-
Medical journals
- Career
Benefits of Individual Imaging Methods for Diagnosis and Monitoring of Activity of Multiple Myeloma
Authors: J. Vanicek 1,5; P. Szturz 2; Z. Rehak 3; B. Kianicka 4; M. Bulik 1,5
Authors‘ workplace: Department of Imaging Methods, International Clinical Research Center, St. Anne’s University Hospital, Brno 1; Department of Internal Medicine – Hematooncology, University Hospital, Brno 2; Department of Nuclear Medicine, Masaryk Memorial Cancer Institute, Brno 3; IHASH2 Clinic of Internal Medicine, St. Anne’s University Hospital, Brno 4; Department of Imaging Methods, Faculty of Medicine of Masaryk University, Brno 5
Published in: Klin Onkol 2012; 25(3): 166-172
Category: Reviews
Overview
Background:
Multiple myeloma pathogenesis, pathology, symptoms and imaging techniques used in clinical diagnostic algorithm, the indications and the differences between currently available imaging methods.Design:
The article describes advantages and disadvantages of basic X-ray imaging and recommended skeleton screening, as the method of first choice, followed by description of the most frequently affected areas and Mirels score. The present golden standard magnetic resonance (MR) imaging, its potential and also recommended MR indications. Concerning computer tomography (CT) imaging, mainly comparison between CT and MR and X-ray imaging its indications and benefits as the interventional instrument are mentioned. The arcticle also focuses on the role of skeleton scintigraphy with Tc-pyrophosphate, which is not recommended today, and the role of positron emission tomography with fluorodeoxyglucose (FDG-PET) in the assessment of the therapy effectiveness and prognosis for patients, its future and present limitations. The next commonly used radioisotope imaging with 99Tc-sestamibi (MIBI) and its comparison to other methods, especially to the FDG-PET and recommended indications for both techniques. Last aim is description of specification of bone tissue density with Dual Energy X-ray Absorption scanning method (DEXA).Conclusion:
These imaging methods are commonly used as additional diagnostic tests in initial diagnostic work-up and in follow-up due to frequent relapses of multiple myeloma.Key words:
multiple myeloma – diagnostic imaging – magnetic resonance – positron emission tomography – scintigraphy – computer tomography – indicationsIntroduction
Multiple myeloma is a malignant disease with assumed malignant mutations on the level of B-lymphocytes, which proliferate and mature into clonal plasmatic cells. In most cases, these cells produce monoclonal immunoglobulin, which may cause damage to the organism and further produce multiple cytokines, which also contribute to the manifestations of the disease and, among other things, activate osteoclasts and cause osteolysis.
The classical triad of symptoms includes fatigue, bone pain and recurrent infections. The most frequent symptoms include back pain or pain in other parts of the skeleton, anemia in 70% of all cases, increased concentrations of creatinine already as soon as the disease is diagnosed, and in 10–20% of patients hypercalcaemia in the diagnostic stage of the disease.
Multiple myeloma may also be manifested by other symptoms (Tab. 1).
1. Symptoms of multiple myeloma. 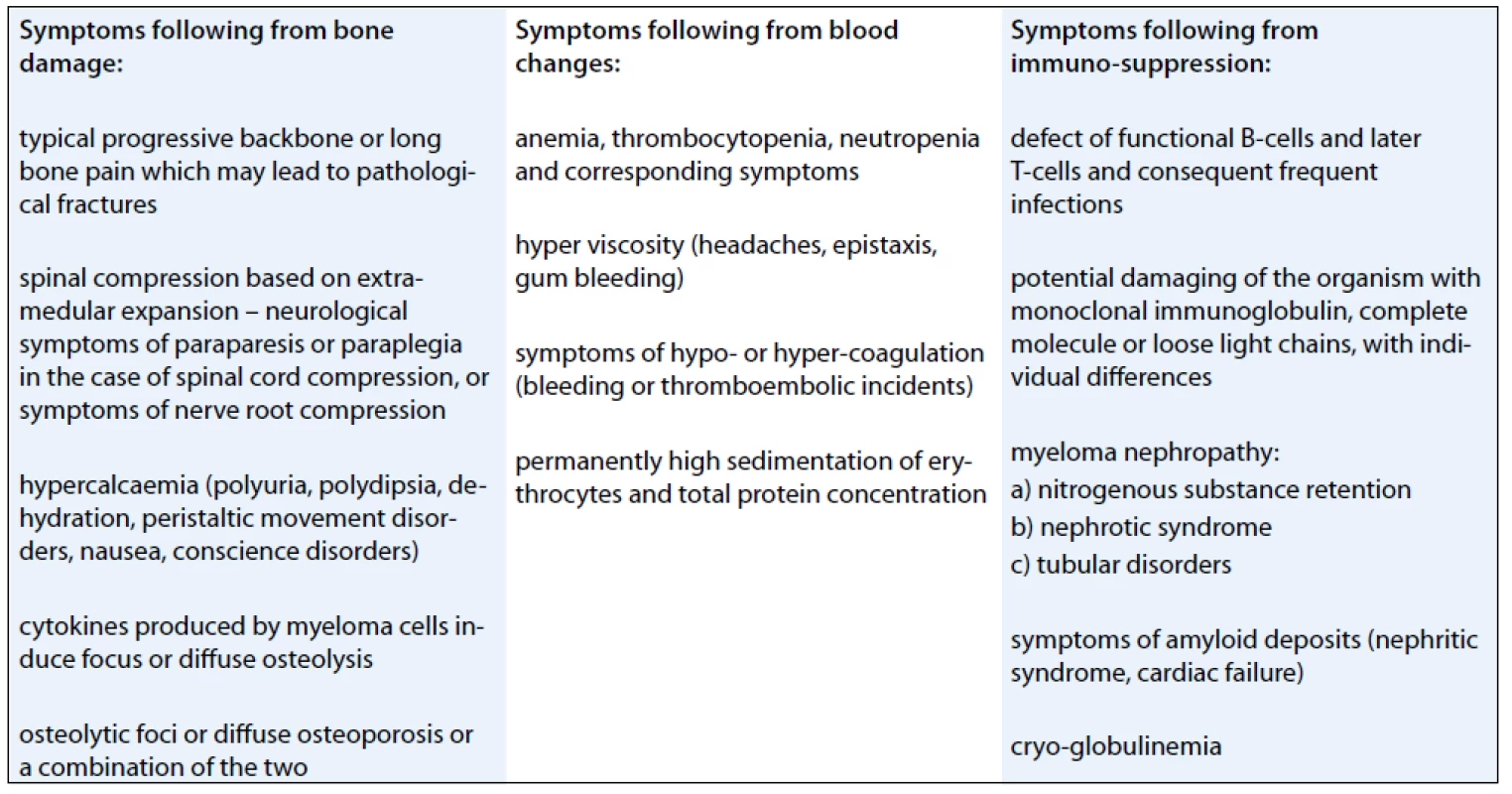
Individual patients may display one of the symptoms or any combination of multiple symptoms.
The purpose of this article is to analyse currently available techniques that are informative about skeleton disorders [1–5].
Skeleton x-Raying
In the case of first suspicion, only the painful target area is x-rayed.
In the case of suspected multiple myeloma skeleton screening is recommended [6]. About 80% of diagnosed myeloma patients show clear osteolytic deposits in their diagnostic skeleton images. The most frequently affected areas include vertebrae (66%), ribs (45%), skull (40%) (Fig. 1), humeral bones (40%), pelvis (30%), long bones (25%) (Fig. 2). Exceptionally, other deposits may also be found in bones distal of the knee or the elbow joint. In case of long bones, the x-ray image may be the starting point for decisions about further progression in the case of osteolytic defects of the bone pursuant to the Mirels score [7] (Tab. 2). The patients with low scores can profit from radiotherapy, while patients with high scores may profit from orthopedic internal fixation. Deposits distorting the cortical bone or deposits with diameter larger than 2/3 of the bone diameter soon cause high percentage of pathological fractures, therefore representing an indication to preventive osteosynthesis.
1. Osteolytic deposit in the skull in a front-rear x-ray image. 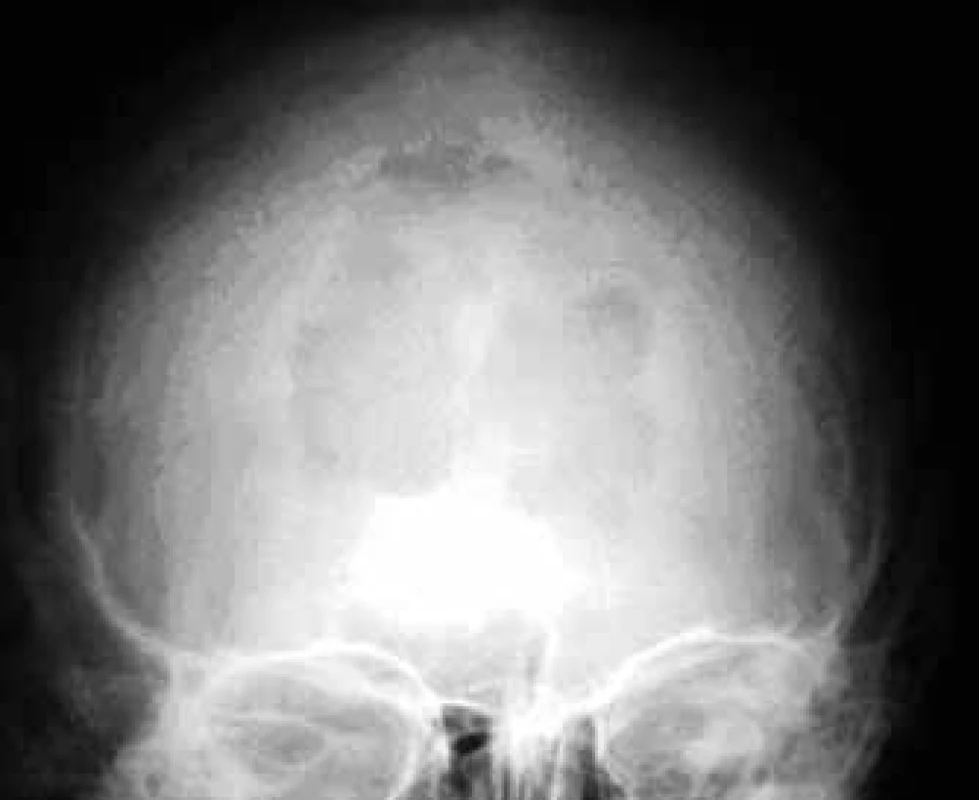
2. Femoral osteolytic deposit on the right of the osteosynthetic material in an x-ray image of the pelvis. 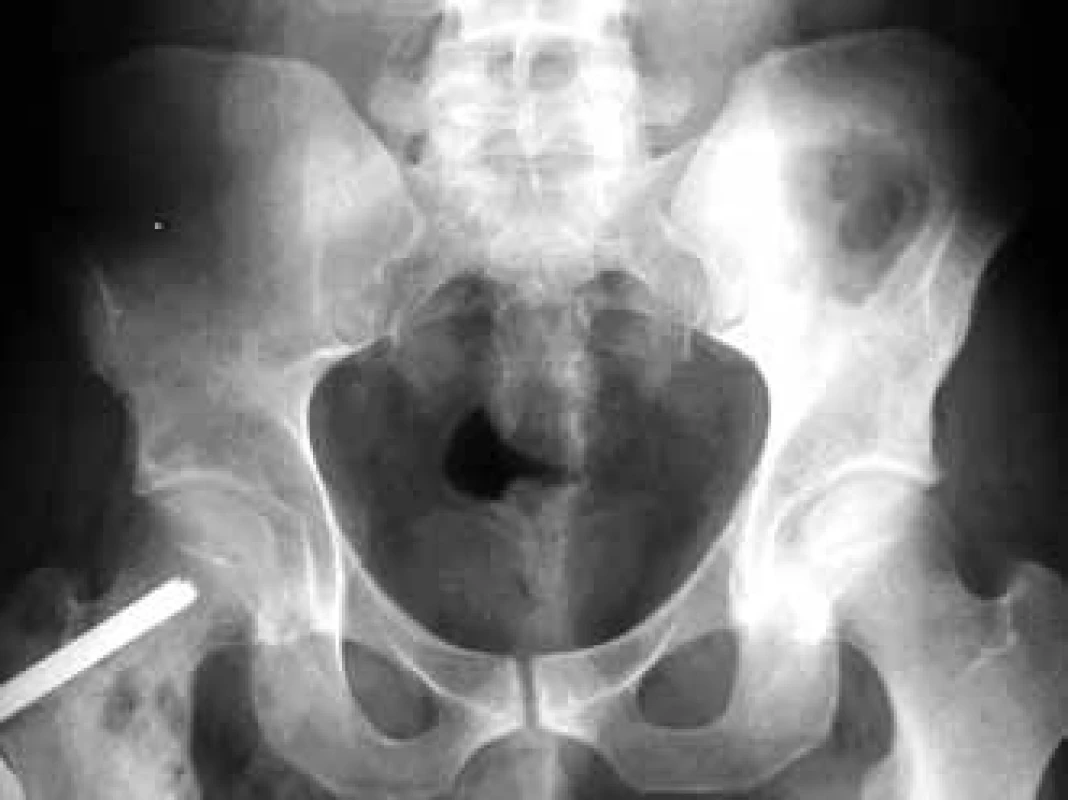
Pursuant to Mirels, the probability of occurrence of pathological fracture is 4% in the case of the score up to 7, 15% in the case of score 8 and 33% in the case of score 9 and higher. Score 9 is always an indication while score 8 is a boundary indication for preventive osteosynthesis.
Patients with a visible deposit in their x-ray image, even though still asymptomatic, are patients with high risk of disease progression towards symptomatic disease with medial interval from a symptomatic deposit find to clinically manifested symptoms of bone damage of 8 months. That is why immediate therapy commencement is recommended after first osteolytic deposit finding despite absence of clinical symptoms. As an alternative to the radiotherapy of the lesions localized in spinal column must be always considered neurosurgical approach [8,9].
Even though the skeleton x-ray screening represents a certain burden for the patient, effective substitution with all-body magnetic resonance imaging is not yet possible for capacity reasons. The benefits of the x-ray screening include quick obtaining of information about the condition of the skeleton. Disadvantages include low sensitivity. Decalcification deposit is only seen after disappearance of at least 30% of hydroxyapatite; in some locations, even higher loss of hydroxyapatite (up to 60%) is needed for deposit visibility.
Another disadvantage of x-ray imaging of the skeleton is represented by the fact that some parts of the skeleton are very difficult to assess on the basis of x-ray images (the sternum, the ribs, the shoulder blades). However, x-ray images cannot be used for bone density assessment, and expression of the level of osteoporosis on the basis of x-ray images is very inaccurate [10,11].
In many cases, osteolytic pain is present and yet the x-ray image of the painful location does not show any pathological finding. This situation indicates MR or CT imaging [12].
In most patients, osteolytic deposits do not heal even under conditions of complete remission, which is why radiographic imaging cannot be used for confirmation of the patient’s response to the applied therapy. But radiography can still be effectively used for discovery of new deposits indicating disease progression.
Recommended scope of skeleton screening for multiple myeloma:
- front-rear image of the breast;
- front-rear and lateral images of C, Th, L of the backbone;
- femoral, humeral and pelvis images;
- skull images;
- potential other areas of suspected occurrence unless covered by the above screening scope.
Distal sections of the skeleton, forearm and shank are not usually imaged in the context of the screening, for deposits in these areas are very rare.
MR Imaging
For early specification of the disease scope, magnetic resonance (MR) represents the most progressive method. MR images show pathological infiltrations of the bone marrow and allow for their distinction from physiological bone marrow long before development of serious damage to the mineral bone structure. Typical myeloma deposits return low signal intensity of T1 weighed imaging and high signal intensity of T2 weighed imaging and STIR (short time inversion recovery) imaging and usually display an enhancement after application of a contrast substance – gadolinium. MR is also able to provide information about the type of damage to the bone marrow (focal, diffuse or a combination of the two).
Low mass of myeloma cells usually shows normal image while large mass often causes diffuse hypointense signal of T1 weighed imaging and diffuse hyperintense signal of T2 weighed imaging with enhancement after application of gadolinium.
The above-mentioned changes are not specific of myeloma; focal or diffuse changes may be variants of the standard condition or result from other pathological processes (iron or amyloid deposits, or reactive hyperplasia of the bone marrow).
Therefore, the potential of MR imaging of the skeleton is information about the level to which the skeleton is affected by the disease and about extramedular propagation of the disease. The results of MIR imaging show prognostic value. For example, a finding of more than 10 deposits in the backbone considerably increases probability of pathological fractures in this area.
Pathological infiltrations in MR images can be found long before occurrence of clear osteolytic deposits seen in x-ray images.
MR is able to distinguish compressive fractures developed as a consequence of osteoporosis or pathological vertebral infiltration (Fig. 3) [13].
3. Multiple compressive fractures of thoracic vertebras in MR image, hypersingal-light – in the case of myeloma infiltration, dark in the case of osteoporosis. 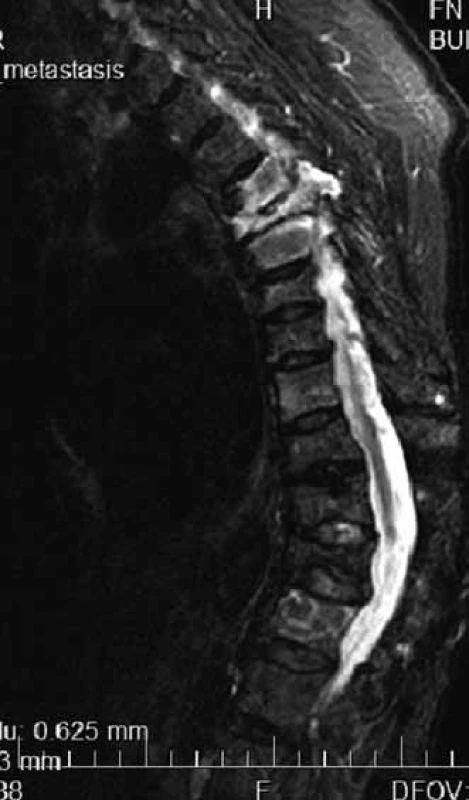
Changes in the bone marrow after treatment are hard to interpret, though, that is why skeleton MR is not usually used for assessment of therapy success, but predominantly for specification of the scope of bone and off-bone damage, at the diagnostic stage and in the case of suspected disease progression (Fig. 4) [6].
4. Infiltration of the sternum with myeloma in MR image. 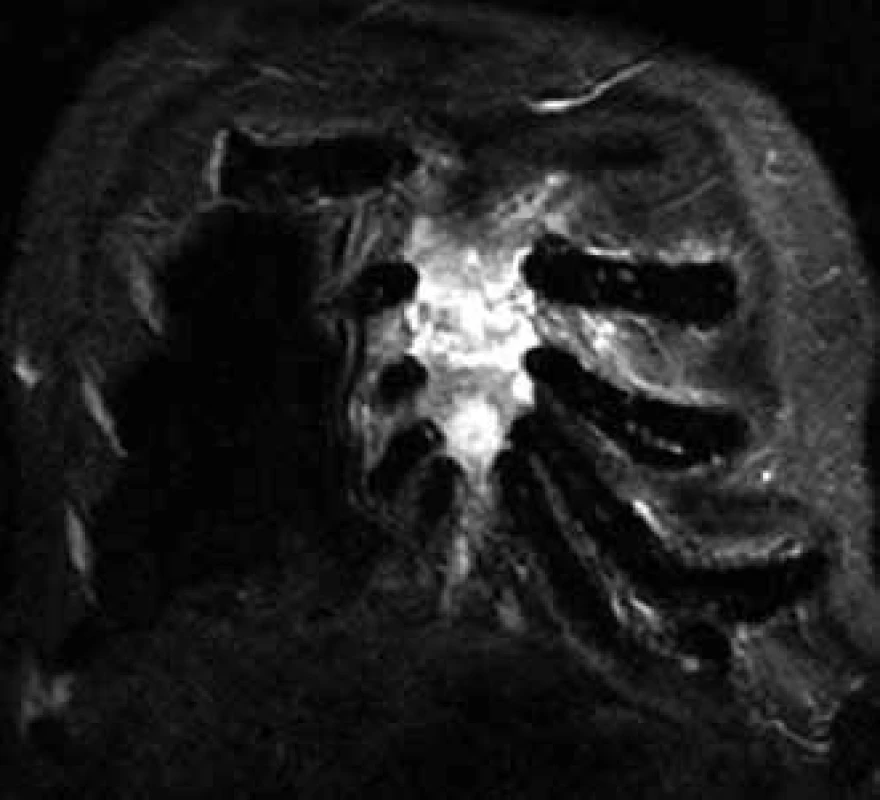
Recommended MR indications:
- acute MR imaging is the method of choice in the case of suspected spinal cord compression in patients with multiple myeloma, even in cases when x-ray images do not show any visible compressive fracture;
- all-spine MR is necessary in patients with solitary plasmocytoma, for this examination can help find other deposits and change classification and therapeutic procedure;
- MR imaging is recommended to focus on areas with suspected damage in multiple myeloma patients with ambiguous or negative find in standard x-ray or CT images, for this examination provides complementary information;
- in the course of the disease progression, MR imaging is recommended for clarification of new symptoms unexplained by previous examinations;
- MR imaging is the optimum approach in case it is needed to prove vascular necrosis of femoral neck and head.
CT Imaging
CT images show mineral structure of hydroxyapatite more accurately than MR but their potential to show pathological soft tissue infiltration of the bone is lower than in the case of MR. They are still able to show extramedular propagation of myeloma, though.
Thanks to the high resolution level, CT can be effectively used for imaging of small lytic deposits not clearly seen in standard x-ray images.
For this high resolution feature, CT is recommended for clarification of ambiguous changes shown by standard x-ray images. Also, in the case of pain in a particular part of the skeleton without visible pathology in a standard x-ray image, CT may help. CT is also the method of choice for imaging of parts of the skeleton which cannot be well visualized by standard x-ray images, such as shoulder blades, ribs and sternum (Figs 5–7).
5. Osteolytic deposits of sternum manubrium in CT image. 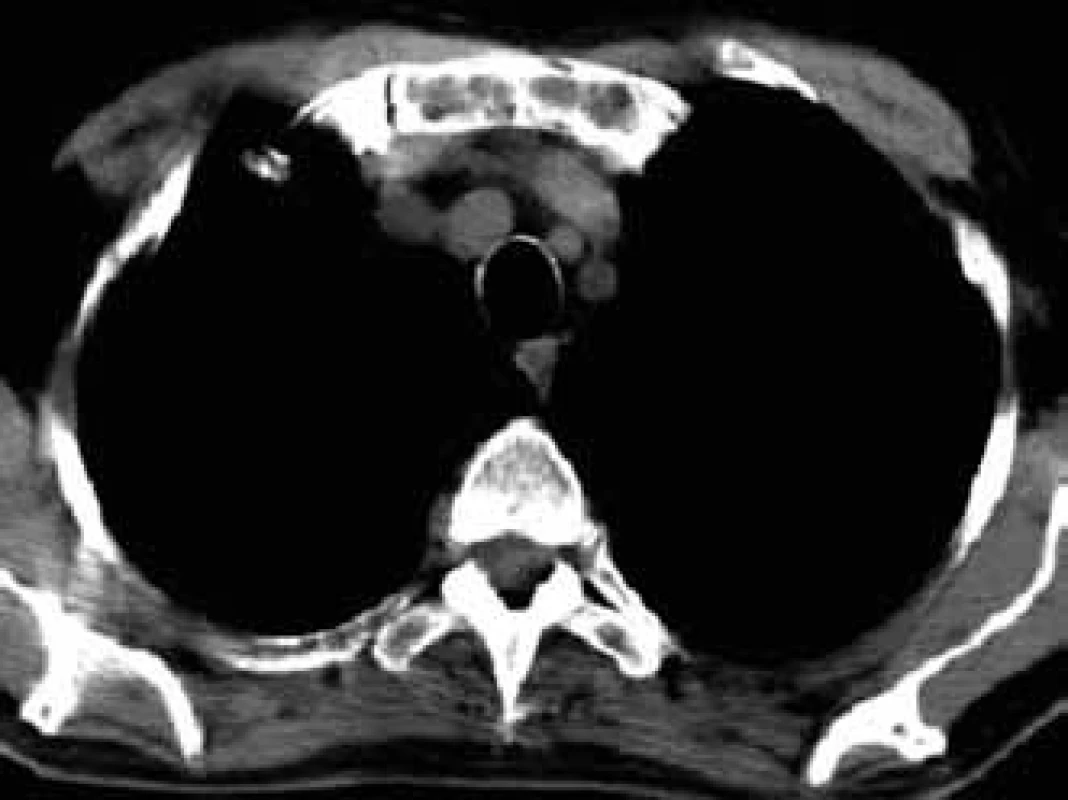
6. A smaller osteolytic deposit in a thoracic vertebra and rib head on the left in CT image. 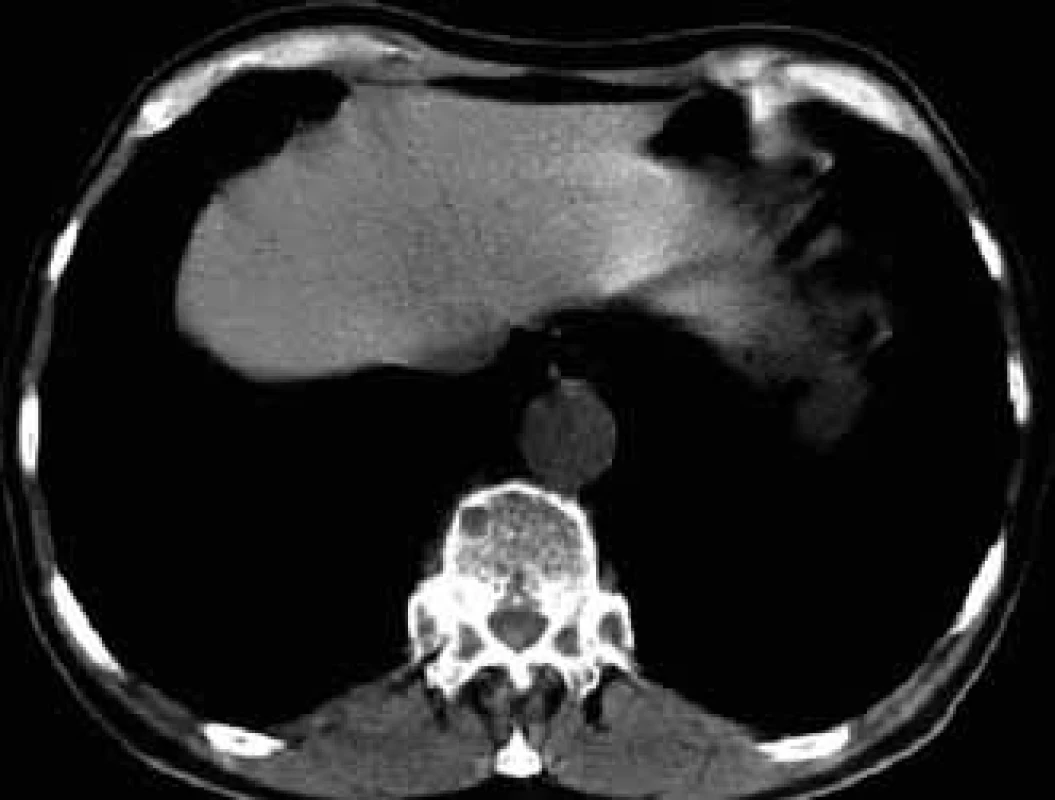
7. MR image shows myeloma deposits in two medial ends of ribs on the left (the same patient as in the case of Fig. 6). 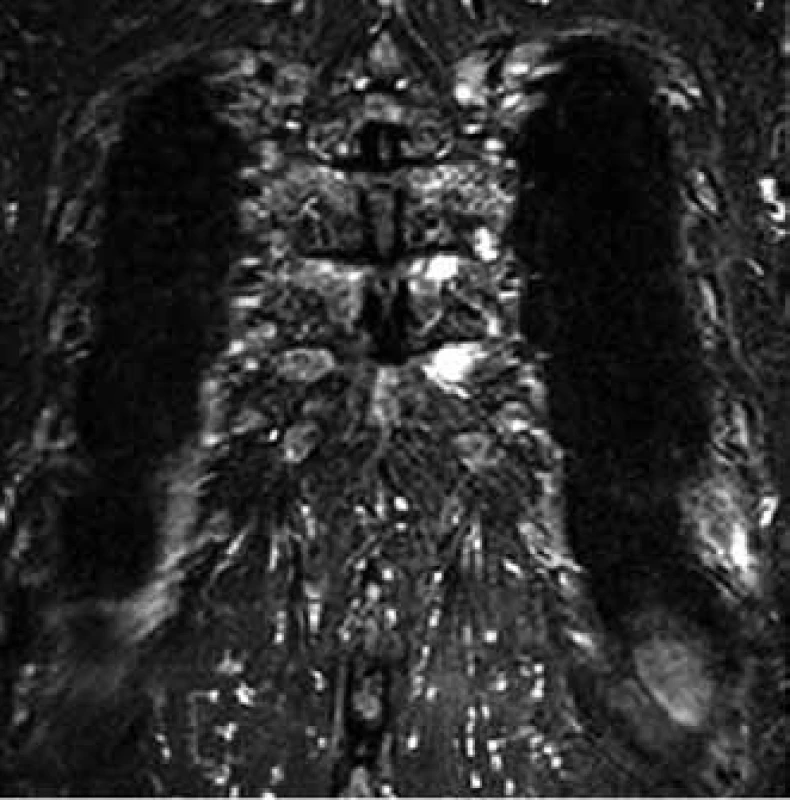
CT also allows for execution of targeted percutaneous puncture of the bone marrow from vertebrae or other structures for diagnostic purposes. This procedure is the optimum method in cases of clear pathological finding in one or more vertebrae when trepanobiopsy of the hip bone blade did not show malignant infiltration and the patient has no visible tumor of the lungs or breast. Timely taking of a histological sample from a pathological deposit is ’a lege artis’ procedure, while the frequently performed search for the primary leasion only means loss of time [6,12,14–17].
Recommended CT Indications:
- suspected spinal cord compression in cases when MR imaging cannot be performed (magnetic metal in the patient’s body or patient’s claustrophobia);
- spinal CT for clarification whether vertebral destruction is present or not in case of pain, including case of negative MR of this area;
- ambiguous findings in standard x-ray images, suspect deposits in areas of skeleton which cannot be clearly imaged by radiographic methods, such as ribs, sternum and scapula (Fig. 6);
- symptoms of bone damage in the case of completely negative standard radiography, where CT image can show what is not visible on a standard x-ray image;
- CT is also recommended in the case of suspect soft tissue infiltration, specification of its scope and the need for CT navigated biopsy of the tissue;
- monitoring of the disease activity is not recommended with the exception of monitoring of extramedular myeloma masses;
- in the course of the disease progression, CT is indicated in the case of unexplained symptoms, suspected disease progression with suspected new fracture.
Skeleton Scintigraphy with Tc Pyrophosphate
Standards scintigraphy of the skeleton is based on proven deposits with increased intensity of mineralization and settlement of applied technetium pyrophosphate. Imaging of malignant deposit with technetium pyrophosphate is therefore conditioned by the presence of osteoneogenesis.
As active osteolysis and no or minimal osteogenesis are typical of bone deposits of multiple myeloma, detection of multiple myeloma deposits in the skeleton show lower sensitivity than detection with standard x-ray imaging [6,18].
Standard scintigraphy of the skeleton sometimes shows deposits in the rib, scapula or sternum areas; imaging of these bones with standard x-ray imaging usually show poor results [19], but CT is more sensitive. That is why skeleton scintigraphy in myeloma patients is not recommended today.
Positron Emission Tomography with Fluorodeoxyglucose
Positron emission tomography with fluorodeoxyglucose (FDG-PET) or PET-CT represents a great benefit for specification of the scope of disease effects [20].
FDG-PET is able to provide information about the scope of skeletal damage and at the same time information about potential extramedular (soft tissue) deposits. Extramedular deposit is a very unfavorable prognostic factor. Findings of duplicate malignity represent an occasional unpleasant surprise.
Reported limitations of FDG-PET include poor imaging of subcentimeter deposits of myeloma cells.
FDG-PET imaging may be used for assessment of effectiveness of radiotherapy in the case of large solitary plasmocytomas. Ongoing post-therapy activities signal unfavorable prognosis and insufficiently effective treatment. Some authors have described assessments of effects of cytostatic therapies and favorable prognoses in patients with normalization of the originally pathological finding by FDG-PET.
Ongoing positivity of PET after completion of high-dose chemotherapy correlated with unfavorable prognoses for these patients [21]. Assessment of disease progression with FDG-PET is not widespread, only being indicated in patients with non-secretory or oligo-secretory myeloma.
Although FDG-PET has been used in myeloma patients for several years now, its indications have not been clearly defined yet. Planning of FDG-PET should respect the recommended interval of 4 weeks after last chemotherapy and 3 months after last radiotherapy [6]. In the case of tumors not showing intensive PET activity, including multiple myeloma, a delay after surgery is recommended for tissue healing may produce false positive results.
FDG-PET examination is a method used selectively; its accurate indication in the case of multiple myeloma is still in progress.
Nowadays, the PET scan is substituted by the PET/CT scan, while PET scan is becoming an abandoned method. The PET/CT scan combines PET metabolic information and CT morphologic information. The new generation of used PET scanners (in PET PET/CT) already allows to identify lesions smaller than 7 mm, shortens the scan time by more than half, and the CT data are not used only for attenuation correction, but bring diagnostic information as well. In case of myeloma CT scans mainly in LD (low - -dose) regime are enough for identifying skeletal lesions. HDCT – high-dose CT is less common and considering the risks with myeloma patients, the use of iodine contrast medium is also rather rare.
Both FDG-PET in combination with low-dose CT and whole-body MRI are more sensitive than skeleton X-ray in screening and diagnosing multiple myeloma. WB-MRI allows assessment of bone marrow involvement but cannot detect bone destruction, which might result in overstaging. Moreover, WB-MRI is less suitable in assessing response to therapy than FDG-PET. The combination of PET with low-dose CT can replace the golden standard, conventional skeletal survey. In clinical practice, this will result in upstaging, due to the higher sensitivity [22].
PET/CT allows identification of high-risk myeloma patients, and extramedullary lesions with the highest SUV (max) – maximum standardized uptake value [23].
On principle, the PET/CT scan can be also done without non-FDG radiotracers.
8. FDG PET /CT , MIP: therapy response monitoring. 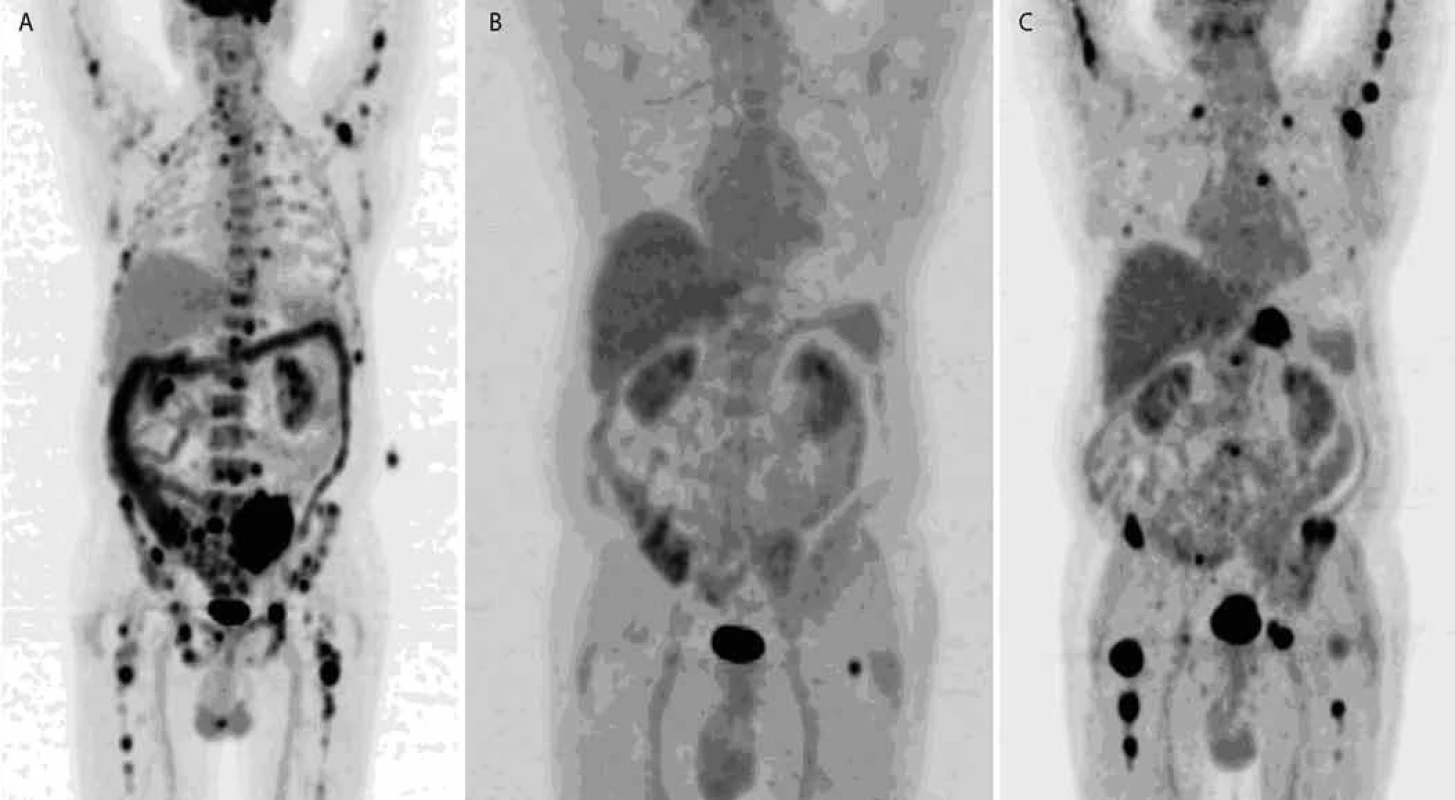
Left: baseline, multiple lesions with high FDG uptake generalized in skeleton. Middle: lesion disappearance, one remaining (in left femoral cervix). Right: multiple lesions with high FDG uptake in skeleton have appeared again which shows evidence of progression. On the basis of increased 11C-Methionine uptake in plasma cells, active multiple myeloma can be imaged with 11C-Methionine PET/CT [25]. Also 11C-Choline PET/CT appears to be more sensitive than 18F-FDG PET/CT for the detection of bony myelomatous lesions [25].
Radioisotope Imaging with 99Tc-sestamibi (MIBI)
Another commonly used radioisotope imaging method uses imaging with sestamibi substance, abbreviated as MIBI (99mTc-methoxy-isobutyril-isonitril), marked with radioactive technetium. Sestamibi is a substance accumulating, among other places, in mitochondria, which are more frequent in myeloma cells than in the surrounding structures. That is why MIBI allows for imaging of the size of multiple myeloma and finding potential extramedular deposits. Results of frequent studies testing MIBI imaging of multiple myelomas have confirmed that MIBI catching correlates with other parameters of the disease [6].
Comparisons of x-ray, MIBI and MR imaging of the spine have shown that the scope of effects of the disease on the spine in MIBI images was lower than in the case of MR imaging, but the overall effects on the skeleton shown by MIBI images were more extensive than in the case of x-ray imaging [26,27].
Comparisons of PET and MIBI imaging have shown that MIBI images were able to detect deposits not visible in x-ray images, and the number of detected deposits was higher than in the case of FDG-PET. MIBI activity better correlated with the number of plasmocytes in the bone marrow than in the case of FDG-PET imaging [28]. Interpretation of MIBI images should consider the fact that MIBI shows presence of myeloma deposits while FDG-PET shows activity of the cells of these deposits. The study comparing PET/CT and MIBI shows that 18F-FDG PET/CT appeared to be a better imaging technique than 99mTc-MIBI scintigraphy in the detection of focal lesions in patients with symptomatic multiple myeloma. 99mTc-MIBI was superior in the visualization of diffuse disease. On the other hand, despite its limited capacity in detecting focal lesions, 99mTc-MIBI scintigraphy still remains the most rapid and inexpensive technique for whole-body evaluation and may be an alternative option when a PET/CT facility is not available [10].
Recommended Indications for FDG-PET and MIBI Imaging:
- solitary plasmocytoma – finding of other deposits may change classification and therapeutic procedure;
- suspect extramedular spread of myeloma masses, unless MR imaging is performed;
- assessment of therapy of deposit plasmocytomas of large sizes (diameter > 5–10 cm), which often poorly respond to therapies;
- neither FDG-PET nor MIBI are recommended in the course of therapies for routine use with all patients. They are only recommended for the selected group of patients with non-secretion or extramedular multiple myeloma.
Specification of Bone Tissue Density with DEXA (Dual Energy x-Ray Absorption Scanning) Method.
DEXA is a standard procedure for measurement of the level of osteoporosis. Low density of lumbar vertebrae increases the risk of pathological fractures. Nevertheless, the found density depends on spondylosis, osteophytes and compressive vertebral fractures. That is why the assessment of results in patients with myeloma is more difficult.
Conclusion
Multiple myeloma is a malignant neoplasm of plasma cells that accumulate in the bone marrow, leading to bone destruction and marrow failure. It is very sensitive to treatment and typically relapses, that is why not only the initial diagnostic work-up, but also an early diagnostics in follow-up are so important. Next to laboratory tests, the role of imaging methods is additional; however, concerning the quality of life of the patient, it is very useful. In clinical indications for follow-up, we use especially MRI, CT or PET/CT scan.
Supported by European Regional Development Fund-Project FNUSA-ICRC (No.CZ.1.05/1.1.00/02.0123).
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.
The Editorial Board declares that the manuscript met the ICMJE “uniform requirements” for biomedical papers.
Martin Bulik, MD
Department of Imaging Methods
International Clinical Research Center
St. Anne’s University Hospital
Pekarska 53
656 91 Brno
Czech Republic
e-mail: bulik@fnusa.cz
Submitted: 2. 10. 2011
Accepted: 18. 2. 2012
Sources
1. Adam Z, Bednařík J, Neubauer J et al. Doporučení pro časné rozpoznání postižení skeletu maligním procesem a pro časnou diagnostiku mnohočetného myelomu. Vnitř Lék 2006; 52 (Suppl 2): 9–13.
2. Anderson KC, Alsina M, Bensinger W et al. National Comprehensive Cancer Network (NCCN). Multiple myeloma. Clinical practice guidelines in oncology. J Natl Compr Canc Netw 2007; 5(2): 118–147.
3. Barosi G, Boccadoro M, Cavo M et al. Management of multiple myeloma and related-disorders: guidelines from the Italian Society of Hematology (SIE), Italian Society of Experimental Hematology (SIES) and Italian Group for Bone Marrow Transplantation (GITMO). Haematologica 2004; 89(6): 717–741.
4. Durie BG, Kyle RA, Belch A et al. Scientific Advisors of the International Myeloma Foundation. Myeloma management guidelines: a consensus report from the Scientific Advisors of the International Myeloma Foundation. Hematol J 2003; 4(6): 379–398.
5. Harrouseau JL, Greil R, Kloke O. ESMO Guidelines Task Force. ESMO Minimum Clinical Recommendations for diagnosis, treatment and follow-up of multiple myeloma. Ann Oncol 2005; 16 (Suppl 1): i45–i47.
6. D’Sa S, Abildgaard N, Tighe J et al. Guidelines for the use of imaging in the management of myeloma. Br J Haematol 2007; 137(1): 49–63.
7. Mirels H. Metastatic disease in long bones. A proposed scoring system for diagnosing impending pathologic fractures. Clin Orthop Relat Res 1989; 249 : 256–264.
8. Chaloupka R, Grosman R. Zásady operačního ošetření maligních nádorů páteře. Acta Spondylologica 2002; 1 : 39–41.
9. Chaloupka R, Vlach O, Grosman R. Dlouhodobé výsledky po operační léčbě maligních nádorů krční páteře. Scripta Medica Brno 1998; 71 (Suppl 5): 154–156.
10. Mysliveček M, Nekula J, Bačovský J. Zobrazovací metody v diagnostice a sledování mnohočetného myelomu. Vnitř Lék 2006; 52 (Suppl 2): 46–54.
11. Neubauer J, Adam Z, Pour L. Jak rozlišit, zda je kompresivní fraktura obratle způsobená osteoporozóu nebo mnohočetným myelomem? Vnitř Lék 2006; 52 (Suppl 2): 83–87.
12. Lecouvet FE, Malghem J, Michaux L et al. Skeletal survey in advanced multiple myeloma: radiographic versus MR imaging survey. Br J Haematol 1999; 106(1): 35–39.
13. Uetani M, Hashmi T, Hayashi K. Malignant and benign compression fractures: differentiation and diagnostic pitfalls on MRI. Clin Radiol 2004; 59(2): 124–131.
14. Horger M, Claussen CD, Bross-Bach U et al. Whole--blood low-dose multidetector row-CT in the diagnosis of multiple myeloma: an alternative to conventional radiography. Eur J Radiol 2005; 54(2): 289–297.
15. Chrastina J, Novák Z. Historie celotělového stereotaktického systému. Rozhl Chir 2008; 87(3): 154–156.
16. Lecouvet FE, Vande Berg BC, Malghem J et al. Magnetic resonance and computed tomography imaging in multiple myeloma. Semin Musculoskelet Radiol 2001; 5(1): 43–55.
17. Neubauer J, Repko M. Metodika kostních biopsií perkutánním způsobem za navigace CT. Vnitř Lék 2006; 52 (Suppl 2): 71–73.
18. Tamir R, Glanz I, Lubin E et al. Comparison of the sensitivity of 99mTc-methyl diphosphonate bone scan whit the skeletal X-ray survey in multiple myeloma. Acta Haematol 1983; 69(4): 236–242.
19. Ludwig H, Kumpan W, Sinzinger H. Radiography and bone scintigraphy in multiple myeloma: a comparative analysis. Br J Radiol 1982; 55(651): 173–181.
20. Adam Z, Bolčák K, Staníček J et al. Přínos fluorodeoxyglukózové pozitronové emisní tomografie (FDG PET) u mnohočetného myelomu. Vnitř Lék 2006; 52(3): 207–214.
21. Durie BG, Waxman AD, D’Agnolo A et al. Whole-body (18)F-FDG PET identifies high-risk myeloma. J Nucl Med 2002; 43(11): 1457–1463.
22. Lütje S, de Rooy JW, Croockewit S et al. Role of radiography, MRI and FDG-PET/CT in diagnosing, staging and therapeutical evaluation of patients with multiple myeloma. Ann Hematol 2009; 88(12): 1161–1168.
23. Haznedar R, Akı SZ, Akdemir OU et al. Value of 18F-fluorodeoxyglucose uptake in positron emission tomography/computed tomography in predicting survival in multiple myeloma. Eur J Nucl Med Mol Imaging 2011; 38(6): 1046–1053.
24. Dankerl A, Liebisch P, Glatting G et al. Multiple Myeloma: Molecular Imaging with 11C-Methionine PET/CT--Initial Experience. Radiology 2007; 242(2): 498–508.
25. Nanni C, Zamagni E, Cavo M et al. 11C-choline vs. 18F-FDG PET/CT in assessing bone involvement in patients with multiple myeloma. World J Surg Oncol 2007; 5 : 68.
26. Alper E, Gurel M, Evrensel T et al. 99mTc-MIBI scintigraphy in untreated stage III multiple myeloma: comparison with X-ray skeletal survey and bone scintigraphy. Nucl Med Commun 2003; 24(5): 537–542.
27. Mirzaei S, Filipits M, Keck A et al. Comparison of Technetim-99m-MIBI imaging with MRI for detection of spine involvement in patients with multiple myeloma. BMC Nucl Med 2003; 3(1): 2.
28. Mileshkin L, Blum R, Seymour JF et al. A comparison of fluorine-18 fluoro-deoxyglucose PET and technetium-99m sestamibi in assessing patients with multiple myeloma. Eur J Haematol 2004; 72(1): 32–37.
Labels
Paediatric clinical oncology Surgery Clinical oncology
Article was published inClinical Oncology

2012 Issue 3-
All articles in this issue
- New and Clinically Used Oncomarkers of Bladder Cancer
- Reproductive Functions in Women after Cancer Therapy
- Genetic Background of Cisplatin Induced Ototoxicity
- Triple-Negative Breast Cancer: Analysis of Patients Diagnosed and/or Treated at the Masaryk Memorial Cancer Institute between 2004 and 2009
- Angioimmunoblastic T-cell Lymphoma as a Very Poor-Prognosis Malignancy – a Single Centre Experience
- Patient with B-CLL with a History of Unrelated Hematopoietic Cells Donation – Retrospective Analysis of CLL Development and Implication for the Recipient
- Benefits of Individual Imaging Methods for Diagnosis and Monitoring of Activity of Multiple Myeloma
- Positron Emission Tomography and Clinical Predictors of Survival in Primary Extragonadal Germ Cell Tumors
- Granulocyte-Colony Stimulating Factor (G-CSF) Accelerates Healing of Radiation Induced Moist Desquamation of the Skin
- Clinical Oncology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Angioimmunoblastic T-cell Lymphoma as a Very Poor-Prognosis Malignancy – a Single Centre Experience
- Triple-Negative Breast Cancer: Analysis of Patients Diagnosed and/or Treated at the Masaryk Memorial Cancer Institute between 2004 and 2009
- New and Clinically Used Oncomarkers of Bladder Cancer
- Granulocyte-Colony Stimulating Factor (G-CSF) Accelerates Healing of Radiation Induced Moist Desquamation of the Skin
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career


![Mirels score [7].](https://www.prolekare.cz/media/cache/resolve/media_object_image_small/media/image/06557182e6c5711cddbe7d3c20379f92.png)