-
Medical journals
- Career
Cognitive Effects of Long-term Treatment with Waking-hour Subcutaneous Apomorphine Infusions in Patients with Advanced Parkinson’s Disease
Authors: R. Kubíkova 1,2; M. Tyrlík 3; I. Rektorová 1,2
Authors‘ workplace: Masaryk University, Brno Movement Disorders Centre, First Department of Neurology, St. Anne’s Hospital, Brno 1; Masaryk University, Brno Research Group of Applied Neurosciences, CEITEC (Central European Institute of Technology) 2; Masaryk University, Brno Faculty of Social Study 3
Published in: Cesk Slov Neurol N 2011; 74/107(4): 463-466
Category: Short Communication
Overview
Aims:
To determine whether long-term treatment with continuous subcutaneous apomorphine infusions (CSAI) is safe for patients with Parkinson’s disease (PD) with a history of hallucinations and/or marked cognitive deficit. Methods: 12 PD patients (9 men, 3 women; age 71.8 ± 6 years) were given neuropsychological assessment prior to continuous administration of CSAI and fourteen months after it. Results: CSAI led to clinical improvement, at least minimal, in all subjects. However, the treatment led to statistically significant impairment in verbal fluency tasks, the Mattis dementia rating scale (MDRS) score and the attention and initiation subtests. No mood changes were detected. Conclusions: We observed significant impairment in “frontal-like” tasks after continuous treatment with CSAI in our PD patients with baseline cognitive impairment. Further longitudinal controlled studies are needed to assess the impact of both CSAI and PD progression. We suggest cautious selection of patients eligible for CSAI therapy with respect to their cognitive profiles and/or neuropsychiatric complications.Key words:
apomorphine – cognitive – affective – Parkinson’s disease – dementiaIntroduction
Apomorphine is a dopamine agonist with a high affinity for D1 and D2 dopaminergic receptors, activating both the direct and indirect pathways. Long-term treatment with CSAI has been shown to reduce levodopa dosage, reduce time spent ‘off’, and improve dyskinesias in fluctuating parkinsonian patients [1]. Apomorphine may be considered a less invasive alternative to pallidotomy or bilateral subthalamic deep brain stimulation (DBS) in controlling levodopa-induced dyskynesias [2,3]. Only a few studies have focused on neuropsychological and neuropsychiatric changes arising out of CSAI treatment. Ruzicka et al [4] reported impairment of visuospatial perception and cognitive slowing after a single dose of apomorphine. With respect to long-term treatment with CSAI, Alegret et al [2] compared the cognitive effects of bilateral subthalamic DBS and CSAI treatment at six-month and one-year follow-ups. The CSAI group did not show any significant change against baseline in neuropsychological evaluation. De Gaspari et al [3] evaluated 13 PD patients on CSAI and 12 PD patients after DBS of the subthalamic nucleus at baseline and after 12 months. Like the Alegret group, [2], they found that CSAI did not affect cognitive or neuropsychiatric functions. In one study, long-term CSAI treatment was shown to improve mood [5]. All these studies included non--demented patients, with no history of hallucinations, who were also eligible for DBS surgery for PD.
The prevalence of dementia in PD is 30% and the approximate 10-year cumulative prevalence is 75%, with an incidence increasing to 4 to 6 times higher than the general age-matched population. In terms of cognitive deficits, those of memory, executive and constructional functions, attention, and visuospatial function are common in PD dementia (PD-D) [6].
The objective of our study was to assess whether long-term use of CSAI might constitute a safe treatment alternative with respect to cognitive function in patients with a history of hallucinations, pronounced cognitive deficit, and/or dementia, i.e. in patients who are not eligible for PD surgery. The possible effects of CSAI on symptoms of depression were studied at the same time.
Patients and Methods
We studied 12 patients with advanced PD and motor complications refractory to oral medication. Nine of them had a history of hallucinations, and all exhibited marked cognitive deficit (MDRS <136) [7]. For demographic data, see Table 1. All patients had completed at least eight years of education. All of them were taking oral dopaminergic medication (L-dopa ± entacapone and/or dopamine receptor agonist).
1. Clinical and demographic variables. 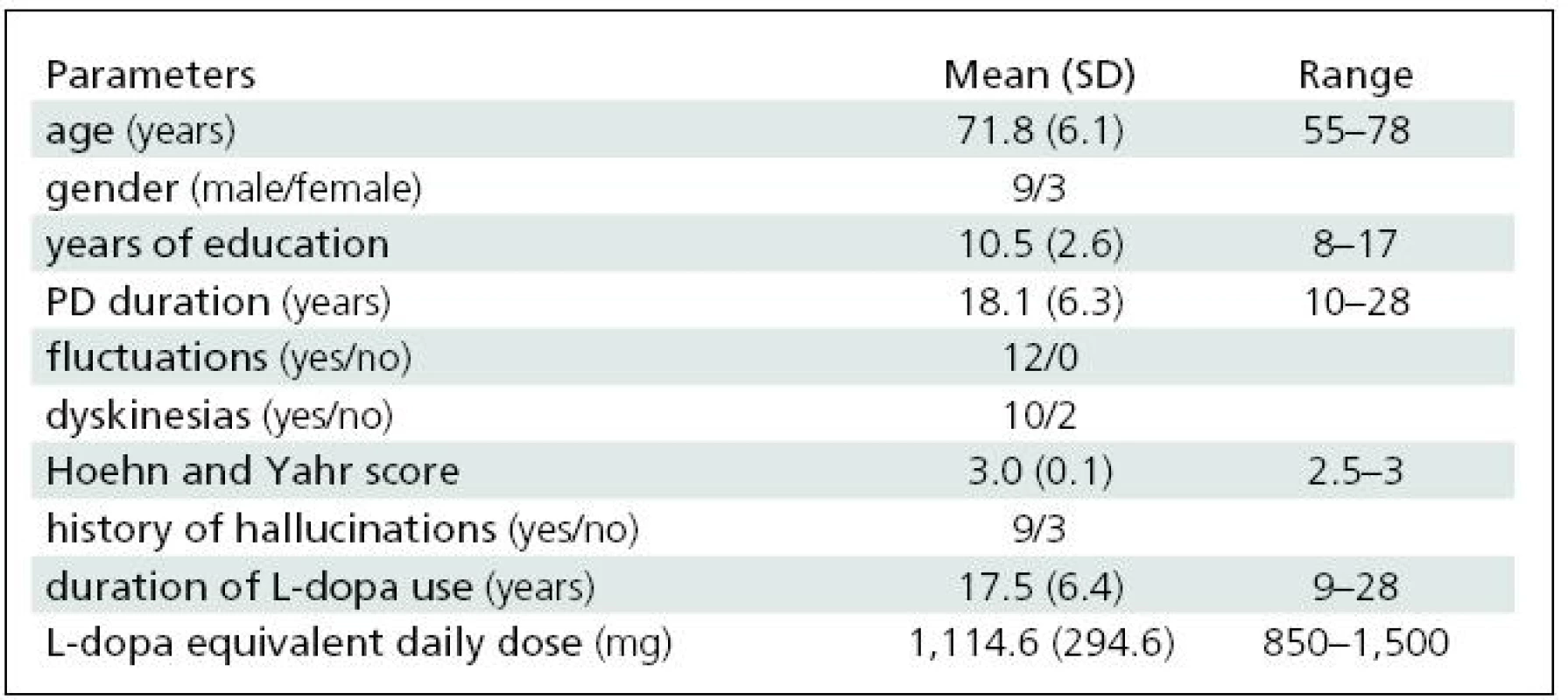
Patients were given a comprehensive neuropsychological assessment in the ‘on’ motor state prior to (V1) and 14 months after (V2) long-term administration of CSAI. Apomorphine was administered during the waking hours (12–16 hours per day) using a small portable pump. Neuropsychological assessment included MDRS, which consists of 5 subscales: attention, initiation, construction, conceptualisation, and memory [7]. In addition, working memory, executive functions and verbal production were also assessed by lexical and semantic verbal fluency tasks; inhibition of habitual responses was measured by Stroop test; and memory was also tested by the word list test (WLT) from the Wechsler memory scale (WMS III – revised) [7].
Symptoms of depression were evaluated by the Montgomery and Asberg depression rating scale (MADRS) [7]. Clinical global impression of change (CGIC) was rated on a scale of 7 points: 1 = very much improved, 2 = much improved, 3 = minimally improved, 4 = no change, 5 = minimally worse, 6 = much worse, 7 = very much worse [7]. Informed consent to participate in the study was obtained from all patients. The study was approved by the local ethics committee.
Statistical analyses were carried out using SPSS-PC v.13.0. The Wilcoxon matched pairs test was used to evaluate differences between V1 and V2. Bonferroni correction was employed to study executive functions (6 multiple comparisons) and posterior cognitive impairment (memory and construction; 5 multiple comparisons). The 12-month approximate percentage change in the respective cognitive tests was calculated from the median test scores at visits 1 and 2.
Results
The daily apomorphine dose was 39.6 ±11.8 mg (range 20–60 mg/day). The L-dopa equivalent daily dose was reduced by 28% (see also Table 1). All of the patients completed the study. No major local reactions at the injection sites were observed. In self-evaluation CGIC ratings, all of the patients claimed slight or clear improvement; no patient reported worsening. Evaluation by the examiner produced similar results. Nine of twelve patients had mild to moderate visual hallucinations, already reported in the medical history prior to study commencement. Reduction of L-dopa and/or the apomorphine dose led to improvement or disappearance of this symptom in all subjects.
We found statistically significant changes (decline) in the following tests between V1 and V2: verbal fluency (semantic and lexical); recognition of verbal material; MDRS total score and its initiation and attention subtests. After Bonferroni correction, only the changes in verbal fluency tasks, MDRS total score and its initiation and attention subtests remained significant. The approximate 12-month percentage change in the total MDRS score was 24% from visit 1. MDRS attention and initiation subtests disclosed changes of 33% and 15.1% respectively, and the semantic and lexical verbal fluency tasks disclosed changes of 54.1% and 51.4 % respectively. We found no mood changes as assessed by MADRS (see Table 2).
2. Neuropsychological test results. 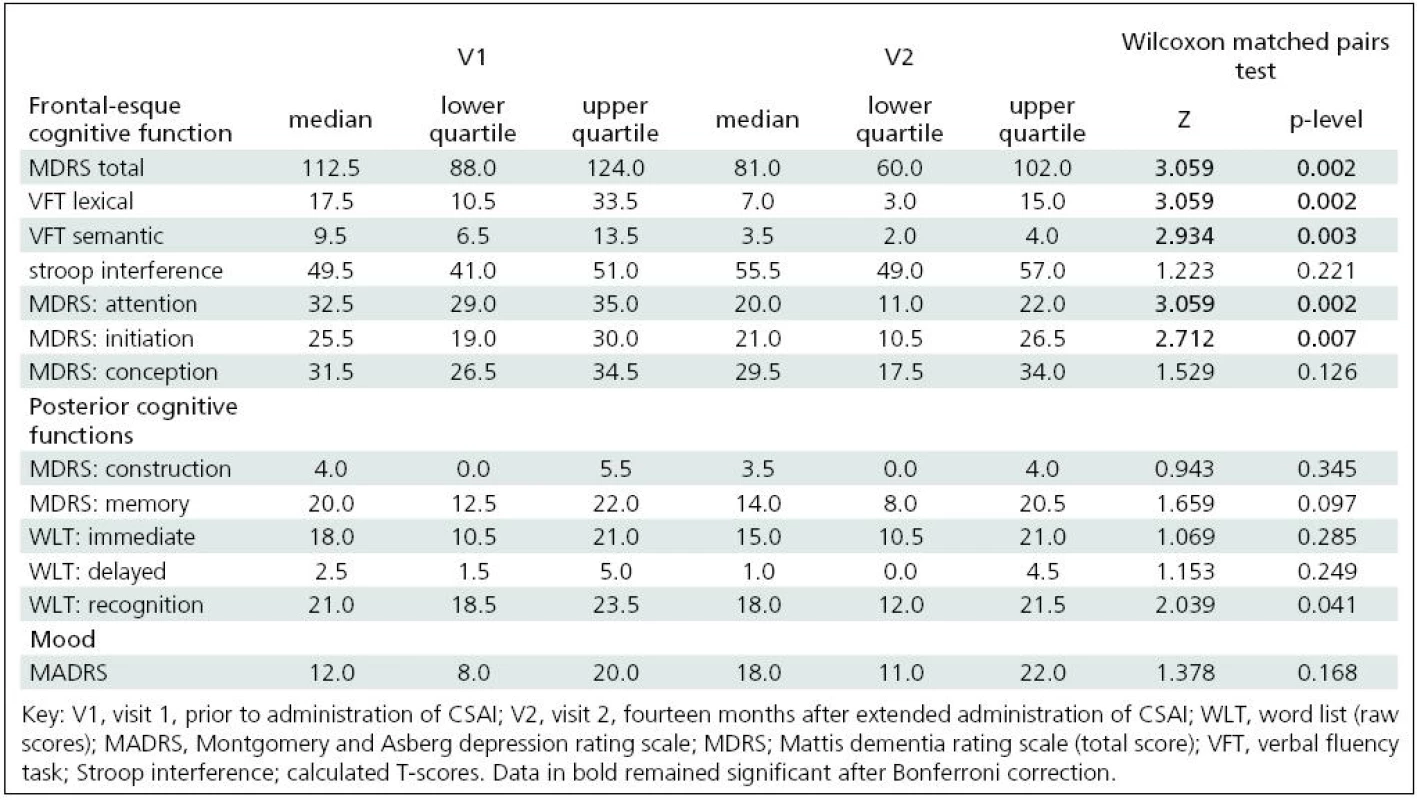
Discussion
This is the first long-term study to evaluate cognitive effects in advanced PD patients with prominent cognitive deficits treated with CSAI for an extended time period. In contrast with existing published studies on the non-demented PD population [1–3,5], we found a significant decline in verbal fluency, MDRS total score and its initiation and attention subscores. In PD dementia, both dysexecutive syndrome and memory functions (including free recall, cued recall, and recognition) are compromised [6]. The contribution of dopamine to cognitive impairment is still a matter of debate. In this respect, the impairment in executive and verbal fluency tasks found in accidentally 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPT--P)-induced parkinsonism in humans may be considered as a pure dopamine deficiency [8]. The results of studies which specifically examined the effect of dopamine agonists on cognition [9] are consistent with the view that insufficient, as well as excessive, dopamine transmission in the prefrontal cortex may impair working memory and executive functions. Conversely, Huber et al [10] found that memory performance was not influenced by the absolute level of dopamine, either high or low, in spite of a significantly slower memory acquisition rate when dopamine levels were low. In view of these findings, our results point rather to a possibly negative role of long-term dopaminergic treatment with CSAI in attention and executive functions in PD.
However, the cholinergic system has also been implicated in cognitive dysfunction in PD and results of recent studies have shown that rivastigmine (an acetylcholinesterase inhibitor) promoted a significant cognitive improvement as compared to placebo in a PD-D population [6]. Furthermore, other neurotransmitter systems, such as the serotoninergic or noradrenergic pathways, display projections to the prefrontal cortex and could also be involved [6]. Therefore, both long--term dopaminergic treatment with CSAI and the effect of progression of PD are brought into question. Unfortunately, the study design (an open label study) does not enable us to determine precisely the specific cause of cognitive impairment observed in our patients.
To the best of our knowledge, no longitudinal data has been made available for MDRS score change in PD-D. According to a large longitudinal study in a PD population [11], the approximate annual percentage change in MMSE was 13.9% in patients who developed dementia by the first follow-up visit. In our study, the approximate 12-month percentage change in the verbal fluency tasks and the MDRS attention subscore was at least twice as high when compared with the above-reported data. However, direct comparison of the two studies is not possible and any differences must be interpreted with great caution. Unlike MMSE, MDRS is a sensitive tool for the measurement of frontal--like cognitive deficit [7]. Furthermore, MMSE does not include evaluation of verbal fluency, and the progression of dementia in PD is not linear [6]. Despite the drawbacks mentioned, we consider that the cognitive decline in our patient group was severe and that long-term treatment with CSAI could have contributed to its magnitude.
Conclusions
Long-term treatment with waking-hour CSAI led to at least minimal clinical motor improvement in all subjects. We observed no mood changes, but there were significant impairments, particularly in frontal--like executive functions. Further controlled studies are required to explore what is behind our results. To the best of our knowledge, this is the first study to focus on the cognitive effects of long-term treatment with CSAI in advanced PD patients with marked baseline cognitive impairment or dementia. The rate of cognitive decline was alarming and leads us to suggest both cautious selection of patients eligible for CSAI therapy, and close monitoring of cognitive profiles in all PD subjects with established cognitive deficits and/or neuropsychiatric complications who are given CSAI.
This study was supported by Research Plan of the Ministry of Education of the Czech Republic ref. no. MSM 0021622404.
Assoc. Prof. MUDr. Irena Rektorova, PhD.
First Department of Neurology
Masaryk University, St. Anne’s Hospital
Pekarska 53
656 91 Brno
e-mail: irena.rektorova@fnusa.czAccepted for review: 30. 7. 2010
Accepted for publication: 14. 2. 2011
Sources
1. Clarke CE, Worth P, Grosset D, Stewart D. Systematic review of apomorphine infusion, levodopa infusion and deep brain stimulation in advanced Parkinson’s disease. Parkinsonism Relat Disord 2009; 15(10): 728–741.
2. Alegret M, Valldeoriola F, Martí M, Pilleri M, Junqué C, Rumia J et al. Comparative cognitive effects of bilateral subthalamic stimulation and subcutaneous sontinuous infusion of apomorphine in Parkinson’s disease. Mov Disord 2004; 19(12): 1463–1469.
3. De Gaspari D, Siri C, Landi A, Cilia R, Bonneti A, Natuzzi F et al. Clinical and neuropsychological follow up at 12 months in patients with complicated Parkinson’s disease treated with subcutaneous apomorphine infusion or deep brain stimulation of the subthalamic nucleus. J Neurol Neurosurg Psychiatry 2006; 77(4): 450–453.
4. Růzicka E, Roth J, Spacková N, Mecír P, Jech R. Apomorphine induced cognitive changes in Parkinson’s disease. J Neurol Neurosurg Psychiatry 1994; 57(8): 998–1001.
5. Morgante L, Basile G, Epifanio A, Spina E, Antonini A, Stocchi F et al. Continuous apomorphine infusion (CAI) and neuropsychiatric disorders in patients with advanced Parkinson’s disease: a follow-up of two years. Arch Gerontol Geriatr Suppl 2004; 9 : 291–296.
6. Rektorová I. Neurodegenerativní demence. Cesk Slov Neurol N 2009; 72/105(2): 97–109.
7. Burns A, Lawlor B, Craig S (eds). Assessment scales in old age psychiatry, 2nd ed. London: Martin Dunitz, Taylor and Francis Group 2004.
8. Stern Y, Tetrud JW, Martin WR, Kutner SJ, Langston JW. Cognitive change following MPTP exposure. Neurology 1990; 40(2): 261–264.
9. Rektorová I. Effects of dopamine agonists on neuropsychiatric symptoms of Parkinson’s disease. Neurodegener Dis 2010; 7(1–3): 206–209.
10. Huber SJ, Shulman HG, Paulson GW, Shuttleworth EC. Dose dependent memory impairment in Parkinson’s disease. Neurology 1989; 39(3): 438–440.
11. Aarsland, D, Kjeld, A, Larsen, JP, Perry R, Tore WL, Lolk A et al. The rate of cognitive decline in Parkinson’s disease. Arch Neurol 2004; 61(12): 1906–1911.
Labels
Paediatric neurology Neurosurgery Neurology
Article was published inCzech and Slovak Neurology and Neurosurgery
2011 Issue 4-
All articles in this issue
- Neural Components of Motivational and Decision-Making Processes in the Human Brain
- Histopathological Diagnosis of Mitochondrial Myopathies – Indications and the Utility of Muscle Biopsy
- Apolipoprotein E Genotype as an Independent Prognostic Factor for Clinical Outcome in Traumatic Brain Injury in Children and the Adolescent Population
- The Incidence of Mutation on the Leucine-rich Repeat Kinase 2 Gene in Patients with Parkinson’s Disease in Slovakia
- Glatiramer Acetate (Copaxone®) in the Treatment of Relapsing/Remitting Cerebrospinal Multiple Sclerosis – Clinical Efficacy and Safety Profile
- Delayed Acute Subdural Hematoma
- Eyelid Implants in Lagophtalmus Therapy
- Congenital Myotonia Caused by Mutations in the CIC-1 Chloride Channel Gene
- Repeated Intradetrusor Application of Botulinum Toxin A in Children with Detrusor Overactivity
- Radiculopathy Caused by Lumbar Epidural Venous Varices – a Case Report
- Facial Canal Dehiscence and Prolapse of the Facial Nerve – Case Reports
- Spinal Complications in Genetic Syndromes – Case Reports
- Neurological Complications of Herpes Zoster – a Case Report
- Attention Deficit/Hyperactivity Disorder
- Cognitive Effects of Long-term Treatment with Waking-hour Subcutaneous Apomorphine Infusions in Patients with Advanced Parkinson’s Disease
- Czech and Slovak Neurology and Neurosurgery
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Attention Deficit/Hyperactivity Disorder
- Delayed Acute Subdural Hematoma
- Neurological Complications of Herpes Zoster – a Case Report
- Congenital Myotonia Caused by Mutations in the CIC-1 Chloride Channel Gene
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

