-
Medical journals
- Career
NKT-like Cells are Expanded in Solid Tumour Patients
Authors: Zdrazilova-Dubska L. 1–3; D. Valík 1,2; E. Budinská 2,4; T. Frgala 6; L. Bacikova 1; R. Demlová 2,3,5
Authors‘ workplace: Department of Laboratory Medicine, Masaryk Memorial Cancer Institute, Brno, Czech Republic 1; Regional Centre for Applied Molecular Oncology, Masaryk Memorial Cancer Institute, Brno, Czech Republic 2; Department of Pharmacology – ACIU, Medical Faculty, Masaryk University, Brno, Czech Republic 3; Institute of Biostatistics and Analyses, Masaryk University, Brno, Czech Republic 4; Department of Clinical Evaluation, Masaryk Memorial Cancer Institute Brno, Czech Republic 5; Unica, Institute for Reproductive Medicine, Brno, Czech Republic 6
Published in: Klin Onkol 2012; 25(Supplementum 2): 21-25
Práce byla podpořena Evropským fondem pro regionální rozvoj a státním rozpočtem České republiky (OP VaVpI – RECAMO, CZ.1.05/2.1.00/03.0101) a projekty Velkých infrastruktur MŠMT LM2011017.
Autoři deklarují, že v souvislosti s předmětem studie nemají žádné komerční zájmy.
Redakční rada potvrzuje, že rukopis práce splnil ICMJE kritéria pro publikace zasílané do bi omedicínských časopisů.
Obdrženo: 12. 10. 2012
Přijato: 7. 11. 2012Overview
CD3+ CD56+ NKT-like cells have been shown to produce substantial amounts of pro-inflammatory cytokines and to mediate lysis of malignant cells. Using flow cytometry, we evaluated the absolute NKT-like cell count in peripheral blood from individuals in a reference population and the median number was 0.085 × 109/l. The average number of NKT-like cells in patients with disseminated cancer was 2.65 fold higher than in the reference population. The number of CD3+ CD56+ cells in solid tumour patients who achieved complete remission was comparable to the reference population. In breast cancer patients with initially (prior to therapy) increased number of NKT-like cells, we observed a trend toward longer disease-free survival. Thus we conclude that CD3+ CD56+ NKT-like cells have potential to suppress tumour evasion and are expanded in peripheral blood of some epithelial tumour patients.
Key words:
NKT-like cells – cancer – CD3+ CD56+ cells – flow cytometry – breast neoplasmsIntroduction
Natural killer T (NKT) cells are unusual lymphocytes coexpressing some NK markers and possessing self-reactivity and capacity to secrete large quantities of cytokines, such as IFN-γ in mice [1,2]. NKT cells represent a heterogeneous group that consists of Type I cells (Classical NKT cells), Type II cells (Non-classical NKT cells) and NKT-like cells (CD1d-in-dependent NK1.1+ T cells) reviewed in [3]. CD3+CD56+ NKT-like cells represent a minor population in peripheral blood [4]. These cells have been shown to mediate lysis of malignant cells and to produce substantial amounts of cytokines [5–8].
We evaluated the NKT-like cell absolute count in our regional reference population and in cancer patients. We aimed to investigate whether an initially increased NKT-like cell count in breast cancer patients favours disease-free survival.
Material and Methods
Patients and Controls
NKT-like cells were analysed in cancer patients, hospital staff and clients of preventive care clinic of Masaryk Memorial Cancer Institute. Evaluated individuals signed the informed consent. The reference/common population consisted of cancer-free controls: 56 individuals from the hospital staff and 41 clients of preventive care. Cancer patients: the group of disseminated cancers consisted of 20 cases of metastatic breast tumours, 16 metastatic colorectal cancers, 14 disseminated kidney tumours, 9 cases of generalised malignant melanoma, 5 pancreatic cancers, 3 cases of metastatic prostate cancer, 3 disseminated testicular tumours, 1 case of metastatic hepatocellular carcinoma and 1 case of penile cancer. The group of patients in remission consisted of 16 cases of breast tumours, 6 colorectal cancers, 3 kidney tumours, 13 cases of malignant melanoma and 3 testicular tumours. Breast cancer evaluation: 31 patients diagnosed for breast cancer between March 2004 and September 2005 were evaluated for NKT-like cell count prior to treatment. The group consisted of patients with various stage of disease; 11 of them with distant metastases. The follow-up for disease progression was performed in August 2012.
Immunophenotype Analyses
Peripheral blood was collected into EDTA test tubes (Sarstedt) and processed within 4 hours after blood withdrawal. Complete blood count with white blood cell differential was measured using Sysmex XE 5000 hematologic analyser. Cell staining for flow cytometry was performed with 50 µl of full blood with the following monoclonal antibodies: CD3-FITC (clone UCHT1, 10 µl), CD8-PE (B9.11, 5 µl), CD56-PC5 (N901, 10 µl), CD4-PC7 (SFCI12T4D11, 5 µl), purchased from Beckman Coulter. After a 15 min incubation in the dark at the room temperature, red blood cells were lysed with 600 µl of VersaLyse (Beckman Coulter) for 15 min and flow cytometric analysis was performed immediately using FC500 instrument (Beckman Coulter). Lymphocytes were gated based on SS/FS properties and the correction was performed using CD3 measured in another test tube together with CD45. NKT--like cells were assessed as CD3+ CD56+ cells and their absolute count was calculated using the number of lymphocytes measured by hematologic analyser.
Statistical Analyses
Survival differences between NKT-like low and NKT-like high population of patients were compared using log-rank test. NKT-like low group consisted of breast cancer patients with NKT-like cell count lower than 25th percentile of the normal population count, while NKT-like high group of the population higher than 75th percentile of common population.
Differences of NKT-like cell counts between two populations were tested using two-sided Mann-Whitney two sample tests. In case of multiple hypotheses testing, p-values were adjusted by Benjamini-Hochberg correction and significance level was set to 10% correlation between age and NKT-like cell count was calculated using Spearman’s rank coefficient.
Results
NKT-like Cells in Reference/Common Population
We enumerated the number of NKT-like cells in peripheral blood of 97 individuals from the reference/common population. The group consisted of 70 women and 27 men. We did not observe statistically significant difference in the CD3+ CD56+ count between men and women (p = 0.089) and the NKT-like cell number was age-independent (Fig. 1A, p = 0.6377). The median number of NKT-like cells in the reference population was 0.085 × 109/l (Fig. 1B).
Fig. 1. NKT-like cells in common population. A. Correlation between age and NKT-like cells number. Each measurement is shown as an open circle. The line represents linear flowline, R is correlation coefficient, p is statistical significance. B. Histogram showing the distribution of NKT-like cell counts. Values for 75<sup>th</sup>, 50<sup>th</sup> and 25<sup>th</sup> percentile are shown. 
NKT-like Cells in Cancer Patients
The number of NKT-like cells was measured in patients with disseminated cancer and the disease in remission. The average number of NKT-like cells in patients with disseminated cancer was 2.65 fold higher than in the reference population (Fig. 2A, p = 0.00003365). The average number of NKT-like cells in the remission group was on the other hand 1.25 fold lower compared to the common population (p = 0.00005043). Within the metastatic group, a non-significantly increased number of NKT-like cells was observed in colorectal carcinomas compared to malignant melanoma (FDR = 0.0722) and to testicular tumours (FDR = 0.0722) (Fig. 2B). When comparing disseminated and remission group within one diagnosis, significantly higher NKT-like count was observed in disseminated tumours in all diagnoses, except testicular cancer (FDR of 0.0499; 0.00027; 0.0452 and 0.0452 for breast, colorectal, melanoma and kidney cancer, respectively, Fig. 2B).
Fig. 2. Distribution of NKT-like cells in cancer patients. Box and whisker plots represent median (horizontal bar), interquartile range (box), 1.5-times interquartile range (whiskers) and outliers (points). For the purpose of readability, the y-axes represent a zoom from the whole scale range (0–1.9133). Figures in original scale are available in supplementary material. A. Comparison of NKT-like cell number in common population, cancer patients with metastatic disease and in cancer patients with disease in remission. B. Differences of NKT-like cells counts between remission and metastatic disease in various cancer diagnoses. 
NKT-like Cell in Breast Cancer Patients
The number of NKT-like cells was evaluated in 31 patients with breast cancer between diagnosis and treatment. Subsequently, the follow-up for disease--free survival was performed. During the 7-year follow-up, 16 patients achieved complete remission of the disease and in 15 cases the disease progressed (Tab. 1). There was a trend towards increased frequency of progression in the NKT-like low group of patients, but it was not significant (Fig. 3).
Fig. 3. Disease-free survival Kaplan-Meier curves in NKT-like low and NKT-like high breast cancer patients. NKT-like low group consists of patients with NKT-like cell number lower that 25<sup>th</sup> percentile of common population and NKT-like high group represents breast cancer patients with NKT-like above 75<sup>th</sup> percentile of common population. 
1. Tab. 1. Summary of tumour and clinical information and number of NKT-like cells in breast cancer patients. 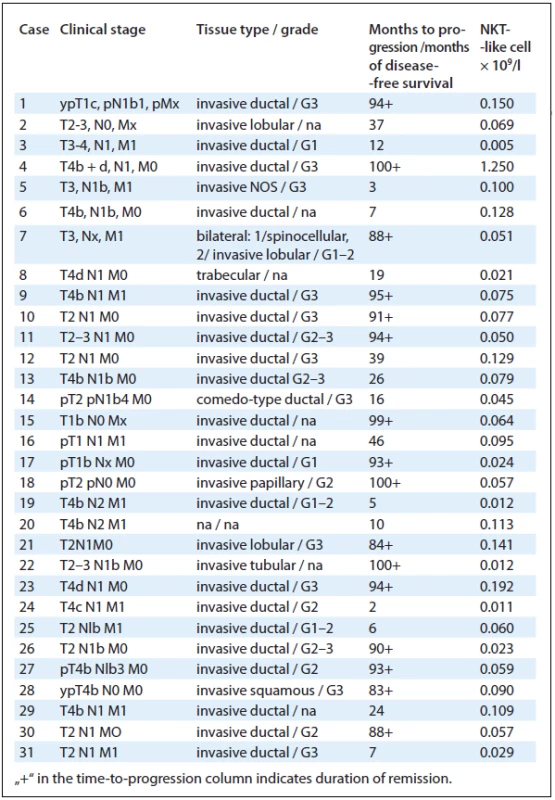
Discussion
NKT-like cells represent a subset of T-lymphocytes expressing some natural killer cell receptors. These cells are considered to be associated with effector-memory and effector T-lymphocyte subpopulations and thus their count to be increased with age [9]. In our study we have not observed age-dependent change in NKT-like cell counts, however this discrepancy might be attributed to the lack of elderly individuals and centenarians in our study population.
The expansion of CD3+CD56+ NKT-like cells in cancer patients with active disease but not with disease in remission may reflect the presence of the active malignancy leading to stimulation of the cytotoxic arm of the immune response including NKT-like cells. This finding is in line with the observation that CD8+ NKT-like cells were expanded in tumour-bearing C57BL/6 mice [10]. On the other hand, we did not observe a correlation between CEA or CA15-3 tumour marker levels and the NKT-like cell count (data not shown) and within the group of breast patients prior to anti-cancer therapy there was no difference in NKT-like cells count in initially metastatic vs localised disease.
Concerning the immunophenotype, the majority of NKT-like cells in our study were CD16 - and expression of CD8 predominated CD4 expression (data not shown). CD8+ NKT-like cells have been shown to produce large amounts of IL-10 and IFN-γ [11] and to lack the production of IL-4 [12]. Anti-tumour activity of NKT-like cell could be mediated by IFN-γ without IL-4 resulting into pro-inflammatory TH1 immune response. Anti-tumour activity of NKT-like cells could be further attributed to several compounds upregulated by NKT-like cells and involved in cytotoxicity, such as perforin, granzymes and TNF family proteins [11]. It was shown that NKT-like cells are an important source of pro-inflammatory cytokines, IFN-γ, TNF-α, IL-2 and IL-17, and granzymes and thus this cell subpopulation might be involved in lung transplant pathology [13]. Our preliminary data from disease-free survival in NKT-like-high breast cancer patients and the pro-inflammatory cytokine production suggest that NKT-like cells suppress solid tumour growth. Similarly, a protective role of NKT-like cells has been described for chronic lymphocytic leukaemia [14].
Focusing on various tumour origin, increased count of NKT-like cells have been observed in breast, colorectal and kidney tumour cases, but not in testicular tumours and malignant melanoma, suggesting that NKT-like cell expansion occurs predominantly in epithelial tumours. Malignant melanoma cells often express molecules suppressing anti-tumour activity of tumour infiltrating lymphocytes, e.g. galectin-3 and galectin-1 [15,16]. Other potent tools of melanoma cells to impair NKT-like cytotoxicity are expression of soluble MHC class I chain-related molecules [17] and expression of NKG2D ligands [18,19]. Absent stimulation of NKT-like cells in testicular tumour might be related to the fact that testes represent an immune privileged site and tumours arising from this tissue are often not accessible by immune cell response.
In conclusion, CD3+ CD56+ NKT-like cells with potential to suppress tumour evasion are expanded in peripheral blood of some epithelial tumour patients.
The study was supported by the European Regional Development Fund and the State Budget of the Czech Republic for RECAMO (Regional Centre for Applied Molecular Oncology; CZ.1.05/2.1.00/03.0101) and by Large Infrastructure Projects of Czech Ministry of Education, Youth and Sports LM2011017.
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.
The Editorial Board declares that the manuscript met the ICMJE “uniform requirements” for biomedical papers.
Lenka Zdrazilova-Dubska, RNDr., Ph.D.
Department of Laboratory Medicine
Masaryk Memorial Cancer Institute
Zluty kopec 7
656 53 Brno
Czech Republic
e-mail: dubska@mou.cz
Submitted: 12. 10. 2012
Accepted: 7. 11. 2012
Sources
1. Taniguchi M, Harada M, Kojo S et al. The regulatory role of Vα14 NKT cells in innate and acquired immune response. Annu Rev Immunol 2003; 21 : 483–513.
2. Smyth MJ, Godfrey DI. NKT cells and tumor immunity – a double-edged sword. Nature Immunol 2000; 1(16): 459–460.
3. Godfrey DI, MacDonald HR, Kronenberg M et al. NKT cells: what‘s in a name? Nat Rev Immunol 2004; 4(3): 231–237.
4. Satoh M, Seki S, Hashimoto W et al. Cytotoxic gammadelta or alphabeta T cells with a natural killer cell marker, CD56, induced from human peripheral blood lymphocytes by a combination of IL-12 and IL-2. J Immunol 1996; 157(9): 3886–3892.
5. Ortaldo JR, Winkler-Pickett RT, Yagita H et al. Comparative studies of CD3 - and CD3+ CD56+ cells: examination of morphology, functions, T cell receptor rearrangement, and pore-forming protein expression. Cell Immunol 1991; 136(2): 486–495.
6. Lu PH, Negrin RS. A novel population of expanded human CD3+CD56+ cells derived from T cells with potent in vivo antitumor activity in mice with severe combined immunodeficiency. J Immunol 1994; 153(4): 1687–1696.
7. Baxevanis CN, Gritzapis AD, Papamichail M. In vivo antitumor activity of NKT-like cells activated by the combination of IL-12 and IL-18. J Immunol 2003; 171(6): 2953–2959.
8. Baxevanis CN, Gritzapis AD, Tsitsilonis OE et al. HER-2//neu-derived peptide epitopes are also recognized by cytotoxic CD3(+)CD56(+) (natural killer T) lymphocytes. Int J Cancer 2002; 98(6): 864–872.
9. Peralbo E, Alonso C, Solana R. Invariant NKT and NKT-like lymphocytes: two different T cell subsets that are differentially affected by ageing. Exp Gerontol 2007; 42(8): 703–708.
10. Stremmel C, Exley M, Balk S et al. Characterization of the phenotype and function of CD8(+), alpha / beta(+) NKT cells from tumor-bearing mice that show a natural killer cell activity and lyse multiple tumor targets. Eur J Immunol 2001; 31(9): 2818–2828.
11. Zhou L, Wang H, Zhong X et al. The IL-10 and IFN-gamma pathways are essential to the potent immunosuppressive activity of cultured CD8+ NKT-like cells. Genome Biol 2008; 9(7): R119.
12. Lee H, Hong C, Shin J et al. The presence of CD8+ invariant NKT cells in mice. Exp Mol Med 2009; 41(12): 866–872.
13. Hodge G, Hodge S, Li-Liew C et al. Increased natural killer T-like cells are a major source of pro-inflammatory cytokines and granzymes in lung transplant recipients. Respirology 2012; 17(1): 155–163.
14. Jadidi-Niaragh F, Jeddi-Tehrani M, Ansaripour B et al. Reduced frequency of NKT-like cells in patients with progressive chronic lymphocytic leukemia. Med Oncol. Epub ahead of print 2012.
15. Nakahara S, Oka N, Raz A. On the role of galectin-3 in cancer apoptosis. Apoptosis 2005; 10(2): 267–275.
16. Zubieta MR, Furman D, Barrio M et al. Galectin-3 expression correlates with apoptosis of tumor-associated lymphocytes in human melanoma biopsies. Am J Pathol 2006; 168(5): 1666–1675.
17. Wang H, Yang D, Xu W et al. Tumor-derived soluble MICs impair CD3(+)CD56(+) NKT-like cell cytotoxicity in cancer patients. Immunol Lett 2008; 120(1–2): 65–71.
18. Oppenheim DE, Roberts SJ, Clarke SL et al. Sustained localized expression of ligand for the activating NKG2D receptor impairs natural cytotoxicity in vivo and reduces tumor immunosurveillance. Nat Immunol 2005; 6(9): 928–937.
19. Schwinn N, Vokhminova D, Sucker A et al. Interferon--gamma down-regulates NKG2D ligand expression and impairs the NKG2D-mediated cytolysis of MHC class I-deficient melanoma by natural killer cells. Int J Cancer 2009; 124(7): 1594–1604.
Labels
Paediatric clinical oncology Surgery Clinical oncology
Article was published inClinical Oncology

2012 Issue Supplementum 2-
All articles in this issue
- RECAMO – ...through Cancer Research towards Applied Molecular Oncology; Where, Why and How
- p63 – an Important Player in Epidermal and Tumour Development
- Detection of Cancer Stem Cell Markers in Sarcomas
- NKT-like Cells are Expanded in Solid Tumour Patients
- Cancer as a Metabolic Disease and Diabetes as a Cancer Risk?
- PThe Regulation of p53 Synthesis
- Protein Quality Control and Cancerogenesis
- The Many Roles of Molecular Chaperones and Co-chaperones in Tumour Biology
- RECAMO – ...through Cancer Research towards Applied Molecular Oncology; Where, Why and How
- The Role of Platelets in Tumour Growth
- Circulating Levels of B-cell Activating Factor in Paediatric Patients with Malignancy With or without Cancer-Related Cachexia
- A Combined Immunoprecipitation and Mass Spectrometric Approach to Determine ΔNp63-Interacting Partners
- Identification and Characterisation of Pro-metastatic Targets, Pathways and Molecular Complexes Using a Toolbox of Proteomic Technologies
- The Biobanking Research Infrastructure BBMRI_CZ: a Critical Tool to Enhance Translational Cancer Research
- Development and Use of Non-FDG PET Radiopharmaceuticals at the Masaryk Memorial Cancer Institute
- New Mechanisms for an Old Drug; DHFR- and non-DHFR-mediated Effects of Methotrexate in Cancer Cells
- Stereotactic Body Radiation Therapy for Colorectal Cancer Liver Metastases; Early Results
- Phase I Trial in Oncology – Theory and Practice
- Clinical Oncology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- p63 – an Important Player in Epidermal and Tumour Development
- NKT-like Cells are Expanded in Solid Tumour Patients
- The Many Roles of Molecular Chaperones and Co-chaperones in Tumour Biology
- Phase I Trial in Oncology – Theory and Practice
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

