-
Medical journals
- Career
Granulocyte-Colony Stimulating Factor (G-CSF) Accelerates Healing of Radiation Induced Moist Desquamation of the Skin
Authors: L. Viswanath 1; J. Bindhu 1; B. Krishnamurthy 1; K. P. Suresh 2
Authors‘ workplace: Department of Radiation Oncology, Kidwai Memorial Institute of Oncology, Bangalore, Karnataka State, India 1; National Institute of Animal Nutrition & Physiology, Bangalore, Karnataka State, India 2
Published in: Klin Onkol 2012; 25(3): 199-205
Category: Original Articles
Overview
Design:
Clinical evidence from a few experimental and randomized trials have implicated the possible benefit of cytokines in prevention and healing of radiation induced mucositis. This pilot study was undertaken to assess the effectiveness of Granulocyte-Colony Stimulating Factor (G-CSF) on healing of radiation induced moist desquamation of the skin.Rationale for the study:
intervention with exogenous growth factors along with conventional treatment practices may stimulate faster skin healing and help the patient in resuming normalcy at the earliest. Materials and Methods: Twenty three patients with established grade III moist desquamation of skin at the site of radiation and during the course of their radiotherapy were recruited for this study. Patients were administered a single dose of Granulocyte-Colony Stimulating Factor (G-CSF – Neupogen® (Filgrastim)) 300 µg subcutaneously at the periphery of the wound as a single session. The rate of skin healing was documented as a function of time from D1 (day of Filgastrim instillation) to the number of days required for complete healing/re-epithelization of the open skin wound.Results:
There was a rapid response and decreased severity of the grade III radiation skin reactions, which extrapolated to an early resumption of radiotherapy treatment. Twenty patients (86%) showed healing of their wounds within 10 days which was notably faster than the expected 2 to 3 weeks anticipated for the severity/grade of the skin reaction. Thirteen patients (56.5%) showed a remarkably rapid response of healing within 5 days. No significant side effects were experienced after the single dose was administered. The mean duration to resolution of moist desquamation was calculated as 6.65 ± 4.73 days. Among the associated parameters, only location of lesion and depth of skin reaction significantly affected the rate of healing. Superficial epidermal erosions showed excellent response with 4.53 ± 2.07 days of re-epithelization & healing (p < 0.001) compared to deep dermal exposure. The results are suggestive of a promising role of G-CSF in the management of Grade III radiation induced skin reaction (moist desquamation).This concept requires structured randomized trials to establish significance of benefit.Conclusion:
G-CSF appears to promote wound healing; this cytokine has the potential to favorably modify the healing process of radiation induced moist desquamation of the skin. This study demonstrates that this dreaded side effect of radiotherapy can by managed in a very simple, convenient and cost effective way, without toxicity & intervention.Key words:
G-CSF – cytokines – skin – radiotherapy – dermatitis – wounds – injuries – head and neck cancer – filgrastimIntroduction
Radiotherapy (RT) remains the mainstay of treatment in most solid malignancies, especially for head and neck and cervical cancers. Other cancers require RT as an integral part of their adjuvant treatment regimens. The RT dose required to treat moderately sensitive histology exceeds the tolerance limits of skin integrity. RT induced skin reactions, manifesting between the 5th and 10th week of treatment. Delay and interruption at this part of treatment hold a high risk of compromising the cure of malignancy. Prolonging the overall treatment time has a documented adverse effect on the radiocurability of head and neck and cervical cancers [1–3].
Radiation skin reactions are inevitable consequence of intense radiotherapy. Clinical experience confirms that moist desquamation reactions in the irradiated skin are distressing and painful to the patient, but sometimes this condition can be extremely difficult to manage. More than 80% of patients experience skin reactions of various grades, approximately 80–90% of patients experience erythematous reactions and around 2–15% of patients experience moist desquamation [4,5].
Skin reactions can range from mild erythema, through dry desquamation (dry, flaky or scaly skin) to confluent moist desquamation, where blistering, peeling and sloughing of the skin occur. The most severe stage of necrosis is rarely seen nowadays.
The skin is composed of two main layers: the epidermis (superficial layer) and the dermis (deep layer). Skin homeostasis is normally achieved by new cells formed in the basal layer of the epidermis. Repopulation of the entire epidermis takes approximately 4 weeks, although this process can be shorter during healing. The basal layer of the epidermis proliferates rapidly, so it is particularly sensitive to radiotherapy. Ionising radiation essentially damages the mitotic ability of clonogenic or stem cells within the basal layer, thus preventing the process of repopulation and weakening the integrity of the skin. Moist desquamation occurs when clonogenic cells in the basal layer are sterilised, thus rendering cells unable to repopulate in time to replace the damaged tissue.
Consequently, the epidermis becomes broken, and it was found that basal cell loss began once the radiation dose reached 20–25 Gy; maximum depletion of basal cells occurred when the patient had received a dose of 50 Gy. Small areas of moist desquamation tend to heal from the basal layer, whereas large areas of broken epidermis require cells to migrate from the surrounding epidermis. Healing becomes visible as islands of epidermal cells expand and reform in central and peripheral regions of desquamation [6–9].
There is very limited data and much conflicting evidence as how skin reactions should be prevented or what are the optimal methods to treat them when they occur. Wound healing is a complex programmed sequence of molecular processes, such as cell migration, angiogenesis, provisional matrix synthesis, collagen deposition etc. Growth factors and cytokines are considered candidate therapeutics because they are synthesized and stimulate cells required for tissue repair (eg. platelet, macrophages, endothelial cells, keratinocytes and fibroblasts) [10–12]. The possible role of exogenous cytokines (G-CSF) in chronic wound healing and management of oral mucositis has been studied in detail in a few randomized trials, showing positive and inconclusive benefit [12,13]. The role of cytokines, such as G-CSF, a hematopoietic growth factor, has been investigated in mucosal protection during cytotoxic chemotherapy [14,15]. More importantly, the risk of stimulating tumor growth by cytokines has been validated by a few studies [16]. However, further evidence may be required before this can be verified. In this pilot study, we have attempted to extrapolate what has been found in chronic wound healing and mucositis to the management of moist desquamation of the skin to promote radiation induced wound healing.
The objectives of the study is to assess the effectiveness of Granulocyte - Colony Stimulating Factor (G-CSF – Neupogen® (Filgrastim)) on healing of radiation induced moist desquamation
Materials and Methods
In this study, 1,070 patients receiving radiotherapy on Telecobalt machine per year were included. Twenty-three (2.14%) patients presented with documented confluent moist desquamation, grade III radiation induced skin reaction (RTOG/EORTC) were recruited for the study after they signed the informed consent form. The treatment was delivered using conventional fractionation and telecobalt gamma rays.
The evaluated parameters included: base line patients’ characteristics, site of lesion, primary tumor, RT dose at which the moist desquamation developed, characteristics of the skin wound (superficial dermal exposure, deep dermal exposure), the size (length vs width in cms) and area (cm2) of skin moist desquamation was noted; all patients were treated with a uniform GCSF treatment protocol. The rate of skin healing was documented as a function of time from D1 (day of Filgastrim administration) to the number of days required for complete healing/re-epithelization of the open skin wound. The above parameters were independently accessed by 3 radiation oncologist, and the data were corroborated and analyzed.
Filgastrim Treatment Protocol
Step 1: Aseptic precautions were taken during the procedure – injection of a single dose of 300 µg of Filgrastim was administered,subcutaneously in the unirradiated normal skin around the periphery of the moist desquamation (2 or 4 quadrant zones).
Step 2: Proper wound care was advised – air drying and rest to the part of the body that is affected for 2 to 3 days, mainly to prevent skin fold from rubbing against the exposed skin and aggravating the lesion and to clothes from sticking to the exposed skin wound.
Step 3: Sprinkling of Neosporin® – antibiotic powder (contains Neomycin & Polymixin B sulfates & Bacitracin zinc) ever 6 hours was advised (instructions were not to touch the exposed skin with cotton swab or apply ointments).
Step 4: Appropriate oral antibiotics (such as Amoxicillin & Cloxacillin for 7 days or Ciprofloxacilllin with or without Tinidazole) when necessary were given to patients
Step 5: Analgesic & anti inflammatory drugs: oral Ibuprofen if subject is symptomatic for 1–2 days.
Statistical Analysis: Student’s test (two-tailed for independent groups) and ANOVA were used to find the significance of number of days needed for healing with respect to various risk factors; regression analysis was used to find predictors for prognosis to heal after treatment.
Results
The mean ages of the subjects were 48.39 (± 10.39) years. Majority of the subjects were females 20 (87%) and 3 (13%) males. Sixteen patients (70%) developed RTOG Gr III moist desquamation of the skin in the fifth week of treatment with external radiotherapy dose equal to or less than 50 Gy (> 40 Gy). Four patients (17.4%) had observed reactions only during the 6th week of treatment. Body site wise, the distribution of the desquamation were on the pelvis (11 patients, 47.8%), thorax (8 patients, 34.8%) and head & neck (4 patients, 17.4%). As for the distribution of cancer site in subjects developing moist desquamation, most often the reactions were seen in subjects receiving RT for cervix and breast cancer, mainly due to skin fold and rubbing of tight clothes against the skin as observed clinically (Tab. 1). At presentation, the size of the confluent skin desquamation in terms of maximum transverse diameter was 5.48 ± 4.07 cm and area 18.04 ± 25.72 cm2 (Tab. 2). Seventeen (74%) of the subjects had superficial dermal exposures, and 6 patients (26%) presented with deep dermal exposure.
1. Cancer site wise distribution of subjects developing moist desquamation. 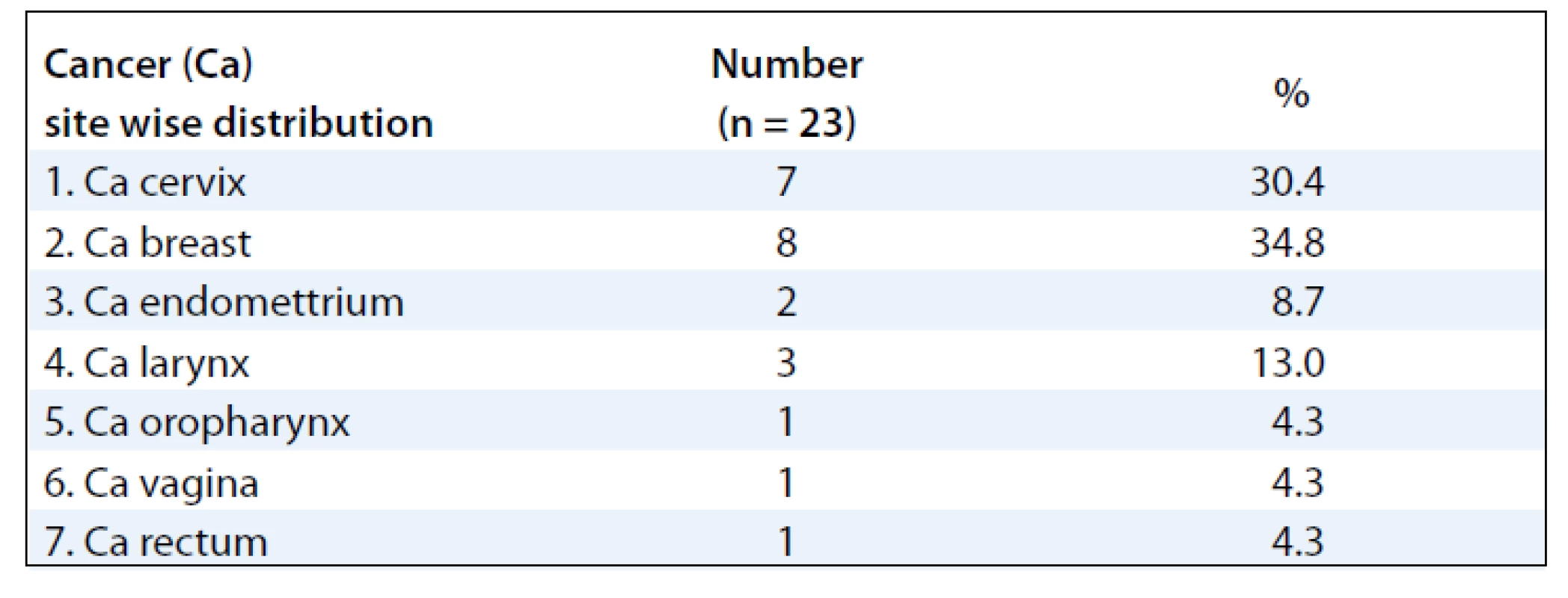
2. Shows the maximum size and area of the confluent skin desquamation. 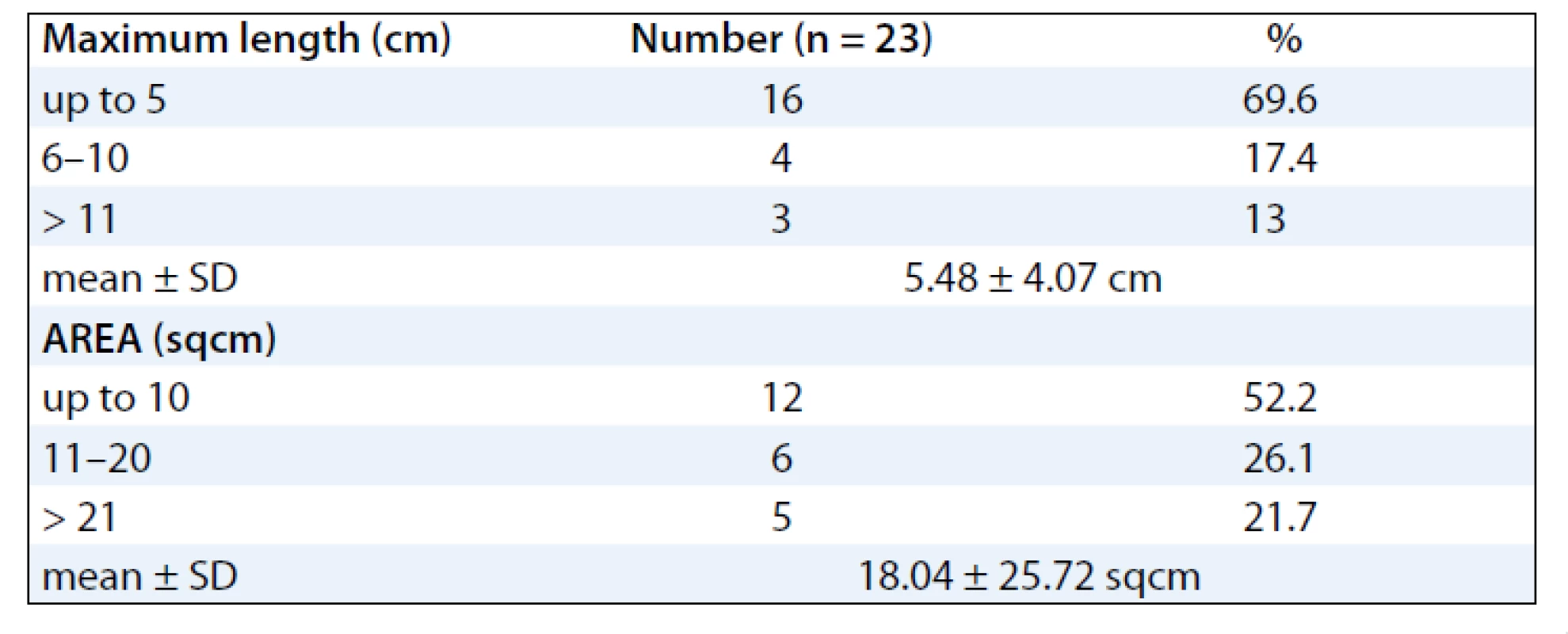
A summary of response assessment to GCSF treatment protocol in terms of number of days required to heal completely is presented in Tab. 3. Positive response was assessable in all 23 patients. There was a rapid reduction in severity of Grade III reaction in 13 (56.5%) patients, who were able to reassume their treatment within 5 days. These patients’ wound had dried, and complete re-epithilisation was evident. Overall, 20 patients (87%) had better and faster healing than anticipated for the severity and grade of reaction, and only 3 (13%) patients required more than 10 days for healing. The mean duration to resolution of moist desquamation was calculated as 6.65 + 4.73 days (Tab. 4). Notably, with site specific evaluation, the rate of healing was more rapid for pelvis and thorax (Tab. 3): with mean number of days required to heal 5.36 ± 3.2 and 5.63 ± 2.77 days, respectively, when compared to head and neck sites. As expected, the depth of dermal exposure was the other significant parameter: deep lesions took a longer time to heal. It was observed that deep dermal exposures required three times as long to heal (12.67 ± 5.13 days) than superficial lesions (4.53 ± 2.07) days (p < 0.001). This was the most significance parameter observed in this study (Graph 1). Age did not prove a significant factor in delayed healing. The rapid rate of healing of moist desquamation with this novel technique has been illustrated by case studies (Fig. 1–3).
3. Response to GCSF treatment protocol in terms of number of days required to heal with respect to various clinical factors. 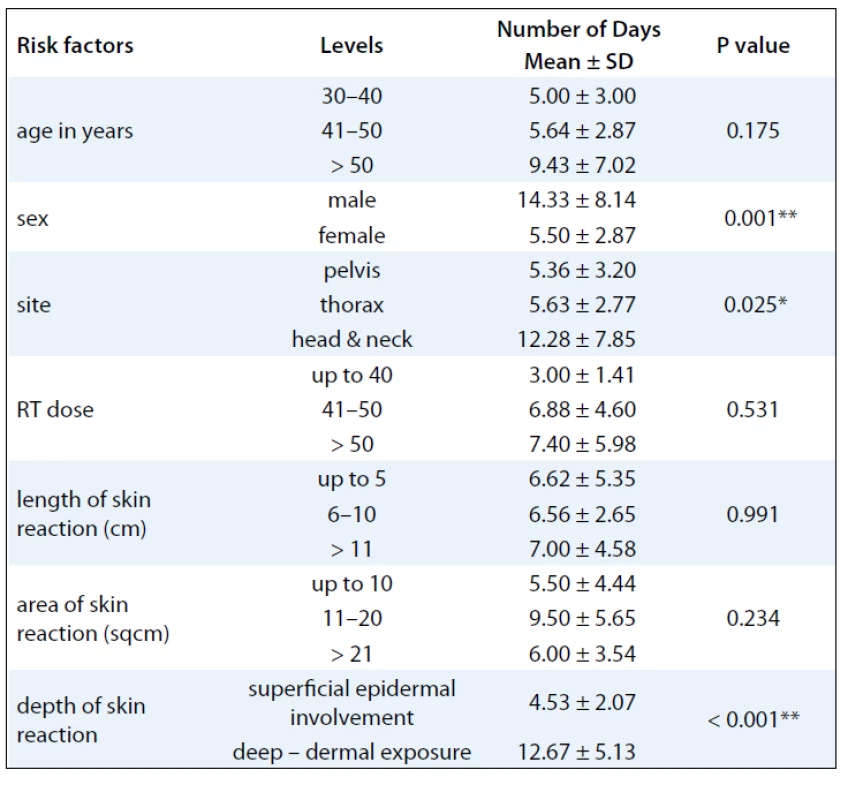
4. Number of days required to heal completely from the time of single dose of Neupogen (Filgrastim) 300 μg injection. 
1. Shows a female patient who completed 50 Gy in 25 fractions radiotherapy for a breast cancer presented with grade III moist dequmation in the axillary fold (Superficial dermal exposure) (1A). The patient was referred to us for cytokine therapy. The skin ulceration healed in the next 4 days after GCSF injection (1B). 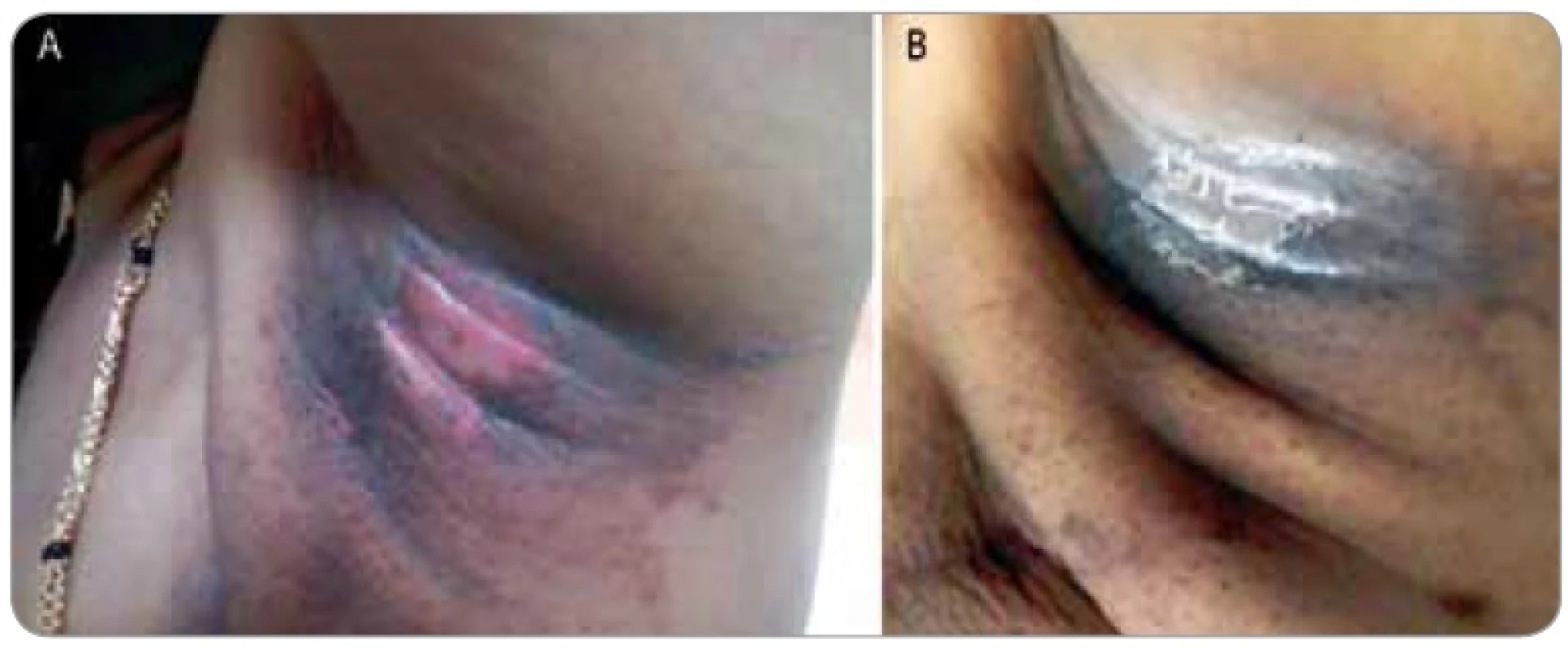
2. Shows a female patient who completed radiotherapy 50 Gy in 25 fractions 5 fractions per week, for Cancer Cervix. She presented with grade III moist desquamation of the skin with deep dermal exposure. The patient was referred to us for cytokine therapy. 2A: before cytokine injection, 2B: day 2, 2C: day 3, 2D: day 4, 2E: day 5. Shows complete healing. 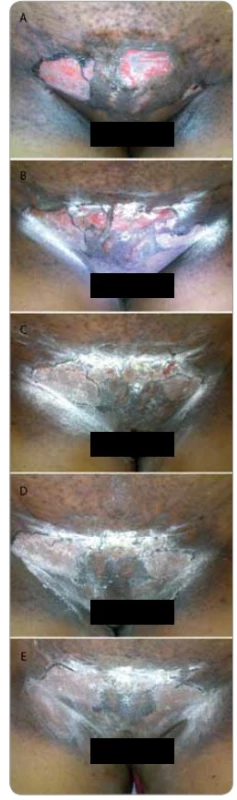
3. Fig. 3A shows a 55-year-old male on treatment with external radiotherapy for a head and neck malignancy with grade III moist desquamation that had developed in the 5th week of treatment and was treated with GV paint for 1 week without any response and the wet skin moist desquamtion persisted and the patient was referred to us for cytokine therapy. Fig. 3B shows the same patient with remarkable resolution of lesion by day 5 after a single dose of GCSF injection. He was able to reassume radiotherapy within a week. 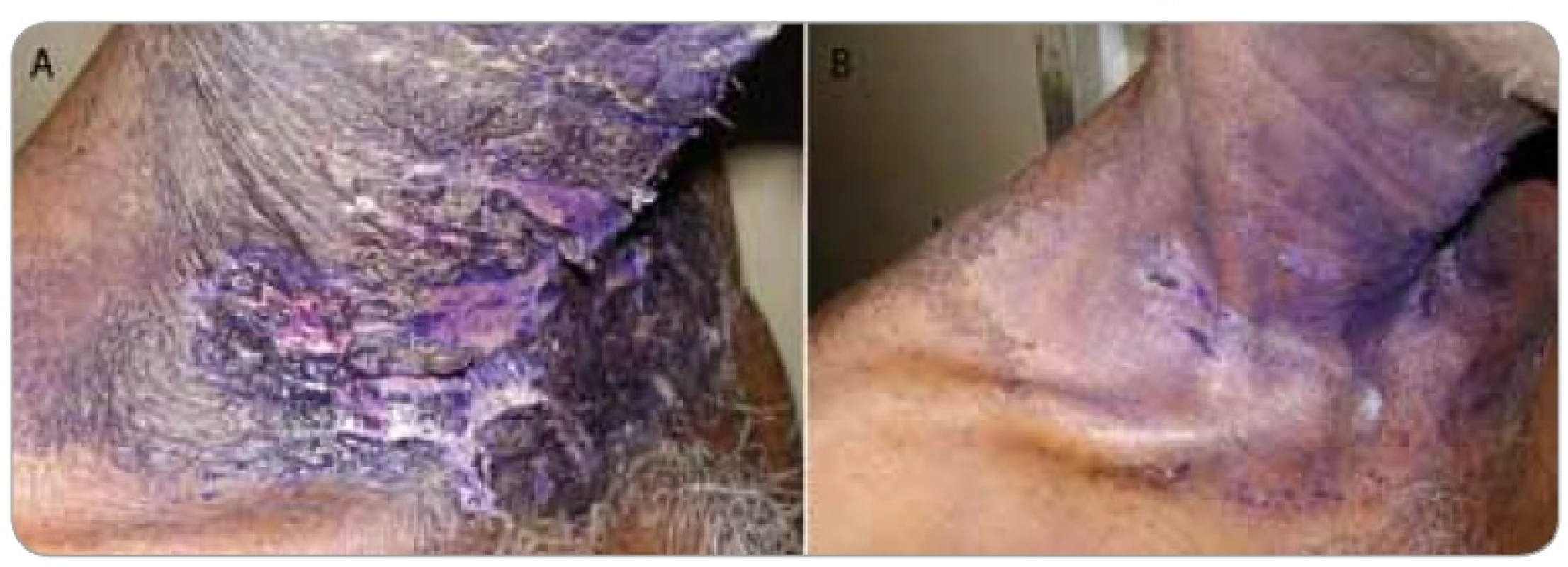
1. Number of days required to heal after G CSF injection to promote wound healing in superficial and deep skin wounds. 
Figures 1–3 show photographs of individual cases of patients who presented with superficial and deep dermal exposure moist desquamation. These patients were treated with a single subcutaneous dose of G-CSF and complete re-eipthelization and healing of the moist desquamation was observed within 1 week.
Discussion
A number of factors influence the severity, onset and duration of radiation skin reactions. In general, moist areas of the body or those that contain skinfolds are more likely to be affected, such as area under the breast, axilla, head and neck, perineum and groins [17].
Intrinsic factors may also play a part, including the baseline characteristics of the patient in terms of general skin condition, nutritional status, age, general health, co-morbid disease and ethnicity. Extrinsic factors, including the dose, energy and fractionation regime (i.e. those prescribed by the radiation oncologist), also affect the degree of experienced skin reaction. Although the skin-sparing effect of modern linear accelerators ensures that the maximum dose of radiotherapy is reached below the basal layer of the skin, certain treatment techniques will increase the likelihood of the skin receiving a dose inducing a visible reaction [18].
The pathogenesis of radiation induced moist desquamation occurs when clonogenic cells in the basal layer are sterilized thus rendering cells unable to repopulate in time to replace the damaged tissue; consequently, the epidermis becomes ulcerated. The basal cell loss begins once radiation dose reaches 20–25 Gy, and the maximum depletion of basal cells occurs when the patient has received a dose of 50 Gy. This would infer an expected Grade II skin reaction around the 4th to 5th week of treatment. This observation held true in our study where 16 (70%) patients developed moist desquamation during this period. Skin care for the management of moist desquamation varied from local application of steroid cream/Gentian violet to the systemic administration of steroids. The evidence to support the use of wound care products is scarce and remains an area of considerable controversy. Many of the currently accepted practices have their limitations and even disadvantages. For example, steroid creams, although useful in reducing the discomfort of inflammation and itching, do not reduce the duration of moist desquamation. The pathogenesis of mucositis is very similar to that of desquamation. More extensive work has to be done in prevention and management of this condition. Notably, this is where the role of cytokines was first evaluated for the management of radiation reaction. Various studies evaluating the role of cytokines as a topical agens and their systemic use were published [19–23].
Granulocyte macrophage colony stimulating factor influences proliferation and differentiation of stem cells and regulates several functions in mature leukocytes, macrophages and dendritic cells in sub-dermis and dermis [23,24]. It has been documented to reduce morbidity associated with burns and to significantly reduce the healing period. G-CSF has also been evaluated for the healing of chemotherapy induced mucositis [25,26]. Therefore, we considered it appropriate to evaluate its role in the healing of radiation induced moist desquamation.
The aim of this study was to evaluate the possible role of G-CSF in accelerating the healing of established moist desquamation. Of the 23 evaluated patients, 22 patients had Gr III moist desquamation mostly occurring in the 4th to 5th week of radiation treatment. This study differs from retrospective series using G-CSF for radiation mucosites in several ways. Firstly, we used a single bolus injection of 300 µg opposed to daily graded injections used in other trials. The injection used in our study was delivered at the unirradiated periphery of the wound with the intention of stimulating a more local reaction. The advantage of this approach would be its cost effectiveness, patient compliance and avoidance of toxicity. In fact, none of patients demonstrated expected toxicity, such as anaphylaxis, skin reaction, fever or bone pain. The study confirms the hypothesis of the benefit of cytokines in wound healing by demonstrating an accelerated time to heal. It was observed that at head and neck sites: although the median length of reaction (5.33 ± 5.77 cms) was only 25% of the median length of reaction in the chestwall (19.57 cm) and pelvic sites (20.11 cm), the number of days to heal was nearly 3 times as long, 12.28 ± 7.85 days versus 5.36 ± 3.20. This was an interesting observation as one would not expect such a discrepancy with a richer skin vasculature in the head and neck region. Skin biopsy would probably shed more light on pathogenesis of this phenomenon. The most significant comparative evaluated parameter was the depth of skin involvement/damage. As expected, deeper lesions took longer time to heal, 12.67 ± 5.13 days, when compared to superficial epidermal reactions, 4.53 ± 2.07 days. This finding was the most significance (p < 0.001) (Tab. 1). However, even these lesions healed at least one week earlier than similar deep reactions in historical control. Although age was also evaluated, it did not prove to be a significant parameter in determining the rate of healing.
Various methods have been advocated to enhance the healing process of radiation therapy induced moist skin reaction. In a study using hydrocolloid occlusive dressing (Duoderm), 20 patients were shown to have mean healing time of 12 days [27]. With amnion as a biological dressing, a study of 14 patients, the median of duration of ulcer healing was 7 days, requiring median of 4 dressings [28]. For hydrogel dressing, the median time to healing was 12 days but for GV paint, the median time to healing was 30 days [29]. GM-CSF(Mielogen®) impregnated gauze with steroid cream, significantly decreased and prevented radiation skin reactions and also promoted healing of GII/III skin damages (4.58 ± 2.93 days versus 6.16 ± 1.91 days for steroid cream alone [30].
Several studies previously examined the ability of GM-CSF to modify radiation induces mucositis. Five randomized controlled trials of GM-CSF reported promising symptomatic improvement in pain and erythema [26,30–32]. However, unlike our study, the reduction of duration of mucositis was not as significant. Neither was the impact of reduction in duration of treatment interruption.
Studies dealing with the use of cytokines in resolving skin desquamation are much more limited [31,33]. Our literature review did not reveal any other studies that have used this novel method of single dose of G-CSF. The authors feel the novel method of single subcutaneous infiltration is more cost effective patient friendly method with a proven benefit. The biological implications, mode of delivery, hypothesis and intervention with exogenous growth factors that may stimulate healing is worth further evaluation.
Conclusion
Radiation induced skin reactions remain a significant problem for patients undergoing radical radiation therapy. Wide variations in practice exist in the management of moist desquamation of the skin. There is a need for more organized research into skin and wound care products, both to prevent and to manage radiation induced skin reactions.
The commonly available Granulocyte-Colony Stimulating Factor (G-CSF – Neupogen® (Filgrastim) appears to promote wound healing. This cytokine has the potential to favorably modify the healing process of radiation induced moist desquamation of the skin. If used judiciously, it may eventually improve therapeutic ratio by avoiding treatment interruption and possibly allow dose escalation. Without the need for any physical intervention and hospitalization for wound management. Our study has demonstrated that this dreaded side effect of radiotherapy can by managed in a very simple, convenient and cost effective way, without toxicity. Further systematic and planned studies are required to evaluate optimal dosage, schedule of treatment and confirm the mechanism of action.
Acknowledgements
Dr. B.L. Preethi, Associate Professor, Department of Physiology, M.S.Ramaiah Medical College, Bangalore.
Dr. Bindu Venugopal. M.D(RT) Student, Department of Radiation Oncology, Kidwai Memorial Institute of Oncology, Bangalore.
Dr. Nikesh. M.D(RT) Student, Department of Radiation Oncology, Kidwai Memorial Institute of Oncology, Bangalore.
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.
The Editorial Board declares that the manuscript met the ICMJE “uniform requirements” for biomedical papers.
Prof. Lokesh Viswanath, MD
Department of Radiation oncology
Kidwai Memorial Institute of Oncology
560029 Bangalore Hosur Road
Karnataka State
India
e-mail: lokpreeth@gmail.com
Submitted: 3. 7. 2011
Accepted: 15. 2. 2012
Sources
1. Bataini JP, Asselain B, Jaulerry C et al. A multivariate primary tumor control analysis in 465 patients treated by radical radiotherapy for cancer of tonsillar region: clinical and treatment parameters as prognostic factors. Radiother Oncol 1989; 14(4): 265–277.
2. Fowler JF, Lindstrom MJ. Loss of local control with prolongation in radiotherapy. Int J Radiat Oncol Biol Phys 1992; 23(2): 457–467.
3. Withers HR, Taylor JM, Maciejewski B. The hazard of accelerated tumor clonogen repopulation during radiotherapy. Acta Oncol 1988; 27(2): 131–146.
4. Porock D, Kristjanson L. Skin reactions during radiotherapy for breast cancer: the use and impact of topical agents and dressings. Eur J Cancer Care (Engl) 1999; 8(3): 143–153.
5. Glean E, Edwards S, Faithfull S et al. Intervention for acute radiotherapy induced skin reactions in cancer patients. J Radiother Pract 2001; 2(2): 75–84.
6. Archambeau JO, Ines A, Fajardo LF. Response of the swine skin microvasculature to acute single exposures of X rays: quantification of endothelial changes. Radiat Res 1984; 98(1): 37–51.
7. Hopewell JW, Nyman J, Turesson I. Time factor for acute tissue reactions following fractionated irradiation: a balance between repopulation and enhanced radiosensitivity. Int. J Radiat Biol 2003; 79(7): 513–524.
8. Hopewell JW. Mechanisms of the action of radiation on skin and underlying tissues. In: Jammet H, Daburon F, Gerber GB et al (eds). Radiation Damage to Skin (BJR Supplement 19). London: British Institute of Radiology 1986 : 39–47.
9. Hopewell JW. The skin: its structure and response to ionizing radiation. Int J Radiat Biol 1990; 57(4): 751–773.
10. Bussolino F, Wang JM, Defilippi P et al. Granulocyte and granulocyte macrophage colony stimulating factors induce human endothelial cells to migrate and proliferate. Nature 1989; 337(6206): 471–473.
11. Mckay IA, Leigh IM. Epidermal cytokines and there role in cutaneous wound healing. Br J Dermatol 1991; 124(6): 513–518.
12. Patni N, Patni S, Bapna A. The optimal use of granulocyte macrophage colony stimulating factor in radiation induced mucositis in head and neck squamous cell carcinoma. J Cancer Res Ther 2006; 1(3): 136–141.
13. Kannan V, Bapsy PP, Anantha N et al. Efficacy and safety of granulocyte macrophage-colony stimulating factor (GM-CSF) on the frequency and severity of radiation mucositis in patients with head and neck carcinoma. Int J Radiat Oncol Biol Phys 1997; 37(5): 1005–1010.
14. Gordon B, Spadinger A, Hodges E et al. Effect of granulocyte-macrophage colony stimulating factor on oral mucositis after hematopoietic stem-cell transplantation. J Clin Oncol 1994; 12(9): 1917–1922.
15. Schuchter LM, Luginbuhl WE, Meropol NJ. The current status of toxicity protectants in cancer therapy. Semin Oncol 1992; 19(6): 742–751.
16. Naylor W, Mallett J. Management of acute radiotherapy induced skin reactions: a literature review. Eur J Oncol Nurs 2011; 5(4): 221–233.
17. Sitton E. Early and late radiation-induced skin alterations. Part I: Mechanisms of skin changes. Oncol Nurs Forum 1992; 19(5): 801–807.
18. Hamilton JA. Colony stimulating factors, cytokines and monocyte macrophages some controversies. Immunol Today 1993; 14(1): 18–24.
19. Lavery BA. Skin care during radiotherapy: a survey of UK practice. Clin Oncol (R Coll Radiol) 1995; 7(3): 184–187.
20. Hejna M, Köstler WJ, Raderer M et al. Decrease of duration and symptoms in chemotherapy induced oral mucositis by topical GM-CSF: result of a prospective randomised trial. Eur J Cancer 2001; 37(16): 1994–2002.
21. Makkonen TA, Minn H, Jekunen A et al. Granulocyte macrophage-colony stimulating factor (GM-CSF) and sucralfate in the prevention of radiation induced mucositis: a prospective randomized study. Int J Radiat Oncol Biol Phys 2000; 46(3): 523–534.
22. Sprinzl GM, Gavlan O, de Vries A et al. Local application of granulocyte-macrophage colony stimulating factor (GM-CSF) for the treatment of oral mucositis. Eur J Cancer 2001; 37(16): 2003–2009.
23. Saarilahti K, Kanjanti M, Joensuu T et al. Comparison of granulocyte-macrophage colony stimulating factor and sucralfate mouthwashes in the prevention of radiation induced mucositis: a double-blind prospective randomized phase III study. Int J Radiat Oncol Biol Phys 2002; 54(2): 479–485.
24. Wagner W, Alfrink M, Haus U et al. Treatment of irradiation induced mucositis with growth factors (rhGM-CSF) in patients with head and neck cancer. Anticancer Res 1999; 19(1B): 799–804.
25. Berdel WE, Danhauser-Riedl S, Steinhauser G et al. Various human hematopoietic growth factors (interleukin-3, GM-CSF, G-CSF) stimulate clonal growth of nonhematopoietic tumor cells. Blood 1990; 73(1): 80–83.
26. Dexter M. Haemopoietic growth factors. Review of Biology and clinical potential. Macclesfield, Cheshire: Gardiner Caldwel Ltd. 1990.
27. Margolin SG, Breneman JC, Denman DL et al. Management of radiation-induced moist skin desquamation using hydrocolloid dressing. Cancer Nurs 1990; 13(2): 71–80.
28. Lobo Gajiwala A, Sharma V. Use of irradiated amnion as a biological dressing in the treatment of radiation induced ulcers. Cell Tissue Bank 2003; 4(2–4): 147–150.
29. Gollins C, Gaffney S, Slade R et al. RCT on gentian violet versus a hydrogel dressing for radiotherapy-induced moist skin desquamation. J Wound Care 2008; 17(6): 268–270.
30. Kouvaris JR, Kouloulias VE, Plataniotis GA et al. Dermatitis during radiation for vulvar carcinoma: prevention and treatment with granulocyte-macrophage colony-stimulating factor impregnated gauze. Wound Repair Regen 2001; 9(3): 187–193.
31. Throuvalis N, Antonadou D, Pulizzi M et al. Evaluation of the efficacy and safety of GM-CSF in the prophylaxis of mucositis in patients with head and neck cancer treated with radiotherapy. Eur J Cancer 1995; Suppl 5: Abstract 431.
32. McAleese JJ, Bishop KM, A’Hern R et al. Randomized phase II study of GM-CSF to reduce mucositis caused by accelerated radiotherapy of laryngeal cancer. Br J Radiol 2006; 79(943): 608–613.
33. Wells M, MacBride S. Radiation skin reactions [online]. Available from: http://www.us.elsevierhealth.com/media//us/samplechapters/9780443064869/9780443064869.pdf.
Labels
Paediatric clinical oncology Surgery Clinical oncology
Article was published inClinical Oncology

2012 Issue 3-
All articles in this issue
- New and Clinically Used Oncomarkers of Bladder Cancer
- Reproductive Functions in Women after Cancer Therapy
- Genetic Background of Cisplatin Induced Ototoxicity
- Triple-Negative Breast Cancer: Analysis of Patients Diagnosed and/or Treated at the Masaryk Memorial Cancer Institute between 2004 and 2009
- Angioimmunoblastic T-cell Lymphoma as a Very Poor-Prognosis Malignancy – a Single Centre Experience
- Patient with B-CLL with a History of Unrelated Hematopoietic Cells Donation – Retrospective Analysis of CLL Development and Implication for the Recipient
- Benefits of Individual Imaging Methods for Diagnosis and Monitoring of Activity of Multiple Myeloma
- Positron Emission Tomography and Clinical Predictors of Survival in Primary Extragonadal Germ Cell Tumors
- Granulocyte-Colony Stimulating Factor (G-CSF) Accelerates Healing of Radiation Induced Moist Desquamation of the Skin
- Clinical Oncology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Angioimmunoblastic T-cell Lymphoma as a Very Poor-Prognosis Malignancy – a Single Centre Experience
- Triple-Negative Breast Cancer: Analysis of Patients Diagnosed and/or Treated at the Masaryk Memorial Cancer Institute between 2004 and 2009
- New and Clinically Used Oncomarkers of Bladder Cancer
- Granulocyte-Colony Stimulating Factor (G-CSF) Accelerates Healing of Radiation Induced Moist Desquamation of the Skin
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

