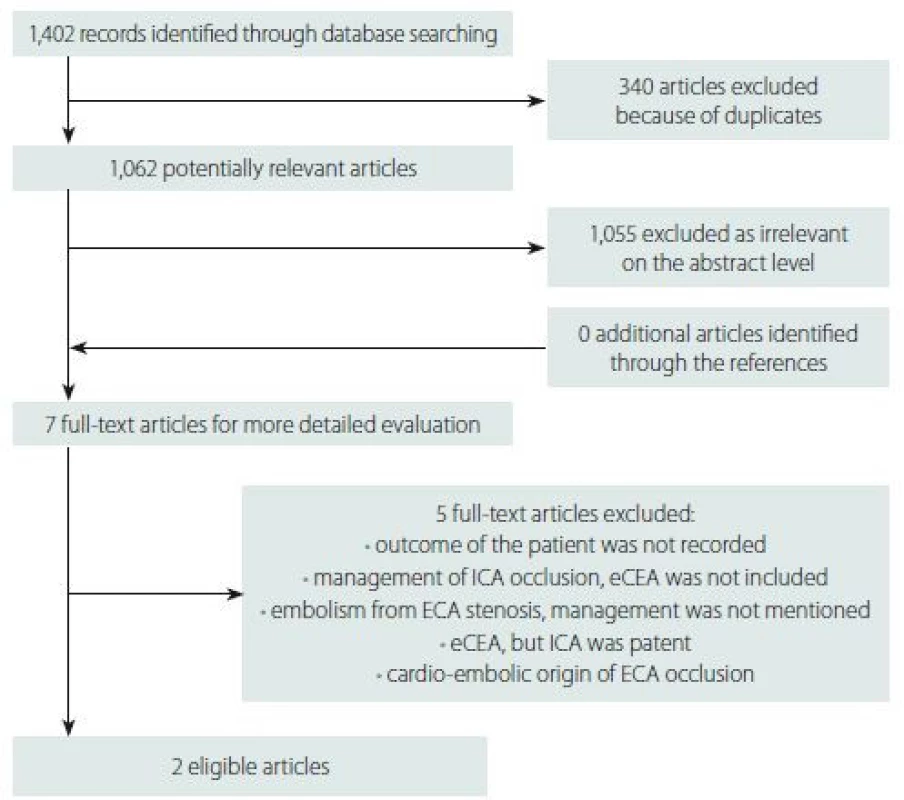
The second publication found presented a retrospective study involving nine patients who underwent eCEA with patching including over-sewing the origin of ICA or CCA-ECA stenting. All patients experienced complete recovery of clinical symptoms. One patient suffered from a myocardial infarction 30 days after the CCA-ECA stenting. One patient was lost to follow-up and none of the remaining eight patients died or suffered from recurrent ischaemic stroke. In one patient, a hybrid procedure – eCEA with retrograde CCA stenting – was performed on both sides. The next patient with eCEA underwent CCA stenting 1 year later with a complete recovery of ocular symptoms after procedure. Only three patients underwent single eCEA. Postoperative duplex US after eCEA was performed only in two patients (13 months and 3 years after eCEA) without detection of significant restenosis in both patients. The authors considered eCEA with surgical treatment of carotid stump syndrome or CCA-ECA stenting safe and effective despite the described procedure heterogeneity, retrospective design and small sample size [3].
Discussion
The management of patients with symptomatic chronic ICA occlusion is still under discussion and remains controversial. A different natural clinical course with a limited prediction of risk of recurrent events are the main reasons. Most patients, with acute ischaemic stroke due to chronic ICA occlusion present with mild or moderate neurological symptoms. In some patients the occlusion is detected accidentally without clinical symptoms or with radiologically documented silent ischaemic strokes on CT or MRI. However, new events may occur later [4]. Several studies have demonstrated that the annual risk of recurrent ischaemic stroke due to symptomatic ICA occlusion ranged from 3.8 to 5.9% despite the best medical treatment [5,6]. Others showed an annual stroke rate up to 10% and the combined rate for TIA and stroke about 20% [1]. For this reason, surgery should be considered as a preventive treatment option. The Carotid Occlusion Surgery Study randomized trial showed no benefit in stroke prevention with EC-IC bypass in patients with symptomatic ICA occlusion when compared to patients with the best medical treatment [1]. This fact renewed the interest in eCEA in patients with symptomatic ICA occlusion and concomitant stenosis of ECA in the carotid
bifurcation.The ECA is an important collateral pathway for cerebral perfusion when the ICA becomes occluded, mainly through the ophthalmic artery. It was demonstrated that the ECA supported up to 30% of the cerebral blood flow in the case of bilateral ICA occlusion [7] and a 12% decrease in middle cerebral artery blood flow velocity was found in ECA clamping during a carotid endarterectomy [8].
In the case of ICA occlusion, significant ECA stenosis can decrease cerebral blood flow and unstable, exulcerative ECA plaque can lead to an embolization. Both may cause neurological or ocular symptoms. Endarterectomy of the external carotid artery may improve cerebral or ocular hypoperfusion and remove a possible embolic source localized within the carotid bifurcation. Increased cerebral blood flow after ECA revascularization has been documented [9].
The low flow, embolization from ECA/ CCA stenosis or from non-occluded proximal remnant (a ‘stump’ or ‘cul-de-sac’) of an occluded ICA through collateral vessels and intracranial embolization or propagation of thrombus from the distal occluded ICA have been established as mechanisms of neurological and ocular symptoms [1]. It is obvious that eCEA can influence the first two mechanisms.
Thrombi or ulcerative plaques were observed in the ICA stump in symptomatic patients even in the absence of significant ECA/ CCA stenosis. Furthermore, these patients showed symptom recovery after eCEA with ICA stump obliteration [1]. Others documented the majority of restenosis or ECA occlusions after eCEA in patients without ICA stump obliteration and the fact that in all patients with recurrent symptoms, the ICA stump was not obliterated [1]. Therefore, obliteration of the ICA stump should be a routine part of the procedure.
Some previous studies showed the superiority of patch arteriotomy closure (venous, prosthetic or ICA transposition flap) to primary closure for patency [1], others demonstrated improved patency after eCEA with ICA transposition flap when compared with other surgical techniques [1].
As reported, the Carotid Occlusion Surgery Study randomized trial failed to show benefits in stroke prevention in patients with-EC-IC bypass [1]. It can be speculated that the benefit of eCEA may relate to the fact that in contrast to an EC-IC bypass, eCEA can not only improve cerebral hypoperfusion, but also eliminate the source of the emboli.
To date, the largest overview of literature concerning the outcomes of eCEA was likely that presented by Fokkema et al in 2013 [1]. The aim of our review was to extend it by adding new papers concerning this topic and to evaluate the potential benefit of eCEA in patients with a chronic ipsilateral ICA occlusion and ECA stenosis within the carotid bifurcation and/ or ICA stump with neurological or ocular symptoms.
Similarly to Fokkema et al [1], we did not include publications concerning patients who underwent eCEA for jaw claudications. In these cases, only the ECA stenosis is the source of symptoms and has no relation to possible ipsilateral chronic ICA occlusion.
Numerous reports demonstrated a low risk of the procedure and recovery or improvement of symptoms in the majority of patients during follow-up with an acceptable rate of restenosis [1–3]. In 17 out of 21 analyzed studies, most of which were also included in the overview by Fokkema et al [1], no patients suffered from stroke or died during the post-operative 30-day follow-up. Recovery or improvement of clinical symptoms was observed in 63–100% of the patients (a 100% effect was achieved in eight of all the analyzed studies). The rate of restenoses was recorded in 9 out of 21 studies; no restenoses were observed in four studies and ranged between 14.0–36.4% in the remaining studies.
However, the reported data are limited due to the retrospective design, study heterogeneity and small sample sizes. Moreover, the majority of these reports were published before 2012 and they did not meet our inclusion criteria. Very limited data about patients with chronic symptomatic ICA occlusion, who underwent eCEA solely, are available. Some reports also included asymptomatic patients [1] or patients with nonrelated or unclear symptoms [1]. Others included patients with recent ICA occlusion [1,10], in whom ICA thrombendarterectomy failed to reopen the ICA and eCEA was performed. In other series, some patients underwent eCEA preceding EC-IC bypass surgery or combined with adjunctive subclavian or CCA-ECA bypass. It is evident that these procedures exposed the patients to a higher operative risk than those receiving eCEA alone [1]. The inclusion of data about the degree of ECA stenosis or type of eCEA in our overview was not possible, as these data were missing from a substantial majority of the analyzed studies.
The majority of authors emphasized a careful selection of patients for eCEA.
Although some previous series included asymptomatic patients, it is evident that a clear indication for the procedure in asymptomatic individuals does not yet exist. In addition, it was suggested that there was no benefit of eCEA in patients with ICA occlusion and without further recurrent neurological or ocular symptoms due to the development of sufficient collateral pathways [1]. This suggestion was based on the finding that some patients did not have recurrent symptoms after eCEA despite ECA restenosis or occlusion [1]. Thus, only recurrent events, especially amaurosis fugax and hemispheric TIA may justify the indication for surgery. In the set of Fokkema et al [1], 77.8% of the patients indicated for eCEA experienced recurrent events. Nevertheless, no direct comparison between patients with a single event and those with recurrent events who have undergone eCEA is available. The ‘non-lateralizing’ symptoms often given in relation to carotid stenosis in previous studies are now considered as unrelated.Duplex ultrasound (or CTA/ MRA) degree of ECA/ CCA stenosis indicated for eCEA is poorly documented. In the available studies, the authors included patients with stenosis ≥ 50% or unstable lesion, i.e., ulceration or thrombus in carotid bifurcation including ICA stump [1]. This means that the indication for eCEA was based on the same approach as the indication for internal CEA.
We suggest that patients with ICA occlusion and severe ECA stenoses should undergo CTA for an exclusion of aortic arch pathology and stenoses of CCA origin, as well as transoesophageal echocardiography and Holter ECG monitoring to exclude major cardiac sources of embolization before indication for eCEA.
Embolization and hypoperfusion are considered the main causes of cerebral or ocular symptoms. An assessment of intracranial collateral patterns using transcranial duplex imaging is very important for eCEA indication in those patients with significant ECA stenosis and presumed cerebral hypoperfusion. Conversely, unstable ulcerative lesions in carotid bifurcation with a high risk of embolization can be indicated for surgery without the evaluation of intracranial collateral patterns.
Some authors prefer contralateral CEA in the case of significant contralateral ICA stenosis in patients with symptomatic ICA occlusion and concomitant ECA/ CCA stenosis for a documented reduction of symptoms and recurrent events [1]. The assessment of collateral flow must precede the indication of contralateral CEA. It is generally accepted that this surgery might improve insufficient collateral flow with hypoperfusion on the side of the occluded ICA.
A specific treatment management should be applied in each individual patient. In the case of plaque with high risk of embolization localized in the carotid bifurcation on the side of ICA occlusion and contralateral significant ICA stenosis, eCEA should be considered as the first step in preventing recurrent ischaemic stroke due to hypoperfusion in the territory of the occluded ICA. Recently, selective arterial spin-labelled perfusion MRI was introduced as a method to assess the contribution of the individual arteries to the perfusion of the brain [1,11]. This method may help better select those patients with presumed hypoperfusion mechanism of cerebral symptoms for eCEA or contralateral CEA.
Last but not least, general conditions such as quality of life, life expectancy and risk of surgery must be considered before eCEA. Patients with a short life expectancy or patients with a high risk for surgery should not be candidates for the procedure.
We should consider that the above mentioned benefit of eCEA is based on case series studies with retrospective design, great heterogeneity and small sample sizes. No relevant study has been performed to date. However, the Society for Vascular Surgery guidelines from 2011 [12] recommended eCEA in symptomatic patients with ICA occlusion (Grade 1, Level of Evidence C); the current guidelines of the European Society for Vascular Surgery [13] provide no recommendation. We have found only two relevant papers since 2012, probably due to the low number of treated patients and low awareness about eCEA worldwide.
Conclusion
Despite numerous previous case series reports with the potential benefit of eCEA in selected symptomatic patients with a chronic ipsilateral ICA occlusion and ECA stenosis, we have still no sufficient evidence favouring eCEA based on randomized or reliable studies. The findings of previous studies are limited due to their insufficient methodology, thus the indication and benefit of eCEA remain unclear.
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.
The Editorial Board declares that the manuscript met the ICMJE “uniform requirements” for biomedical papers.
Přijato k recenzi: 10. 4. 2019
Přijato do tisku: 29. 4. 2019
doc. MUDr. Daniel Šaňák, Ph.D., FESO
Komplexní cerebrovaskulární centrum
Neurologická klinika LF UP
a FN Olomouc
I. P. Pavlova 6
775 20 Olomouc
e-mail: daniel.sanak@fnol.cz
Sources
1. Fokkema M, Reichmann BL, den Hartog AG et al. Selective external endarterectomy in patients with ipsilateral symptomatic internal carotid artery occlusion. J Vasc Surg 2013; 58(1): 145–151. doi: 10.1016/ j.jvs. 2012.12.059.
2. Hrbac T, Benes V, Sirucek P et al. Safety and efficacy of surgical treatment of carotid stump syndrome: pilot study. Ann Vasc Surg 2012; 26(6): 797–801. doi: 10.1016/ j.avsg.2011.11.034.
3. Dulai M, Elsherif M, Tawfick W et al. Outcome following open and endovascular intervention for carotid stump syndrome. SAGE Open Med Case Rep 2018; 6 : 2050313X18779744. doi: 10.1177/ 2050313X18779744.
4. Lee JI, Jander S, Oberhuber A et al. Stroke in patients with occlusion of the internal carotid artery: options for treatment. Expert Rev Neurother 2014; 14(10): 1153–1167. doi: 10.1586/ 14737175.2014.955477.
5. Grubb RL Jr, Powers WJ. Risks of stroke and current indications for cerebral revascularization in patients with carotid occlusion. Neurosurg Clin N Am 2001; 12(3):
473–487.6. Klijn CJ, van Buren PA, Kappelle LJ et al. Outcome in patients with symptomatic occlusion of the internal carotid artery. Eur J Vasc Endovasc Surg 2000; 19(6):
579–586. doi: 10.1053/ ejvs.2000.1129.7. Fields WS, Bruetman ME, Weibel J. Collateral circulation of the brain. Monogr Surg Sci 1965; 2(3): 183–259.
8. Fearn SJ, Picton AJ, Martimer AJ et al. The contribution of the external carotid artery to cerebral perfusion in carotid disease. J Vasc Surg 2000; 31(5): 989–993. doi: 10.1067/ mva.2000.104598
9. Zarins CK, Del Beccaro EJ, Johns L et al. Increased cerebral blood flow after external carotid artery revascularization. Surgery 1981; 89(6): 730–734.
10. Welling RE, Cranley JJ, Krause RJ et al. Surgical therapy for recent total occlusion of the internal carotid artery. J Vasc Surg 1984; 1(1): 57–61.
11. Dang Y, Wu B, Sun Y et al. Quantitative assessment of external carotid artery territory supply with modified vessel-encoded arterial spin-labeling. AJNR Am J Neuroradiol 2012; 33(7): 1380–1386. doi: 10.3174/ ajnr.A2978.
12. Ricotta JJ, Aburahma A, Ascher E et al. Updated Society for Vascular Surgery guidelines for management of extracranial carotid disease. J Vacs Surg 2011; 54(3):
832–836. doi: 10.1016/ j.jvs.2011.07.004.13. Naylor AR, Ricco JB, de Borst GJ et al. Editor’s choice – management of atherosclerotic carotid and vertebral artery disease: 2017 clinical practice guidelines of the European Society for Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg 2018; 55(1): 3–81. doi: 10.1016/ j.ejvs.2017.06.021
Labels
Paediatric neurology Neurosurgery Neurology
Article was published inCzech and Slovak Neurology and Neurosurgery

2019 Issue 3-
All articles in this issue
- Neuromuscular diseases and pregnancy
- Are late complications of Parkinson’s disease really late? YES
- Are late complications of Parkinson’s disease really late? NO
- Are late complications of Parkinson’s disease really late? COMMENT
- Obstructive sleep apnea and cerebral blood flow
- Brief analysis of the frequency of use and spectrum of animal models in stroke research
- Factors affecting the school life of children with epilepsy
- Circadian system disturbances in Huntington’s disease – implications for light therapy
- Experiences with an electrophysiological diagnosis of occupational ulnar nerve lesions at elbow
- Coin in the Hand Test for detection of malingering memory impairment in comparison with mild cognitive impairment and mild dementia in Alzheimer‘s disease
- Neuropathic pain component in patients with myotonic dystrophy type 2 – a pilot study
- Can endarterectomy of the external carotid artery be beneficial? A critical overview
- Inpatient multidisciplinary rehabilitation programme for postural and gait stability in Huntington’s disease – a pilot study
- Optical coherence tomography measurements of the optic nerve head and retina in newly diagnosed idiopathic intracranial hypertension without loss of vision
- Equivalence of Montreal Cognitive Assessment alternate forms
- Frameless and fiducial-less method for deep brain stimulation
- Effect of vacuum-compression therapy for carpal tunnel syndrome as a part of physiotherapy – pilot study
- Anterior choroidal artery aneurysm
- Czech and Slovak Neurology and Neurosurgery
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Coin in the Hand Test for detection of malingering memory impairment in comparison with mild cognitive impairment and mild dementia in Alzheimer‘s disease
- Neuromuscular diseases and pregnancy
- Optical coherence tomography measurements of the optic nerve head and retina in newly diagnosed idiopathic intracranial hypertension without loss of vision
- Effect of vacuum-compression therapy for carpal tunnel syndrome as a part of physiotherapy – pilot study
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

