-
Medical journals
- Career
Circulating Levels of B-cell Activating Factor in Paediatric Patients with Malignancy With or without Cancer-Related Cachexia
Authors: J. Bienertova-Vasku 1,2,4,5; A. Lungova 4; P. Bienert 2; F. Zlamal 2; J. Tomandl 3; M. Tomandlova 3; Z. Splichal 2; J. Sterba 4,5
Authors‘ workplace: Department of Laboratory Medicine, Masaryk Memorial Cancer Institute, Brno, Czech Republic 1; Department of Pathological Physiology, Faculty of Medicine, Masaryk University, Brno, Czech Republic 2; Department of Biochemistry, Faculty of Medicine, Masaryk University, Brno, Czech Republic 3; Department of Pediatric Oncology, University Hospital Brno, Brno, Czech Republic 4; Regional Centre for Applied Molecular Oncology, Masaryk Memorial Cancer Institute, Brno, Czech Republic 5
Published in: Klin Onkol 2012; 25(Supplementum 2): 58-63
Práce byla podpořena výzkumným záměrem MZ ČR MZ0MOU2005 a Evropským fondem pro regionální rozvoj a státním rozpočtem České republiky (OP VaVpI – RECAMO, CZ.1.05/2.1.00/03.0101).
Autoři deklarují, že v souvislosti s předmětem studie nemají žádné komerční zájmy.
Redakční rada potvrzuje, že rukopis práce splnil ICMJE kritéria pro publikace zasílané do bi omedicínských časopisů.
Obdrženo: 10. 10. 2012
Přijato: 5. 11. 2012Overview
Background:
Cancer-related cachexia is a multifactorial syndrome characterised by progressive loss of body weight and it affects a large proportion of patients with advanced cancer. Cachexia is associated with reduced treatment tolerance, response to therapy, quality of life and duration of survival, whereas some of its mechanisms are shared across the whole continuum of diseases in the population, either cancer-related or non-cancer related e.g. systemic inflammation, increased lipolysis, insulin resistance and reduced physical performance. However, so far there has been only little effort to utilise the integrative physiology of adipose tissue to achieve therapeutic gain. B cell-activating factor (BAFF) is a novel member of the TNF ligand superfamily, is mainly produced by myeloid cells and has recently been shown to participate in B-cell survival and B - and T-cell maturation, but also in adipogenesis. Therefore, it represents an elegant candidate molecule linking the immune system and adipose tissue metabolism, both being involved deeply in the pathogenesis of cachexia. Moreover, it has been described very recently that BAFF directly influences secretion of IL-6 and IL-10.Material and Methods:
In this study, pre-treatment circulating levels of BAFF were investigated in a cohort of 83 paediatric patients with malignancy (0–18 y) with or without cancer-related cachexia using ELISA-based methodology.Results:
Apart from logical significant associations of BAFF circulating levels with disease severity in B-lineage malignancies (ALL or B-cell lymphomas), we observed significant elevation of BAFF in adolescent patients with Ewing sarcoma and rhabdomyosarcoma, compared to the circulating levels appropriate for given age.Conclusion:
To the best of our knowledge, this is so far the first study focusing on BAFF in paediatric malignancies with or without cancer-related cachexia. More research into whether BAFF can represent a useful circulating biomarker for detection and monitoring of the cancer-related cachexia is imperative.Key words:
B cell-activating factor – cachexia – cancer – paediatricsIntroduction
Cachexia is a multifactorial syndrome characterised by progressive loss of body weight, often, but not always, accompanied by anorexia [1]. The consequences of cachexia are detrimental and considered to be the direct cause of approximately 20% of cancer deaths. Loss of fat stores as well as muscle mass in cancer cannot be explained by reduced appetite alone as it often precedes the onset of anorexia and is more severe in animal model of cachexia than that of food restriction [2]. Cancer-related cachexia affects more than 40% of paediatric patients with malignancy [3] and represents an important factor influencing the tolerance of treatment as well as prognosis.
The importance of white adipose tissue in the control of adiposity has been recognised with the discovery of adipocyte-secreted adipokines which regulate body weight [4]. Adipose tissue mass is also influenced by adipogenesis that involves the recruitment of new adipocytes (preadipocyte differentiation) and adipocyte maturation [5]. However, the role of adipocytes in the pathogenic cascade resulting in malignancy-related lipoatrophy is still elusive.
B cell-activating factor (BAFF) is a novel member of the TNF ligand superfamily, mainly produced by myeloid cells. BAFF has been shown to participate in B-cell survival and B - and T-cell maturation [6]. BAFF was recently characterised as a novel member of the TNF ligand superfamily and is also referred to as BLyS, THANK, TALL-1 or TNFSF13B. BAFF has three receptors that belong to the TNF receptor superfamily: B-cell maturation antigen (BCMA), transmembrane activator and CAML interactor (TACI), and BAFF receptor (BAFF-R) [7]. These receptors are primarily expressed in B-cells, but TACI and BAFF-R are known to be expressed by T-cell subsets as well [8,9]. However, it was recently reported that adipocytes are also capable of producing BAFF and it was proposed that autocrine or paracrine BAFF and BAFF-receptor (BAFF-R) interactions in visceral adipose tissue leads to impaired insulin sensitivity via inhibition of insulin signalling pathways and alterations in adipokine production [10].
Very recently, it has been reported that BAFF enhances interleukin-6 and interleukin-10 production by human B-cells stimulated via oligodeoxynucleotides [11]. Many cancers that induce cachexia were reported to have elevated systemic IL-6 levels. The relationship of IL-6 to cancer cachexia has been well documented [12]. Crucial evidence for a role of IL-6 in the development of cancer cachexia has come from studies using the murine colon-26 adenocarcinoma, where increasing levels of IL-6 correlated with the development of cachexia, and treatment with a neutralising antibody to IL-6, but not TNF-α or interferon (IFN), attenuated the development of weight loss and other key parameters of cachexia [13].
On the other hand, IL-10 can potentially inhibit the production of pro-inflammatory cytokines including IL-6 and Fujiki et al reported already in 1997 that the inoculation of IL-10-transfected cells kept IL-10 mRNA expression at tumour sites and induced the elevation in serum IL-10 levels without affecting the growth rates of colon 26 cells both in vitro and in vivo [14].
Taking into account that BAFF was previously demonstrated to regulate IL-6 and IL-10 production in human B-cells, it can be hypothesised that it is an elegant candidate for linking the pro-inflammatory state typical for end-stage cachexia with metabolism of adipose tissue and therefore can be directly involved in the pathogenic cascade in cancer-related cachexia.
The aim of the study was to investigate the circulating BAFF levels in a Central-European paediatric population of patients with malignancies and to investigate a possible association of BAFF levels with cachexia at the first presentation of the patients.
Materials and Methods
Subjects
This cross-sectional study included a total of 83 children (M/F: 49/34, mean age at diagnosis 7.5 y ± 5.9) with various paediatric malignancies, either haematological diseases or solid tumours. The study cohort included patients with B-precursor cell acute lymphoblastic leukaemia (Bcp-ALL), acute myeloid leukaemia, Hodgkin lymphoma, non-Hodgkin lymphoma, neuroblastoma, ependymoma, medulloblastoma, Wilms tumour, Ewing sarcoma, rhabdomyosarcoma and Langerhans histocytosis that were diagnosed at the Department of Paediatric Oncology of the University Hospital Brno between January and June 2011. All sampling had been performed before treatment was initiated according to respective protocols (Interim AIEOP BMF ALL 2000, Interim AIEOP BFM ALL 2009, Interfant 06, Interim AIEOP BFM 2011, AHOD031, AHOD0431, AHOD0831, B-NHL BFM 2004, INT-B-NHL-2010, EURO EWING 99, ARET 0321, COG ANBL02P1, EpSSG RMS 2005 SIOP 2001, ANBL 0531, SJMB 96, ACNS 0223, ACNS 0126, ANBL 00P3).
The study was conducted according to the guidelines of the Declaration of Helsinki; all procedures involving human subjects were approved by the local Committee for Ethics of Medical Experiments on Human Subjects.
Cancer-related cachexia was defined as a history of weight loss of at least 5% reported by the parents at the first presentation of the patient or a drop in growth rate two or more percentile ranks on standard growth charts or a weight for height less than the tenth percentile on standard growth charts [15].
Biochemistry
Blood samples for BAFF plasma analysis were collected after overnight fasting into K-EDTA tubes and were immediately centrifuged at 1700× g for 20 min and then stored at –80 ºC until analysis. Plasma BAFF levels were measured by a commercially available ELISA (R&D Systems, Minneapolis, MN, USA) with the intra - and inter-assay precisions < 6.0 and 9.0%, respectively (plasma samples were diluted 5-fold before analysis).
Statistics
Where applicable, it was first determined whether the variable under consideration had a normal distribution using the Kolmogorov–Smirnov test, and in cases of skewed variables, logarithmic transformation and further normality testing were performed. For descriptive purposes, mean values and standard deviations are presented using untransformed values.
Statistical analysis was performed using the Mann-Whitney U-test, Krus-kal-Wallis test, Fisher’s exact test; post hoc Bonferroni’s correction for multiple comparisons was employed where required. Univariate linear modelling assessed the relationship between BAFF and quantitative variables; multivariate linear models investigated the predictive role of BAFF on anthropometric and nutritional parameters.
Results
The basic demographic and clinical description of the study cohort is given in Tab. 1.
1. Distribution of BAFF levels across the whole studied cohort (values given as mean ± SD). 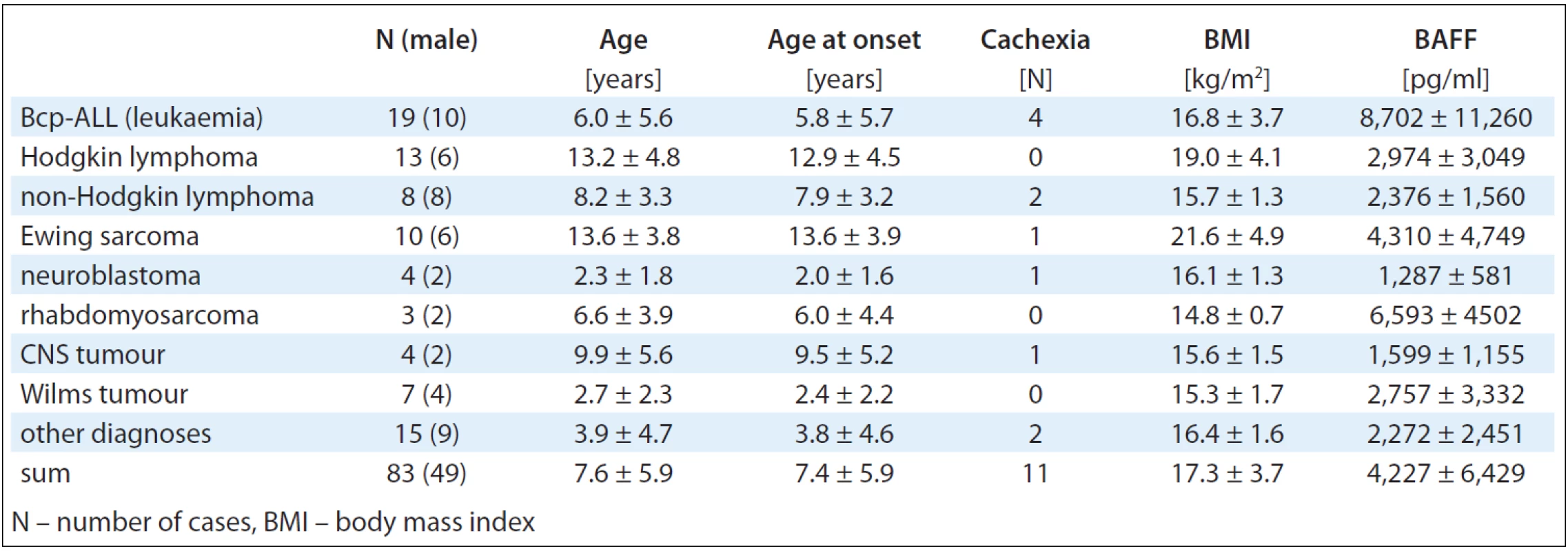
Evaluation of the BAFF Levels Across the Whole Studied Cohort
The distribution of the BAFF levels across the whole studied cohort is presented in Fig. 1. The highest circulating levels of BAFF were observed in Bcp-ALL (8,702 ± 11,260 pg/ml), rhabdomyosarcoma (6,593 ± 4,502 pg/ml), Ewing sarcoma (4,311 ± 4,750 pg/ml) and Wilms tumour (2,757 ± 3,332 pg/ml) cases.
Fig. 1. Distribution of BAFF levels across the whole studied population (values given as mean ± SD). *Diagnoses with very low frequency were excluded from the figure. 
Evaluation of BAFF Levels in B-lineage Malignancies
We observed significant differences in circulating levels of BAFF between B-ALL and B-cell lymphoma patients (Bcp-ALL: 8,702 ± 11,260 pg/ml, Hodgkin lymphoma: 2,974 ± 3,049 pg/ml; non-Hodgkin lymphoma 2,376 ± 1,561 pg/ml; p = 0.0268). When analysing the Hodgkin-lymphoma patients against the non-Hodgkin lymphomas, we did not observe significant differences between these two sub-cohorts (p = 0.64).
Evaluation of Prediction Effect of BAFF for Presence of Cancer-related Cachexia
The distribution of BAFF levels across the diagnoses included in the study in relation to cancer-related cachexia is presented in Fig. 2. In the multivariate logistic regression modelling with cancer-related cachexia as a dependent variable and age, BAFF levels and diagnosis as independent variables, none of the independent variables served as a predictor for the presence of cancer-related cachexia (AUC = 0.5, β = 0.67, p = 0.77; AUC = area under the curve, β = partial regression coefficient). When constructing two different models for males and females across the whole range of diagnosis, no prediction role of BAFF on presence of cachexia was observed either (AUC = 0.5, β = 0.54; p = 0.76). In a different multivariate model with BMI as the dependent variable, the only independent variable that exerted a significant prediction for BMI was age (p < 0.001), while BAFF did not play any predictive role for BMI (β = 0.88; p = 0.78).
Fig. 2. Distribution of BAFF levels across the studied population in relation to presence of cachexia (values given as mean ± SD). *Diagnoses with very low frequency were excluded from the figure. 
Discussion
Cachexia is a common but very challenging problem in the paediatric population with cancer. Malnutrition adversely impacts a patient’s quality of life and above all his/her ability to tolerate aggressive therapeutic interventions and thus can represent a factor limiting treatment aggressiveness associated with better survival.
Adipose tissue was for a long time regarded as a silent and passive organ, storing excess energy as triglycerides and releasing energy as fatty acids [16]. Loss of adipose tissue in cachexia is primarily due to an increased lipolysis, since there is an increased turnover of both glycerol and free fatty acids compared with normal subjects or cancer patients without weight loss [12]. Lipolysis is increased by approximately 40% in cachexia patients [17], moreover, adipocytes in cachectic subjects have approximately 3 fold increased response to natriuretic peptide, independently of the basal lipolytic rate [18]. However, the underlying mechanisms for triggering excessive lipolysis in the adipose tissue of cancer patients are unclear, as well as the individual contribution of pro-inflammatory cytokines such as IL-6 to this cascade.
B-cell activating factor is an important regulator of B-cell immunity and there are many reports of increased serum BAFF level in haematopoietic malignancies [9]. However, BAFF seems to play an important role not only in differentiation and maturation of B-cells, but also in adipose tissue where it is capable of stimulating synthesis of various pro - as well as anti-inflammatory cytokines, e.g. IL-6 or IL-10 [11]. The exact nature of the mechanisms linking the immune system and adipose tissue is unknown, however, novel promising studies were published recently: e.g. a study by Zonca et al [19] demonstrating that the BAFF secretion is differentially enhanced by CXCL12 and interferon (IFN)-γ, that are implicated in human adipose-derived stem cell-mediated migration and immunosuppression, respectively. Moreover, BAFF induces rapid phosphorylation of extracellular signal-regulated kinases 1/2 (ERK1/2) and Akt kinases and promotes an increase in hASC proliferation, without affecting the immunosuppressive capacity of these cells [19]. The authors also suggest that the PI3K transduction pathway is involved in hASC basal growth and that BAFF-mediated effects are ERK-dependent.
Our study is the first to investigate circulating levels of BAFF in paediatric population with cancer. Moreover, this is the first study that investigated relationships between BAFF levels and the presence of cancer-related cachexia during the pre-treatment period. Not surprisingly, we observed the highest levels of BAFF in the Bcp-ALL patients, which can be explained by the robust proliferation based on the B-cells in these patients. However, we observed markedly elevated levels of BAFF in patients with different types of sarcomas, both rhabdomyosarcomas and Ewing sarcoma, whereas these levels exceeded significantly levels reported for the healthy population of the corresponding age [20]. The observed increase of BAFF in sarcoma patients cannot be explained solely by the proliferation of B-cells, typical for B-lineage malignancies, and the values remain significantly elevated after appropriate adjustment for age. It could be suggested that Ewing sarcoma patients are substantially older than patients with Bcp-ALLs and it is well-known that the BAFF levels are age-dependent, however, this does not offer any explanation for such robust elevation of BAFF in adolescent patients with Ewing sarcoma, whose circulating levels of BAFF exceed those of the healthy population more than 2.5 times. It must also be taken into account that BAFF levels tend to decrease with increasing age. Also, most of the enrolled patients with sarcomas did not present with cachexia at the pre-treatment clinical investigation, therefore cachexia does not serve as a suitable explanation for such elevation.
In 2008, Kohno et al [21] reported expression of BAFF-R in the human fibrosarcoma cell line HT108, not on the cell surface, but also in the cytoplasm. Moreover, the expression of BAFF was also detected and the reduction of endogenous BAFF or BAFF-R by siRNA decreased basal NF-kappaB activity. Expression of BAFF-R and BAFF was also demonstrated in osteosarcoma [21]. Therefore, it could be suggested that there is a BAFF/BAFF-R-dependent autocrine mechanism in sarcomas that may play a role in the development of certain types of non-haematopoietic tumours.
Conclusion
To conclude, this is the first study of BAFF distribution in paediatric patients with malignancy, both in the presence or absence of cancer-related cachexia. The study does not provide significant evidence for association of BAFF with cancer-related cachexia across the whole range of diagnosis, however, significantly elevated BAFF concentrations were observed in numerous paediatric patients with solid tumours, the mechanism of which is currently unknown. Therefore, more research into the role of BAFF in host (mal)adaptation to the presence of tumour is urgently needed.
Although limited in the number of cases, our study provides a potential basis for further evaluation of BAFF in a wide range of malignancies across the whole continuum of the paediatric population with cancer.
In addition, it still seems to be important to further investigate whether BAFF can represent a useful circulating biomarker for detection and monitoring of the cancer-related cachexia.
Acknowledgement
Each author has made an important scientific contribution to the study and has assisted with the drafting or revising of the manuscript, in accordance with the definition of an author as stated by the International Committee of Medical Journal Editors.
Abbreviations
ALL – acute lymphoblastic leukaemia
BAFF – B cell-activating factor
BAFF-R – receptor for B cell-activating factor
BCMA – B cell maturation antigen
Bcp-ALL – B-cell precursor acute lymphoblastic leukaemia
BLyS – B-lymphocyte stimulator
BMI – body mass index
CXCL12 – chemokine (C-X-C motif) ligand 12
ERK1/2 – extracellular signal-regulated kinases 1/2
hASC – human adipose stem cells
IL-6, IL-10 – interleukin 6, interleukin 10
INF-γ – interferon-γ
NFκB – nuclear factor kappa-light-chain-enhancer of activated B cells
TACI – transmembrane activator and CAML interactor
TALL-1 – TNF - and APOL-related leukocyte expressed ligand
TNF – tumour necrosis factor
This study was supported by the scientific program of the Czech Ministry of Health MZ0MOU2005 and by the European Regional Development Fund and the State Budget of the Czech Republic (RECAMO, CZ.1.05/2.1.00/03.0101).
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.
The Editorial Board declares that the manuscript met the ICMJE “uniform requirements” for biomedical papers.
Julie Bienertova-Vasku, M.D., Ph.D.
Department of Pathological Physiology and Department of Paediatric Oncology
Kamenice 5, A18
625 00 Brno
Czech Republic
email: jbienert@med.muni.cz
Submitted: 10. 10. 2012
Accepted: 5. 11. 2012
Sources
1. Bosaeus I, Daneryd P, Svanberg E et al. Dietary intake and resting energy expenditure in relation to weight loss in unselected cancer patients. Int J Cancer 2001; 93(3): 380–383.
2. Bing C, Brown M, King P et al. Increased gene expression of brown fat uncoupling protein (UCP)1 and skeletal muscle UCP2 and UCP3 in MAC16-induced cancer cachexia. Cancer Res 2000; 60(9): 2405–2410.
3. Norton J, Peter J. Nutritional supportive care in principles and practices of pediatric oncology. Philadelphia, PA: Lippincott and Co 1989.
4. Trayhurn P, Bing C. Appetite and energy balance signals from adipocytes. Philos Trans R Soc Lond B Biol Sci 2006; 361(1471): 1237–1249.
5. Bing C, Russell S, Becket E et al. Adipose atrophy in cancer cachexia: morphologic and molecular analysis of adipose tissue in tumour-bearing mice. Br J Cancer 2006; 95(8): 1028–1037.
6. Kim YH, Choi BH, Cheon HG et al. B cell activation factor (BAFF) is a novel adipokine that links obesity and inflammation. Exp Mol Med 2009; 41(3): 208–216.
7. Gross JA, Johnston J, Mudri S et al. TACI and BCMA are receptors for a TNF homologue implicated in B-cell autoimmune disease. Nature 2000; 404(6781): 995–999.
8. Huard B, Schneider P, Mauri D et al. T cell costimulation by the TNF ligand BAFF. J Immunol 2001; 167(11): 6225–6231.
9. Ng LG, Sutherland AP, Newton R et al. B cell-activating factor belonging to the TNF family (BAFF)-R is the principal BAFF receptor facilitating BAFF costimulation of circulating T and B cells. J Immunol 2004; 173(2): 807–817.
10. Hamada M, Abe M, Miyake T et al. B cell-activating factor controls the production of adipokines and induces insulin resistance. Obesity (Silver Spring) 2011; 19(10): 1915–1922.
11. Yehudai D, Snir A, Peri R et al. B Cell-Activating Factor Enhances Interleukin-6 and Interleukin-10 Production by ODN-Activated Human B Cells. Scand J Immunol 2012; 76(4): 371–377.
12. Tisdale MJ. Mechanisms of cancer cachexia. Physiol Rev 2009; 89(2): 381–410.
13. Strassmann G, Fong M, Kenney JS et al. Evidence for the involvement of interleukin 6 in experimental cancer cachexia. J Clin Invest 1992; 89(5): 1681–1684.
14. Fujiki F, Mukaida N, Hirose K et al. Prevention of adenocarcinoma colon 26-induced cachexia by interleukin 10 gene transfer. Cancer Res 1997; 57(1): 94–99.
15. Couluris M, Mayer JL, Freyer DR et al. The effect of cyproheptadine hydrochloride (periactin) and megestrol acetate (megace) on weight in children with cancer/treatment-related cachexia. J Pediatr Hematol Oncol 2008; 30(11): 791–797.
16. Alexaki VI, Notas G, Pelekanou V et al. Adipocytes as immune cells: differential expression of TWEAK, BAFF, and APRIL and their receptors (Fn14, BAFF-R, TACI, and BCMA) at different stages of normal and pathological adipose tissue development. J Immunol 2009; 183(9): 5948–5956.
17. Legaspi A, Jeevanandam M, Starnes HF Jr et al. Whole body lipid and energy metabolism in the cancer patient. Metabolism 1987; 36(10): 958–963.
18. Agustsson T, Ryden M, Hoffstedt J et al. Mechanism of increased lipolysis in cancer cachexia. Cancer Res 2007; 67(11): 5531–5537.
19. Zonca M, Mancheno-Corvo P, DelaRosa O et al. APRIL and BAFF proteins increase proliferation of human adipose-derived stem cells through activation of Erk1/2 MAP kinase. Tissue Eng Part A 2012; 18(7–8): 852–859.
20. Jin R, Kaneko H, Suzuki H et al. Age-related changes in BAFF and APRIL profiles and upregulation of BAFF and APRIL expression in patients with primary antibody deficiency. Int J Mol Med 2008; 21(2): 233–238.
21. Kohno T, Daa T, Otani H et al. Aberrant expression of BAFF receptor, a member of the tumor necrosis factor receptor family, in malignant cells of nonhematopoietic origins. Genes Cells 2008; 13(10): 1061–1073.
Labels
Paediatric clinical oncology Surgery Clinical oncology
Article was published inClinical Oncology

2012 Issue Supplementum 2-
All articles in this issue
- RECAMO – ...through Cancer Research towards Applied Molecular Oncology; Where, Why and How
- p63 – an Important Player in Epidermal and Tumour Development
- Detection of Cancer Stem Cell Markers in Sarcomas
- NKT-like Cells are Expanded in Solid Tumour Patients
- Cancer as a Metabolic Disease and Diabetes as a Cancer Risk?
- PThe Regulation of p53 Synthesis
- Protein Quality Control and Cancerogenesis
- The Many Roles of Molecular Chaperones and Co-chaperones in Tumour Biology
- RECAMO – ...through Cancer Research towards Applied Molecular Oncology; Where, Why and How
- The Role of Platelets in Tumour Growth
- Circulating Levels of B-cell Activating Factor in Paediatric Patients with Malignancy With or without Cancer-Related Cachexia
- A Combined Immunoprecipitation and Mass Spectrometric Approach to Determine ΔNp63-Interacting Partners
- Identification and Characterisation of Pro-metastatic Targets, Pathways and Molecular Complexes Using a Toolbox of Proteomic Technologies
- The Biobanking Research Infrastructure BBMRI_CZ: a Critical Tool to Enhance Translational Cancer Research
- Development and Use of Non-FDG PET Radiopharmaceuticals at the Masaryk Memorial Cancer Institute
- New Mechanisms for an Old Drug; DHFR- and non-DHFR-mediated Effects of Methotrexate in Cancer Cells
- Stereotactic Body Radiation Therapy for Colorectal Cancer Liver Metastases; Early Results
- Phase I Trial in Oncology – Theory and Practice
- Clinical Oncology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- p63 – an Important Player in Epidermal and Tumour Development
- NKT-like Cells are Expanded in Solid Tumour Patients
- The Many Roles of Molecular Chaperones and Co-chaperones in Tumour Biology
- Phase I Trial in Oncology – Theory and Practice
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

