-
Medical journals
- Career
Positron Emission Tomography and Clinical Predictors of Survival in Primary Extragonadal Germ Cell Tumors
Authors: T. Büchler 1; P. Dusek 2; A. Brisuda 2; K. Simonova 3; P. Fencl 3; Jiří Jarkovský 4; M. Babjuk 2; J. Abrahámová 1
Authors‘ workplace: Department of Oncology, Thomayer University Hospital and 1 Faculty of Medicine, Charles University, Prague, Czech Republic 1; Department of Urology, 2 Faculty of Medicine, Charles University, and Motol University Hospital, Prague, Czech Republic 2; Departments of Nuclear Medicine and PET Centre, Na Homolce Hospital, Prague, Czech Republic 3; Institutes of Biostatistics and Analyses, Masaryk University, Brno, Czech Republic 4
Published in: Klin Onkol 2012; 25(3): 178-183
Category: Original Articles
Overview
Background:
Primary extragonadal germ cell tumors (EGTs) are an uncommon malignancy accounting for 2–4% of all germ cell neoplasms in adult males. Their prognosis is worse than that for testicular germ cell tumors because of their relative chemoresistance and frequent presentation with widely disseminated metastases. We have studied the role of fluorodeoxyglucose positron emission tomography (FDG-PET) for outcome prediction of patients with EGTs. Patients and Methods:
We have retrospectively analysed 36 men with germ cell tumors originating in the mediastinum or the retroperitoneum. All patients were treated between 1994 and 2010. Negative result of testicular ultrasonographic examination and/or testicular biopsy was required for diagnosis of EGT. Platinum-based systemic therapy was used in all cases, and resectable residual tumor masses were removed surgically.Results:
Overall survival at one and three years was 81% (95% confidence interval [CI]: 68–94%) and 55% (CI: 38–71%), respectively. None of the patients who had positive FDG-PET findings after first line chemotherapy survived at three years after diagnosis. In contrast, 69% and 20% of patients with positive tumor markers, and 90% and 67% of patients with negative tumor markers after first line chemotherapy survived at one and three years, respectively. Negative FDG-PET after completion of treatment was also a powerful predictor of long-term survival with 100% patients surviving three years and 89% surviving five years after diagnosis.Conclusions:
Negative FDG-PET after first-line chemotherapy or after the completion of systemic treatment and resection of residual tumor masses strongly predicts long-term event-free survival in patients with EGTs.Key words:
extragonadal – germ cell – tumor – outcome – positron emission tomographyBackgrounds
Primary EGTs are uncommon malignant tumors that account for approximately 2–5% of germ cell neoplasms in men. They usually originate in the retroperitoneum, the mediastinum, or the pineal gland. The prognosis of EGTs is worse than that of primary testicular germ cell tumors (TGCTs) due to their relative chemoresistance and advanced stage at diagnosis, often with non-lung organ metastases [1,2].
The pathogenesis of EGTs has not been fully elucidated. It is likely that these tumors arise from pluripotent stem cells that are located in normal tissues or that persist in midline structures as a consequence of failed migration during embryonic development [3].
Some patients (pts) are mistakenly diagnosed with EGT when a primary testicular germ cell tumor metastasizes to retroperitoneum or mediastinum and subsequently undergoes necrosis, usually producing haemosiderin scar in the testis (so-called burnt-out testicular tumors). These tumors are probably prognostically more favourable than true EGTs of similar stage and histology.
Histological subtypes of EGTs are similar to those of primary TGCT. However, the proportion of seminomas is only 15% in EGTs, contrasting to approximately 55% for testicular tumors [2]. Another common property of EGTs and TGCTs is the secretion of tumor markers including alpha-fetoprotein (AFP) and chorionic gonadotropin (HCG) and presence of i(12p) isochromosome [4]. Mediastinal nonseminomatous EGTs are associated with haematological malignancies and the Klinefelter syndrome [5]. EGT pts are at an increased risk of subsequent TGCTs and have a relatively high incidence of intratubular germ cell neoplasia [6,7].
The standard TNM staging system is not applicable to EGTs. Most pts with retroperitoneal EGT will fall into intermediate or high-risk group according to the IGCCC system and mediastinal primary tumors are high-risk by definition [8].
Treatment of pts with EGTs follows guidelines for risk-adjusted TGCT therapy and there is only one prospective trial enrolling exclusively EGT pts [9].
In a retrospective study, we have analysed therapy outcomes of men with primary extragonadal germ cell tumors (EGTs) of the retroperitoneum or the mediastinum treated between 1994 and 2010. Our principal objectives were to evaluate the impact of selected baseline characteristics on the treatment results and to assess a possible role of 18-fluorodeoxyglucose positron emission tomography (FDG-PET) scanning for prediction of treatment outcome.
Patients and Methods
Patients
Medical records and electronic databases of pts with germ-cell malignancies were used to identify male pts with extracranial primary EGT treated at one of two major Czech urology and oncology centres (the Thomayer University Hospital and the Motol University Hospital) between 1994 and 2010. All tumors were proven histologically after biopsy of the primary lesion or an accessible metastatic lesion. Histological diagnosis was confirmed by second reading carried out by a pathologist specialising in germ cell tumors. All pts in the cohort had high-sensitivity ultrasound testicular examination to exclude testicular primary, and some pts (n = 9) also underwent testicular exploration with biopsy or orchiectomy if the mediastinal or retroperitoneal tumor mass was thought to be lateralised and//or if the testicular ultrasound result was considered equivocal. The presence of testicular changes such as mature teratoma or haemosiderin deposits on histology was an exclusion criterion because these findings are associated with spontaneously regressed testicular primary tumor. Pts with cryptorchic testis presenting with abdominal tumor were also excluded as tumor originating from retained testis was considered more likely than true primary EGT. Complete response (CR) was defined as a normalisation of all morphological abnormalities and tumor markers. Modified RECIST criteria were used to define partial response (PR) and progressive disease (PD).
Positron Emission Tomography
A weight-adjusted dose of 2-deoxy-2-(18F)fluoro-D-glucose (FDG) was injected to the patients fasting at least 4 hours. Glycaemia was measured prior the FDG application. Per-oral and/or intravenous iodine contrast was administered. FDG-PET image acquisition was carried out after an accumulation time of at least 50 minutes using Siemens Biograph 40 TruePoint TrueView PET scanner. Iterative data reconstruction taking into account transmission correction was performed using low-dose CT images. Diagnostic dose CT was used when iodine contrast was given intravenously for contrast-enhanced CT. Standard whole-body investigation included the area from oral cavity to upper thighs in corrected and non-corrected PET images. Evaluation of fused FDG-PET and CT images was carried out by a nuclear medicine specialist.
Clinical Response Evaluation
Response evaluation was carried out using modified RECIST criteria. Complete response (CR) was defined as normalisation of all radiological and biochemical abnormalities, including normalisation of FDG-PET findings. Pts who had residual tumor mass after chemotherapy that was subsequently completely removed with no evidence of viable tumor on histological evaluation were also classified as being in CR. Partial response (PR) was defined as at least 30% reduction of longest diameters of evaluable tumors. PR as assessed by CT was further subdivided according to tumor marker levels (M – PR and M+ PR) and according to metabolic activity of respective lesions as detected by FDG-PET (PET – PR and PET+ PR). Progressive disease (PD) was defined as at least 20% increase in the sum of longest diameters of evaluable tumors or the appearance of new tumor lesion(s).
Prognostic Parameters and Statistical Analysis
We have studied the impact on overall survival (OS) of the following baseline parameters: age, presence/absence of constitutional symptoms, mediastinal versus non-mediastinal primary, seminoma versus nonseminoma, presence//absence of choriocarcinoma histology, LDH elevation, AFP elevation, HCG elevation, S stage, bulky tumor, lung involvement, liver involvement, and presence/absence of residual mass resection. In addition, we have analysed whether OS is associated with any of the following post-treatment results: FDG-PET response after first-line of therapy, FDG-PET response after completion of therapy, marker response after first-line of therapy, marker response after the completion of therapy, retroperitoneal nodal dissection as a part of treatment.
Overall survival in the cohort was estimated using the Kaplan-Meier method and differences between subgroups were evaluated by log-rank test. Cox proportional hazards model was applied for the evaluation of hazard ratios associated with prognostic factors; backward stepwise algorithm was used for the final selection of predictors in the multivariate model. Overall survival data were censored if death occurred due to a cause unrelated to tumor or treatment.
Results
Baseline Characteristics and Presenting Symptoms
We have identified 36 pts with unequivocal diagnosis of EGT. Baseline characteristics of the cohort are shown in Tab. 1. Median age at diagnosis was 35 years (range 18–66 years). The most common presenting symptoms were abdominal pain and back pain which occurred in 11 (31%) and 12 (33%) pts, respectively, and symptoms of thoracic involvement such as cough, breathlessness, and haemoptysis that were present in 11 pts (31%). Constitutional symptoms of advanced cancer such as fevers, arthralgias, myalgias, fatigue, and weight loss were present in as many as 13 (36%) of our pts. None of the pts had central nervous system involvement on presentation.
1. Baseline characteristics of 36 patients with primary extragonadal germ-cell tumours. 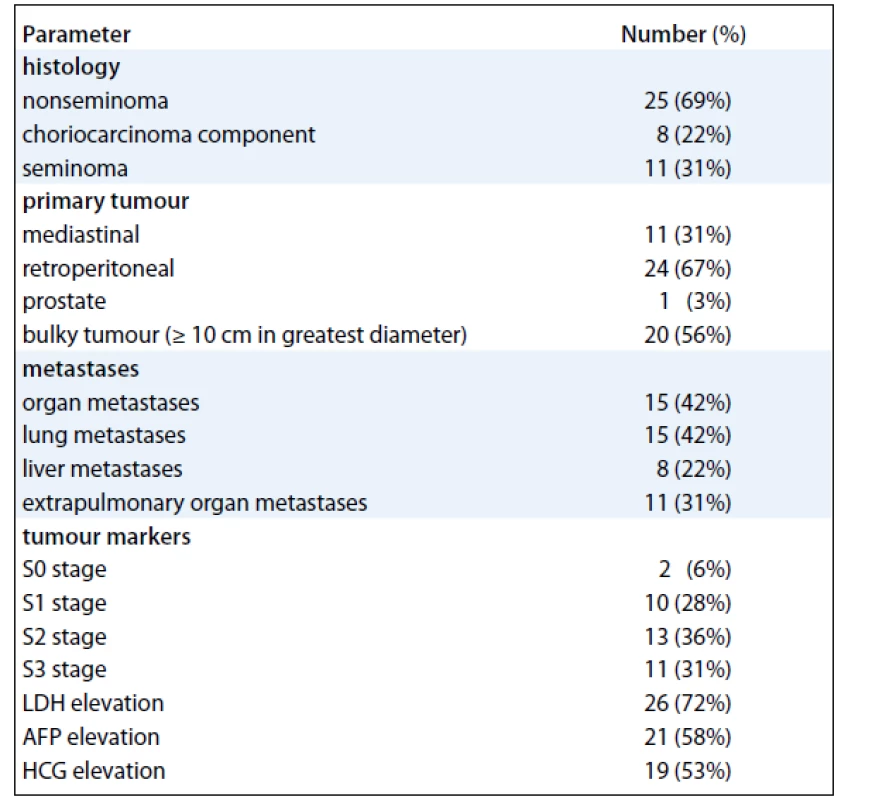
Treatment
All pts received first-line treatment with platinum based chemotherapy, including the standard BEP (bleomycin//etoposide/cisplatin) regimen in 35 pts (97%) and the PVB (cisplatin/vinblastin/bleomycin) regimen in 1 patient (3%). Three (8%) of the 36 pts received additional chemotherapy as a part of the first-line treatment (PVB/BEP hybrid regimen, high-dose methotrexate chemotherapy, and high-dose carboplatin/etoposide chemotherapy with stem cell support in one patient each, respectively).
Four pts (11%) underwent primary resection of the tumor because the previous biopsy had yielded equivocal results failing to establish the germ cell origin of the primary tumor and the masses were assessed as resectable by imaging review. Upon confirmation of germ cell histology (two seminomas, one embryonal carcinoma, one yolk sac tumor), all these pts subsequently received treatment with BEP chemotherapy and are alive and disease-free.
In 12 pts (33%), removal of residual tumor mass and/or retroperitoneal nodal dissection was carried out after chemotherapy. Viable germ cell malignancy was found in six patients (50%) of whom two survive in complete remission after receiving further chemotherapy. Six patients (50%) had necrotic/scar tissue in their resected residual mass and four of them are disease-free.
Salvage chemotherapy for progression, insufficient response, or relapse was given to 21 pts (58%) and consisted usually of four cycles of VeIP (vinblastin/ifosfamide/cisplatin) chemotherapy. Five pts (14%) received high-dose salvage chemotherapy with autologous hematopoietic stem cell support.
Treatment Outcomes
Ten of 36 pts (28%) achieved CR after first-line chemotherapy. Seven pts (19%) had marker (M)-negative partial response (PR), 14 pts (39%) M-positive PR, 2 pts stable disease, 1 patient progression and 2 pts died of their disease progression during first-line therapy.
The median follow-up was 32 months after diagnosis of EGT (range: 5–152 months). Of the 17 surviving pts (47%), sixteen are disease-free. Of the 36 pts, 19 (53%) have died including 18 pts dying of EGT progression or treatment-related complications and one patient dying of an unrelated cause (a traffic accident). Disease-specific OS at one and three years was 81% (95% confidence interval [CI]: 68–94%) and 55% (CI: 38–71%), respectively (Fig. 1).
1. Overall survival of 36 patients with primary extragonadal germ-cell tumours. 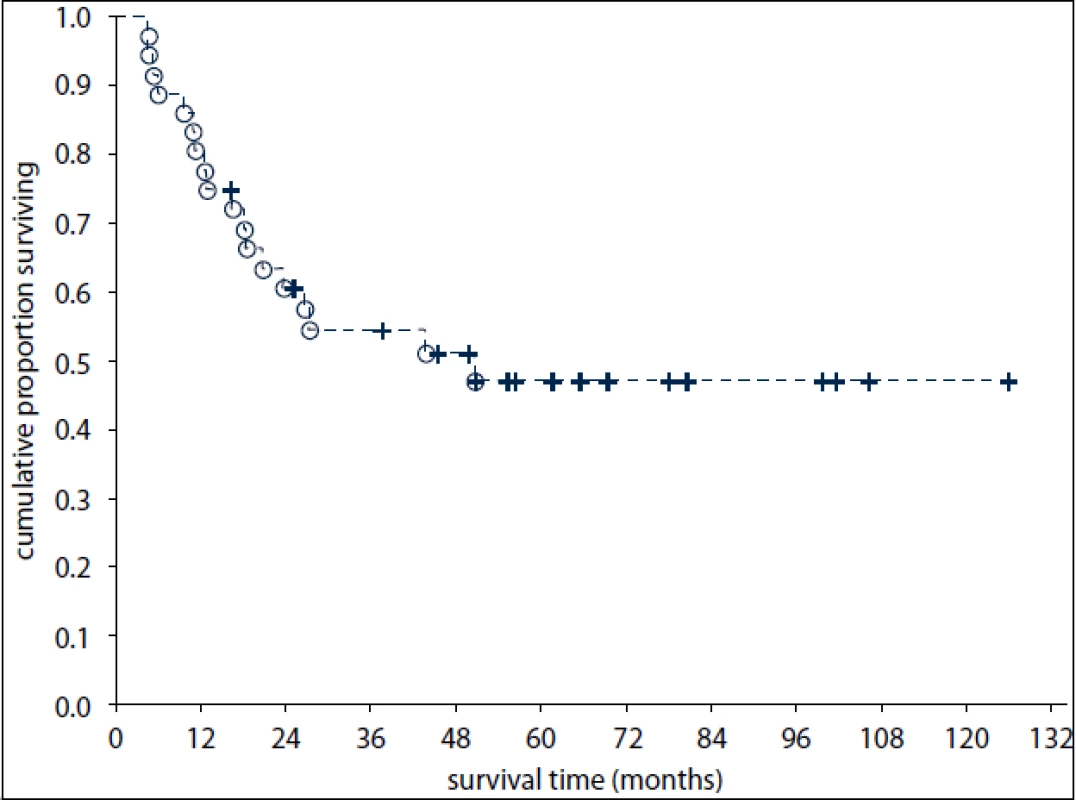
Analysis of Potential Prognostic Factors
Of the studied baseline parameters, none were significantly prognostic for OS although there was a trending association of seminoma histology and the absence of AFP elevation with favourable prognosis (p = 0.067 and p = 0.095, respectively). Resection of residual mass after chemotherapy was not a significant predictor of survival in our cohort.
Negative FDG-PET after completion of treatment was a powerful predictor of long-term survival with 100% pts surviving three years and 89% surviving five years after diagnosis.
None of the patients who had positive FDG-PET findings after 1st line chemotherapy survived at three years after diagnosis (Fig. 2). In contrast, 69% and 20% of patients with positive tumor markers and 90% and 67% of patients with negative tumor markers after first line chemotherapy survived at one and three years, respectively (Fig. 2).
2. Overall survival of patients according to FDG-PET result after the first line chemotherapy (A) and after the completion of treatment (B) and according to tumour marker results after the first line chemotherapy (C) and after the completion of treatment (D). 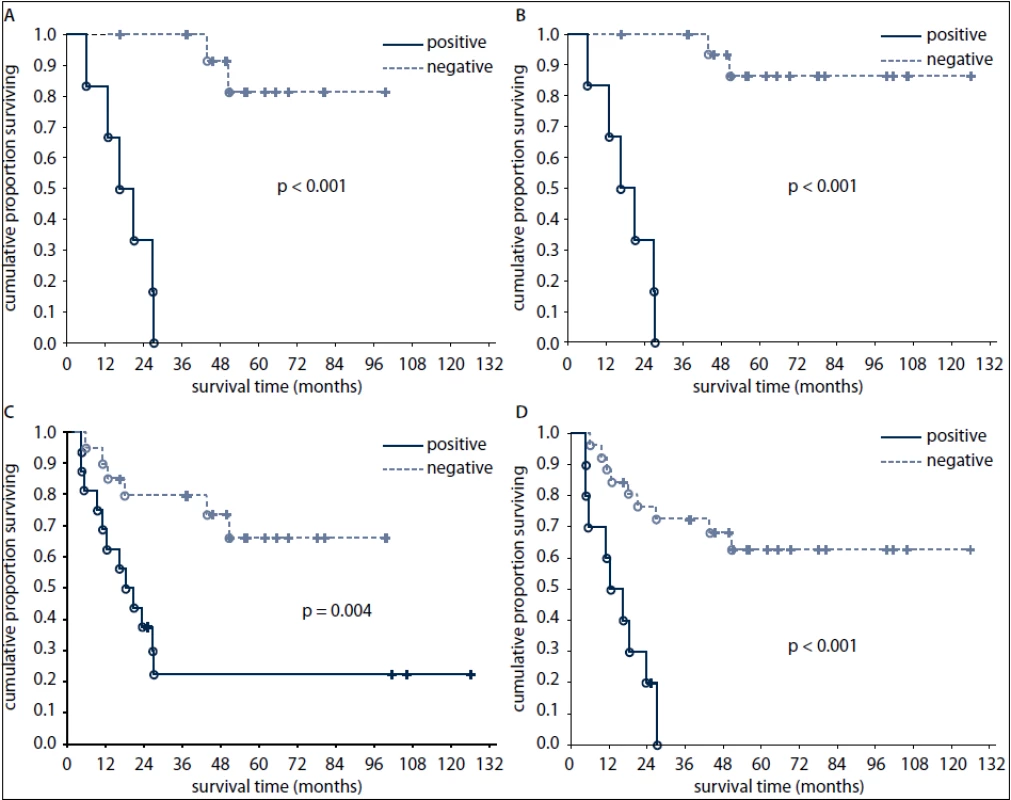
Negative predictive value of post-treatment FDG-PET for OS was better than that of post-treatment markers (89% versus 65%, respectively). All pts with negative post-treatment FDG-PET findings had at the same time negative tumor markers. Of the pts with marker-producing tumors, four pts achieved marker negativity after therapy but remained FDG-PET-positive. All these pts later died of disease progression.
Discussion
EGTs are rare malignancies with poor outcome compared to testicular GCTs. Our knowledge of the clinical course and prognostic parameters of EGTs principally derives from a multicentre retrospective study that included 635 pts aged 14 to 79 years treated at 11 centres in US and Europe from 1975 to 1996 [4]. Adverse prognostic factors for OS of EGT pts identified in a multivariate analysis included nonseminomatous histology, mediastinal primary, involvement of the central nervous system, liver or bone, and HCG elevation [4]. OS in this large study was somewhat better than that in smaller published studies [10,11], as well as in our cohort, probably due to different disease extent at diagnosis. Of our nonseminoma patients, 56% had lung metastases and 32% liver metastases compared to 37% and 16%, respectively, in the multicentre study [3]. Strict criteria for the exclusion of primary testicular tumor were applied for our cohort and could in part explain this discrepancy. Historically, exclusion of testicular tumor was done solely by physical examination. However, true EGTs only represent a minority of generalised germ-cell malignancies with normal findings on palpation of the testes, as demonstrated by Scholtz et al [12].
It is not known whether treatment strategies recommended for TGCTs are optimal for EGT pts. High-dose chemotherapy (HDCT) incorporated into the 1st line regimen achieved promising results in a small study [13]. If given as a part of salvage treatment, HDCT induces remission in up to 43% of pts with retroperitoneal EGTs but only 14% of those with mediastinal primary according to European Bone Marrow Transplant group data [14].
FDG-PET is not thought to be useful for the staging or follow up of nonseminomatous germ-cell tumors although it can reliably identify viable residues of seminoma [15–17]. Early detection of pts with chemoresistant germ-cell tumors by FDG-PET has been proposed but as yet remains experimental [18]. Recently, in a multicentric, prospective trial FDG-PET was not shown to be useful in predicting the presence or absence of viable malignancy or mature teratoma in residual masses after primary chemotherapy in patients with advanced nonseminomatous germ cell tumors. Thus, removal of all resectable residual masses should be performed even if post-chemotherapy FDG-PET findings are negative [19].
Our results suggest that EGT patients achieving FDG-PET negativity after therapy have favourable long-term out-come and thus FDG-PET can be used to guide clinical decisions in these cases, especially the mode and frequency of follow-up. Similar findings have been recently published for patients with testicular nonseminomatous tumors [20]. In our hands, FDG-PET positivity after first-line chemotherapy was associated with extremely poor survival even if salvage therapy was administered. In contrast, patients with negative FDG-PET result after the first-line chemotherapy had relatively favourable prognosis.
These results do not contradict the findings of Oechsle et al [19] because removal of all resectable residual lesions was undertaken in our patients as a part of therapy and our analysis is survival-based whereas the German prospective trial studied the value of FDG-PET in a different setting and indication prior to resection of residual masses [19].
In conclusion, complete responses in primary EGT pts are seldom achieved by first-line chemotherapy but long-term survival is achievable after combined-modality treatment. Negative FDG-PET findings after chemotherapy were strongly associated with favourable long-term survival and FDG-PET appears to be a useful adjunct to other diagnostic methods used to determine treatment outcomes in EGTs.
Supported by grant G9005 (NS10420-3/2009) from the Department of Health, the Czech Republic.
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.
The Editorial Board declares that the manuscript met the ICMJE “uniform requirements” for biomedical papers.
Tomas Buchler, MD
Department of Oncology
Thomayer University Hospital
Videnska 800
140 59 Prague
Czech Republic
e-mail: tomas.buchler@ftn.cz
Submitted: 11. 11. 2011
Accepted: 4. 12. 2011
Sources
1. McKenney JK, Heerema-McKenney A, Rouse RV. Extragonadal germ cell tumors: a review with emphasis on pathologic features, clinical prognostic variables, and differential diagnostic considerations. Adv Anat Pathol 2007; 14(2): 69–92.
2. Schmoll HJ. Extragonadal germ cell tumors. Ann Oncol 2002; 13 (Suppl 4): 265–272.
3. Oosterhuis JW, Stoop H, Honecker F et al. Why human extragonadal germ cell tumors occur in the midline of the body: old concepts, new perspectives. Int J Androl 2007; 30(4): 256–263.
4. Bokemeyer C, Nichols CR, Droz JP et al. Extragonadal germ cell tumors of the mediastinum and retroperitoneum: results from an international analysis. J Clin Oncol 2002; 20(7): 1864–1873.
5. Hartmann JT, Nichols CR, Droz JP et al. Prognostic variables for response and outcome in patients with extragonadal germ-cell tumors. Ann Oncol 2002; 13(7): 1017–1028.
6. Daugaard G, Rørth M, von der Maase H et al. Management of extragonadal germ-cell tumors and the significance of bilateral testicular biopsies. Ann Oncol 1992; 3(4): 283–289.
7. Buchler T, Freeman A, Harland S. Contralateral intratubular germ cell neoplasia in a patient with testicular cancer. Nat Clin Pract Urol 2008; 5(5): 284–288.
8. International Germ Cell Cancer Collaborative Group. International Germ Cell Consensus Classification: a prognostic factor-based staging system for metastatic germ cell cancers. J Clin Oncol 1997; 15(2): 594–603.
9. Bukowski RM, Wolf M, Kulander BG et al. Alternating combination chemotherapy in patients with extragonadal germ cell tumors. A Southwest Oncology Group study. Cancer 1993; 71(8): 2631–2638.
10. Hsu YJ, Pai L, Chen YC et al. Extragonadal germ cell tumors in Taiwan: an analysis of treatment results of 59 patients. Cancer 2002; 95(4): 766–764.
11. Goss PE, Schwertfeger L, Blackstein ME et al. Extragonadal germ cell tumors. A 14-year Toronto experience. Cancer 1994; 37(7): 1971–1979.
12. Scholz M, Zehender M, Thalmann GN et al. Extragonadal retroperitoneal germ cell tumor: evidence of origin in the testis. Ann Oncol 2002; 13(1): 121–124.
13. Kumano M, Miyake H, Hara I et al. First-line high-dose chemotherapy combined with peripheral blood stem cell transplantation for patients with advanced extragonadal germ cell tumors. Int J Urol 2007; 14(4): 336–338.
14. De Giorgi U, Demirer T, Wandt H et al. Second-line high-dose chemotherapy in patients with mediastinal and retroperitoneal primary non-seminomatous germ cell tumors: the EBMT experience. Ann Oncol 2005; 16(1): 146–151.
15. De Santis M, Pont J. The role of positron emission tomography in germ cell cancer. World J Urol 2004; 22(1): 41–46.
16. Karapetis CS, Strickland AH, Yip D et al. Use of fluorodeoxyglucose positron emission tomography scans in patients with advanced germ cell tumor following chemotherapy: single-centre experience with long-term follow up. Intern Med J 2003; 33(9–10): 427–435.
17. Hain SF. Positron emission tomography in uro-oncology. Cancer Imaging 2005; 5(1): 1–7.
18. Bokemeyer C, Kollmannsberger C, Oechsle K et al. Early prediction of treatment response to high-dose salvage chemotherapy in patients with relapsed germ cell cancer using [(18)F]FDG PET. Br J Cancer 2002; 86(4): 506–511.
19. Oechsle K, Hartmann M, Brenner W et al. [18F]Fluorodeoxyglucose positron emission tomography in nonseminomatous germ cell tumors after chemotherapy: the German multicenter positron emission tomography study group. J Clin Oncol 2008; 26(36): 5930–5935.
20. Buchler T, Simonova K, Fencl P et al. Clinical outcomes of patients wtih nonseminomatous germ cell tumours and negative postchemotherapy positron emission tomography. Cancer Invest 2012. Epub ahead of print.
Labels
Paediatric clinical oncology Surgery Clinical oncology
Article was published inClinical Oncology

2012 Issue 3-
All articles in this issue
- New and Clinically Used Oncomarkers of Bladder Cancer
- Reproductive Functions in Women after Cancer Therapy
- Genetic Background of Cisplatin Induced Ototoxicity
- Triple-Negative Breast Cancer: Analysis of Patients Diagnosed and/or Treated at the Masaryk Memorial Cancer Institute between 2004 and 2009
- Angioimmunoblastic T-cell Lymphoma as a Very Poor-Prognosis Malignancy – a Single Centre Experience
- Patient with B-CLL with a History of Unrelated Hematopoietic Cells Donation – Retrospective Analysis of CLL Development and Implication for the Recipient
- Benefits of Individual Imaging Methods for Diagnosis and Monitoring of Activity of Multiple Myeloma
- Positron Emission Tomography and Clinical Predictors of Survival in Primary Extragonadal Germ Cell Tumors
- Granulocyte-Colony Stimulating Factor (G-CSF) Accelerates Healing of Radiation Induced Moist Desquamation of the Skin
- Clinical Oncology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Angioimmunoblastic T-cell Lymphoma as a Very Poor-Prognosis Malignancy – a Single Centre Experience
- Triple-Negative Breast Cancer: Analysis of Patients Diagnosed and/or Treated at the Masaryk Memorial Cancer Institute between 2004 and 2009
- New and Clinically Used Oncomarkers of Bladder Cancer
- Granulocyte-Colony Stimulating Factor (G-CSF) Accelerates Healing of Radiation Induced Moist Desquamation of the Skin
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

