-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Two-year impact of community-based health screening and parenting groups on child development in Zambia: Follow-up to a cluster-randomized controlled trial
In a two year follow-up of a cluster randomized trial, Peter Rockers and colleagues examine the longer term impact of a community-based health screening and parenting group intervention on child development in Zambia.
Published in the journal: . PLoS Med 15(4): e32767. doi:10.1371/journal.pmed.1002555
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1002555Summary
In a two year follow-up of a cluster randomized trial, Peter Rockers and colleagues examine the longer term impact of a community-based health screening and parenting group intervention on child development in Zambia.
Introduction
Children in low - and middle-income countries continue to be exposed to a large number of risk factors affecting healthy development, ranging from exposure to malnutrition and infectious diseases to lack of appropriate stimulation and learning opportunities in their home environment and community [1,2]. According to the latest estimates, 40% of Zambian children under age 5 years are stunted and 6% are wasted [3]. More than 60% of the country’s population lives below the national poverty line, and the under-5 mortality rate is 64 per 1,000 live births [4]. Only 32% of Zambian children receive any form of early childhood care and education by age 6 years [5]. Efforts are currently underway to address the country’s malnutrition burden, most notably through the Scaling Up Nutrition funding mechanism [6].
While developmental deficits early in life have been shown to persist well into adulthood [7], a growing body of evidence suggests that the negative impact of early adversity in the short and medium run can be mitigated through appropriate early-life interventions [8]. In several settings, coaching caregivers on appropriate play-based activities and providing nutrition counseling have been shown to improve physical growth and neurocognitive development [9–11]. Community-based parenting groups have shown promising results in a few settings, and may be an effective and low-cost platform for delivering interventions to improve child health and development [12–17]. A set of recent studies in Uganda have provided evidence of the positive potential of community-based parenting interventions for improving child development in sub-Saharan Africa [18–20].
We conducted a cluster-randomized controlled trial to test the impact of a community-based parenting group intervention in a rural area of Southern Province, Zambia. As part of the trial, households in communities randomized to the intervention were invited to attend fortnightly parenting group meetings covering a diverse curriculum with content on cognitive stimulation, child nutrition, and self-care. The full intervention period lasted 2 years. An initial assessment of the intervention after 12 months found positive changes in caregiver behavior, but did not show statistically significant impacts on developmental outcomes [21]. In this paper we present the results of a year 2 in-depth follow-up of the original study cohort.
Methods
Study design and setting
The study was a cluster-randomized controlled trial implemented in the catchment areas of 5 health facilities in Choma and Pemba districts, Southern Province, Zambia.
Participants
Households were randomly selected in 3 stages of sampling. In the first stage, 5 rural health centers (RHCs) were purposefully selected. These RHCs were part of a previous research study conducted by the study team [22]. In the second stage, 6 health zones were randomly selected from each of the 5 health centers. In the third stage, villages were randomly selected with probability proportional to size to reach the target sample of 18 eligible caregiver–child dyads in each health zone. The aim was to enroll 524 dyads in the study at baseline. Villages with fewer than 2 eligible children were excluded to ensure a sufficient number of potential participants in group meetings. In selected villages, all households were screened for eligibility. Eligible households were provided with study information as part of the informed consent procedure and decided whether to enroll at that time.
To be eligible for the study, a household had to have a child between 6 and 12 months of age at the time of enrollment. Caregivers reported their child’s birthdate during eligibility screening, and the reported date was confirmed by reviewing the child’s health card when available; 96% of households had the child health card for review. Caregivers younger than 15 years of age were excluded. All caregivers provided informed consent prior to study initiation. The study was approved by the Institutional Review Board at Boston University (protocol number H-32726) and by the ethics board at ERES Converge in Zambia (protocol number 2013-Dec-010) prior to the enrollment of participants.
The initial target sample size of 524 caregiver–child dyads was powered to detect a 0.5–standard deviation (SD) difference in standardized child development scores with 90% power, assuming 30 clusters of equal size, 5% loss to follow-up, and an intracluster correlation coefficient of 0.1.
Randomization and masking
Health zones (clusters) were randomized prior to baseline enrollment with equal probability to either the intervention or the control group. Prior to randomization, health zones were matched in pairs within RHC catchment areas based on distance to the health center and village population. Within each matched pair, 1 health zone was randomly assigned to the intervention group. Group assignment was masked from all assessors, but masking of participants was not possible.
Procedures
The original study was funded for 1 year, and all study activities stopped at the end of that year, in November 2015. The study team secured funding for a second-year extension at the end of 2015, and study activities resumed in March 2016, at which time households were also re-consented to participate.
During the original 1-year study, intervention households received 2 services. First, they received a fortnightly visit by a child development agent (CDA), a community-based health worker employed full time by the project. During home visits, CDAs screened and referred children for infections and acute malnutrition, and encouraged caregivers to use routine child health services. Second, caregivers were invited to attend fortnightly parenting group meetings, where they were taught a diverse curriculum that included content on cognitive stimulation and play practices, child nutrition and cooking practices, and self-care for good mental health. During the year 2 study extension, the household visit component of the intervention was dropped, while facilitation of the fortnightly parenting group meetings continued. This change was motivated by the findings from the year 1 assessment, which suggested that the parenting groups were the primary driver of observed positive behavior change [21].
CDAs facilitated the organization of fortnightly parenting group meetings in intervention communities. Each health zone had 2 CDAs who each covered half of its communities. CDAs were selected through consultation with communities, and all had previous experience providing community-based health services, some as part of the formal health system. Prior to the start of the study, CDAs were trained on how to support group meetings. A local child development curriculum, containing age-appropriate activities covering health, nutrition, and early stimulation activities, as well as content related to caregiver mental health, was developed for these meetings. The curriculum was newly developed for the local context by the research team and included elements from existing child development programs, including the Care for Child Development package [23] and the Essential Package from Care International [24]. Content from these existing packages was adapted using an interactive theater approach. The curriculum training manual is provided in S1 Text. During the study period, CDAs were trained on the curriculum 3 meeting rounds at a time every 6 weeks. Groups were also encouraged to meet during intervening weeks without a formal curriculum. Meetings were held at multiple sites within each health zone. Meeting locations were chosen by group members to minimize travel distances. Each group meeting was run by a local “head mother” who was selected by the members of the group. CDAs met with head mothers before each round of meetings and provided them with training and resources according to the planned curriculum for that round. CDAs did not regularly attend the meetings themselves. Each meeting focused on a different topic; the topics included child nutrition, forms of play, cognitive stimulation, and language activities. All female caregivers in study communities with children under 5 years of age were invited to attend meetings. Caregivers were encouraged to bring children to group meetings, as aspects of the curriculum involved interactions with children. Based on consultation with local community leaders, male caregivers were not invited to attend group meetings.
Household survey data were collected at 4 time points: baseline (August/September 2014), year 1 follow-up (October/November 2015), re-consent (March 2016), and year 2 follow-up (November/December 2016). In this paper, we focus on data collected at baseline, re-consent, and the year 2 follow-up; a full analysis of data from the year 1 follow-up has been published previously [21]. Baseline, year 1 follow-up, and re-consent data collection was conducted at children’s homes. To ensure a controlled environment for the administration of the Bayley Scales of Infant and Toddler Development–Third Edition (BSID-III) at the year 2 follow-up, caregivers were invited to bring their child to the nearest health center, where the assessment was conducted one child at a time in a private room.
Outcomes
Primary outcomes were children’s stunting (defined as height-for-age z-score [HAZ] < −2) and neurocognitive development. Children’s height and weight were measured by trained assessors at baseline and the year 2 follow-up using standard anthropometric assessment kits. Length of all children under 2 years of age was collected in a lying position; height of older children was measured in a standing position. Length and height boards were rented from the Zambia National Food and Nutrition Commission (NFNC). Child weight was measured using digital scales also rented from the NFNC. Study interviewers were carefully trained for the anthropometric assessment, and a member of the NFNC attended the training to ensure correct use of the boards and scales. Anthropometric data were converted to z-scores using WHO child growth standards [25]. Child neurocognitive development was assessed using the BSID-III, which has previously been used and validated in Zambia [26]. A team of assessors attended a 2-week training on the BSID-III led by an accredited trainer prior to the start of data collection. Following the training, assessors spent 1 week pilot testing assessment procedures in the field. The BSID-III assesses 5 domains of development: cognition, language, motor, adaptive behavior, and social-emotional. Composite scores were determined for each domain using Bayley-III Scoring Assistant software [27] according to a 3-step procedure: first, raw scores were established by summing the number of items successfully completed for each sub-domain; second, scaled scores were constructed for each sub-domain by age-standardizing raw scores using the BSID-III reference population and rescaling to a range of 1 to 19, a mean of 10, and a standard deviation of 3; and third, composite scores were constructed by summing sub-domain scaled scores within domains and rescaling to a range of 40 to 160, a mean of 100, and standard deviation of 15. In order to show Cohen’s d estimates, composite scores were then converted to z-scores by standardizing within the study population.
Other outcome measures collected included HAZ, caregiver–child interactions, caregiver mental health, and child diet. Data on caregiver–child interactions were collected at baseline, year 1 follow-up, re-consent, and year 2 follow-up, using the 6-item Multiple Indicator Cluster Survey (MICS) module [28]. For child diet, a diet diversity score was constructed, using year 2 data, based on the number of food groups children had consumed the previous day, as per the method described in Steyn et al. [29]. Caregiver mental health was assessed using the 20-item WHO Self Reporting Questionnaire (SRQ) [30]. Caregiver depression was defined as reporting 7 or more symptoms on the SRQ, as per standard procedure [31]. Household demographics and asset information were collected at baseline. Caregivers reported on ongoing attendance at group meetings in the year 2 follow-up survey.
Statistical analysis
First, we compare baseline characteristics of the intervention and control groups, and present data on attrition by treatment arm. We then estimate the impact of the intervention on the primary outcomes of interest using intention-to-treat analysis. For continuous outcome variables, ordinary least squares regression models were fit to estimate unadjusted and adjusted impacts (β estimates). For dichotomous outcome variables, logistic regression models were fit to estimate unadjusted and adjusted odds ratios (ORs). Unadjusted models included controls for the cluster matching variables (population size and distance from the nearest health center) and, when available, the outcome variable measured at baseline. Adjusted models included a set of controls selected according to the following procedure: first, the outcome measured at the year 2 follow-up was regressed on the outcome measured at baseline, when available, and a set of demographic characteristics measured at baseline following a backward stepwise selection process with a drop threshold set at p = 0.2. The baseline demographic variable set included child HAZ, child weight-for-age z-score, child mid-upper arm circumference, child age, child sex, caregiver age, caregiver’s education, father’s education, and child motor skills according to the CREDI Scale [32]. Interviewer fixed effects were also included in the stepwise selection models. The resulting subset of variables from each model was included in the adjusted model for each outcome. For all models, standard errors were adjusted to account for clustering.
As a robustness check on the primary results, we conducted a per-protocol analysis, stratifying the treatment group according to household attendance at parenting groups. We also present data on caregiver–child interactions at each round of data collection, including the re-consent visit after a 5-month interruption in the delivery of the intervention. Finally, we estimate the impact of the intervention on other outcomes of interest, including caregiver mental health and child diet diversity. Cluster-robust standard errors were used to account for within cluster correlation. All analyses were conducted using Stata statistical software [33]. This trial was registered on ClinicalTrials.gov prior to baseline data collection (NCT02234726). Data were deposited in the Dryad repository: https://doi.org/10.5061/dryad.3340hc4 [34].
Results
Study population
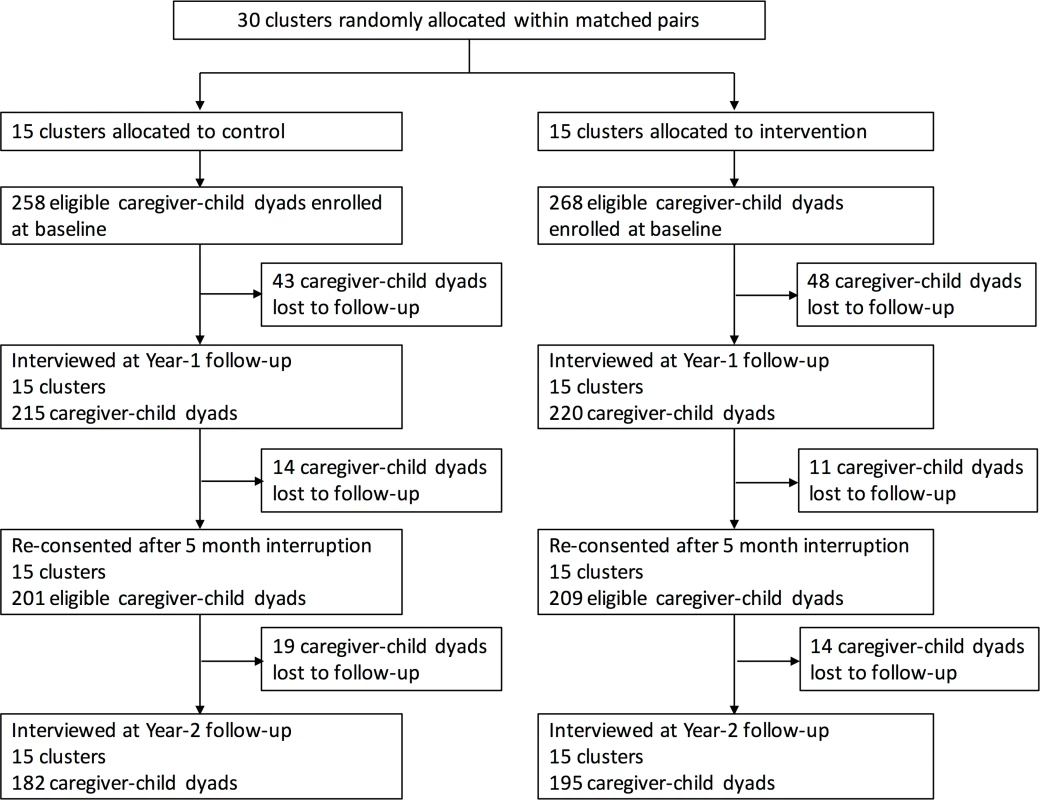
Thirty clusters were randomly assigned to intervention (15 clusters, 268 caregiver–child dyads) or control (15 clusters, 258 caregiver–child dyads). Overall, 5% of eligible households approached at enrollment refused to participate in the study. During the first year of the study, 48 dyads in the intervention group and 43 dyads in the control group were lost to follow-up. After a 5-month interruption in the study intervention, 209 intervention dyads and 201 control dyads were re-consented to participate in the second year of the study. At the end of the second year (last visit December 23, 2016), 195 caregiver–child dyads (73%) remained in the intervention group, while 182 dyads (71%) remained in the control group (Fig 1). Baseline characteristics of enrolled caregivers and children were similar in the intervention and control groups (Table 1). Nearly all (97%) caregivers in the study population were the child’s mother.
Tab. 1. Baseline characteristics of study participants. 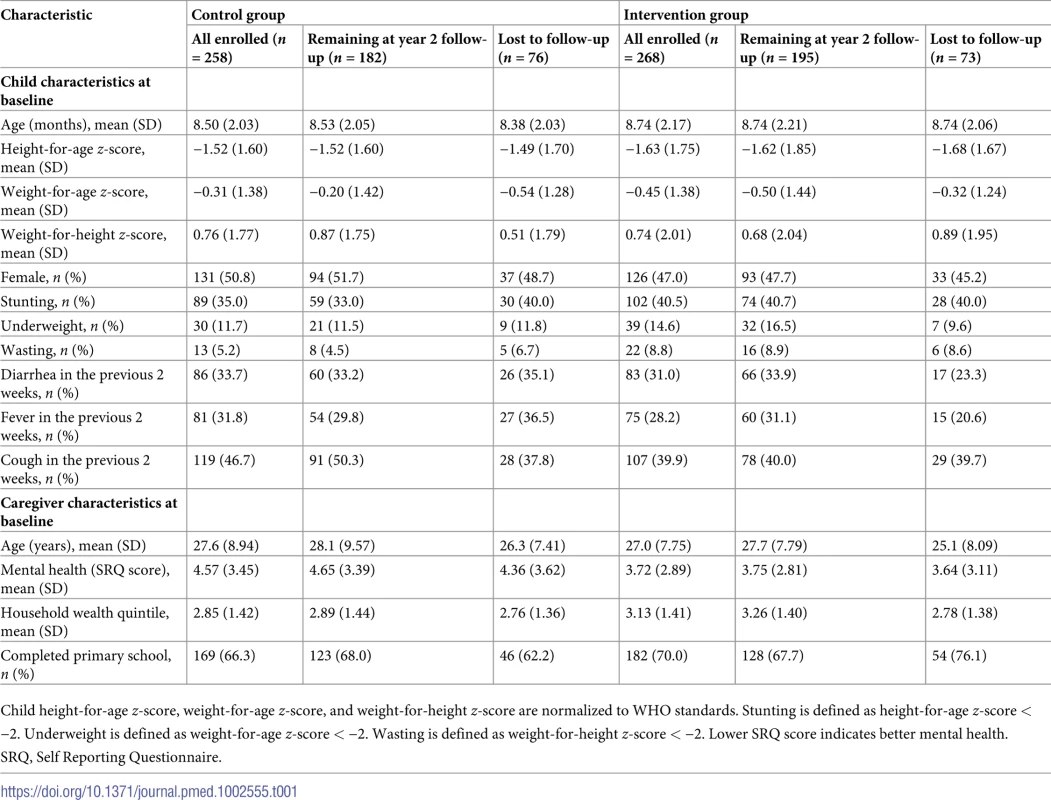
Child height-for-age z-score, weight-for-age z-score, and weight-for-height z-score are normalized to WHO standards. Stunting is defined as height-for-age z-score < −2. Underweight is defined as weight-for-age z-score < −2. Wasting is defined as weight-for-height z-score < −2. Lower SRQ score indicates better mental health. Children were on average 8 months old at the start of the study, with a relatively uniform distribution within the eligible age range (6 to 12 months). Height was on average well below the international reference median, with a mean HAZ of −1.5 at baseline. More than one-third of children were stunted at baseline. Study caregivers were on average 27 years old at baseline, and slightly fewer than half had completed primary school.
Impact of intervention on primary outcomes
Controlling for a set of baseline characteristics (Table 2), the intervention significantly reduced the odds of stunting (OR 0.45 [95% CI 0.22 to 0.92]; p = 0.028). Probability density functions for HAZ at the year 2 follow-up in the intervention and control groups are presented in Fig 2. Corresponding cumulative density functions are provided in S1 Fig. The intervention had a significant positive impact on child language (β 0.14 [95% CI 0.01 to 0.27]; p = 0.039). There was no impact on cognition (β 0.11 [95% CI −0.06 to 0.29]; p = 0.196), motor skills (β −0.01 [95% CI −0.25 to 0.24]; p = 0.964), adaptive behavior (β 0.21 [95% CI −0.03 to 0.44]; p = 0.088), or social-emotional development (β 0.20 [95% CI −0.04 to 0.44]; p = 0.098). We found no evidence to suggest that physical growth mediates these impacts (S1 Table). Raw and scaled BSID-III subtest scores are provided in S2 Table.
Fig. 2. Probability density function for height-for-age <i>z-</i>score at year 2 follow-up. 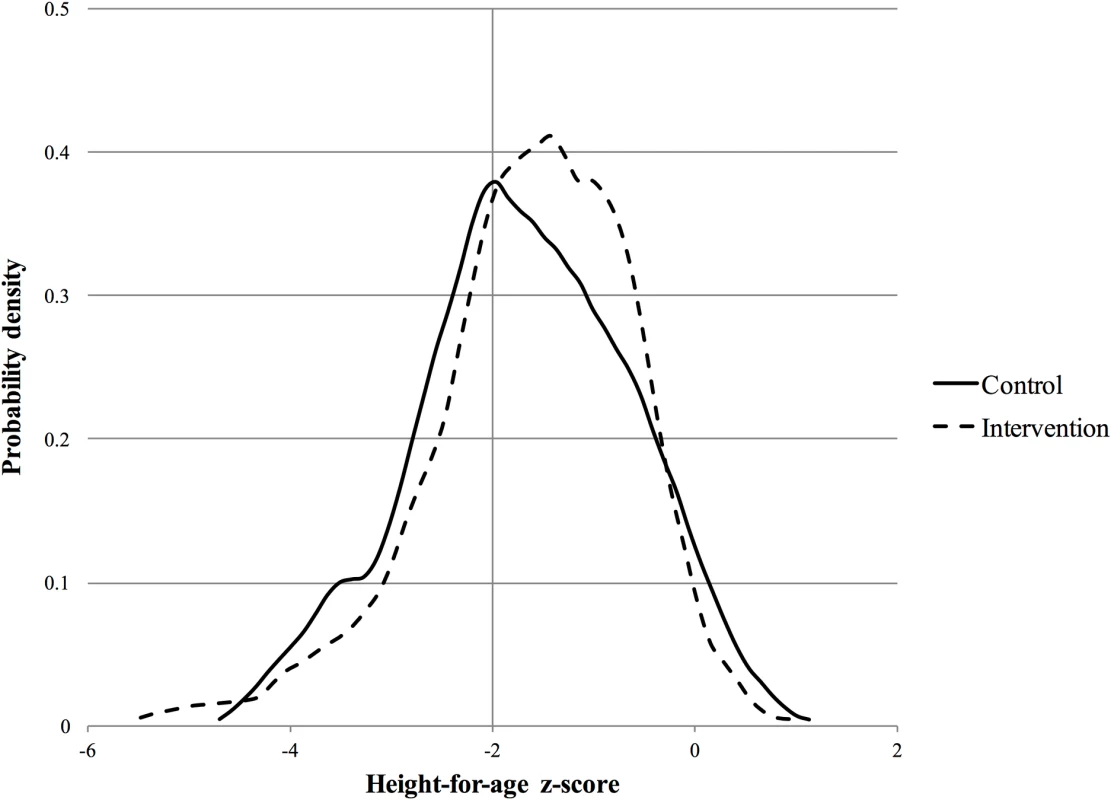
Tab. 2. Impact of the intervention on primary outcomes at year 2 follow-up. 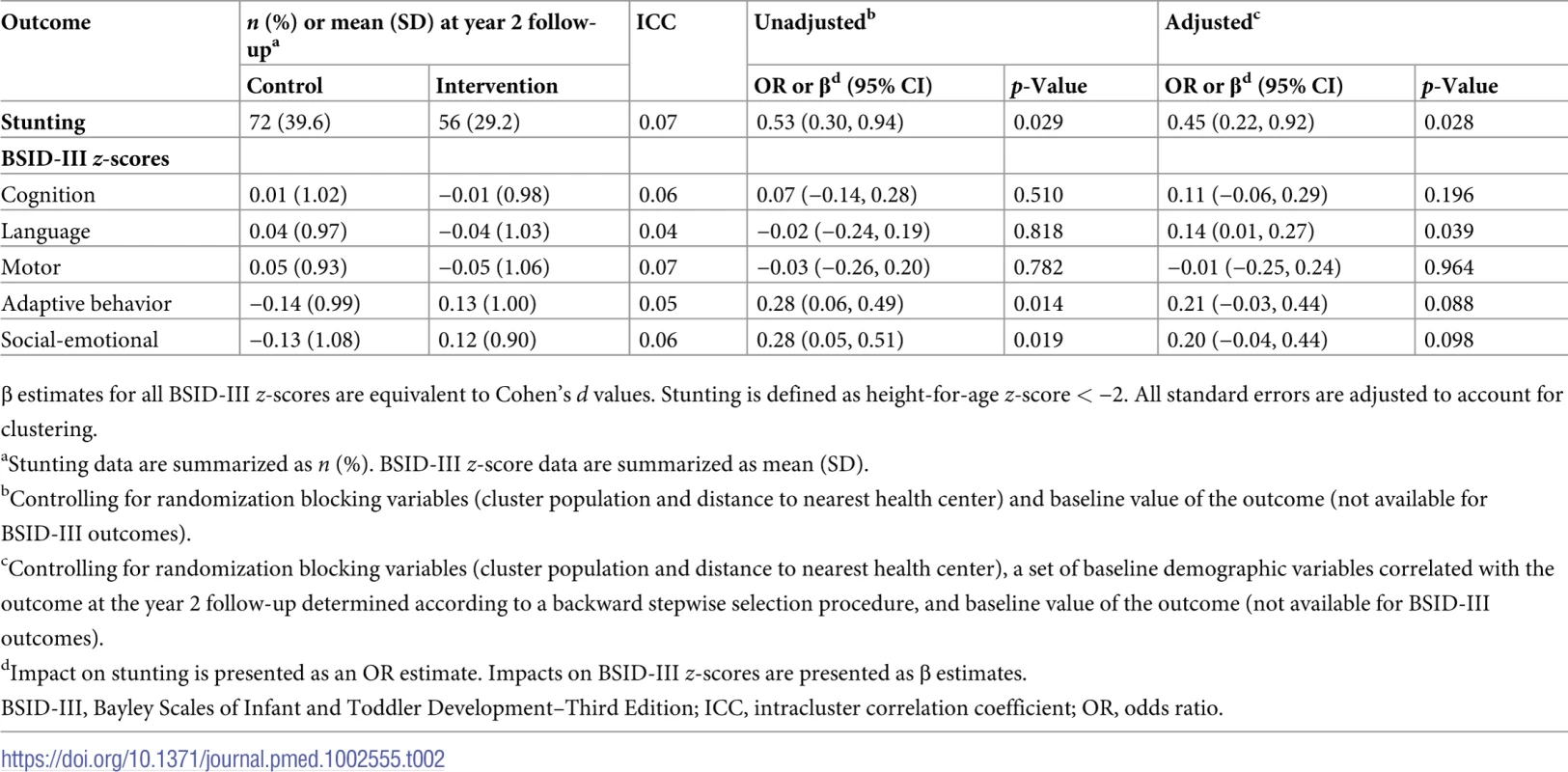
β estimates for all BSID-III z-scores are equivalent to Cohen’s d values. Stunting is defined as height-for-age z-score < −2. All standard errors are adjusted to account for clustering. In S2 Fig, we show reported attendance at parenting group meetings. In total, there were 27 parenting groups in intervention communities, and on average 6 caregivers attended each meeting. A majority of caregivers reported attending parenting group meetings either 2 to 3 (22%) or 4 or more times (35%) per month, indicating that many groups took the initiative to meet on their own in the time between fortnightly planned program meetings. Around one-third of caregivers in the intervention group reported that by the end of the study period they did not attend meetings. In Table 3, we present a per-protocol analysis. Treatment effects were significant for stunting, language, and adaptive behavior among compliers, i.e., those who attended 2 or more group meetings per month; no significant impacts were found among non-compliers.
Tab. 3. Per-protocol analysis of impact of the intervention on primary outcomes. 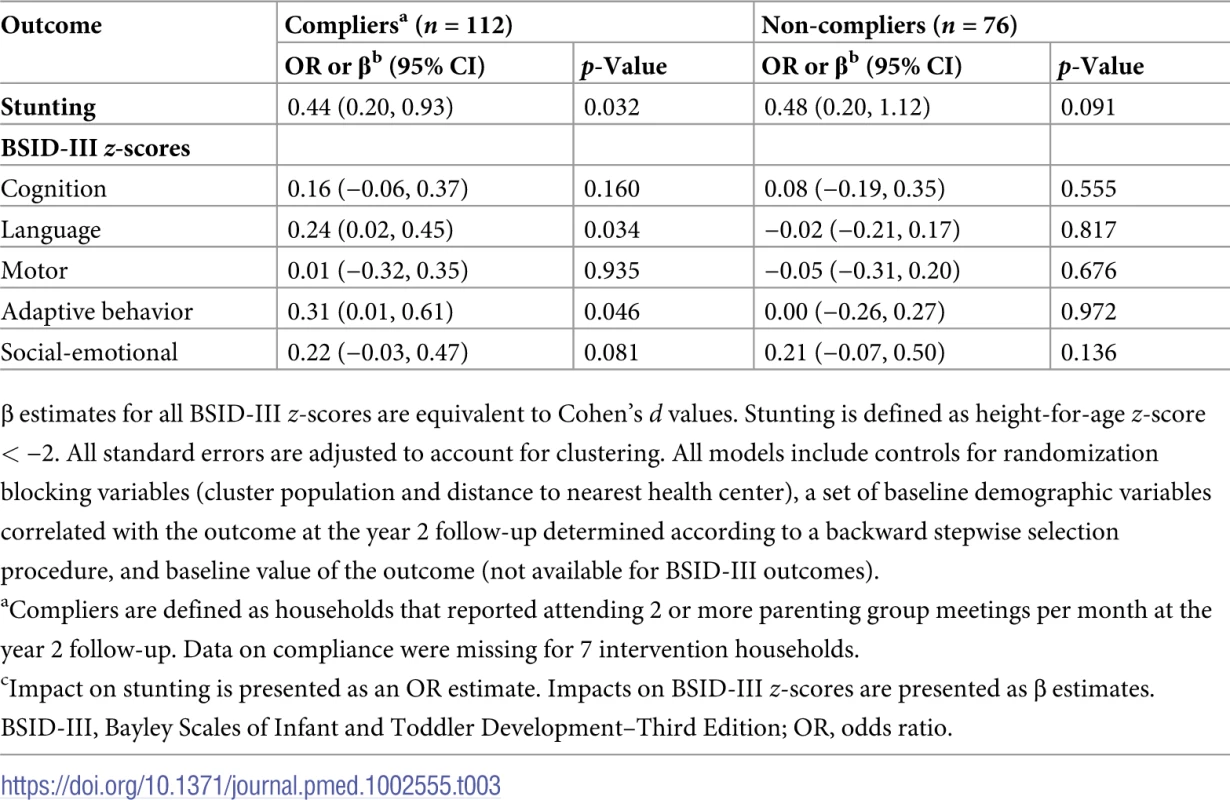
β estimates for all BSID-III z-scores are equivalent to Cohen’s d values. Stunting is defined as height-for-age z-score < −2. All standard errors are adjusted to account for clustering. All models include controls for randomization blocking variables (cluster population and distance to nearest health center), a set of baseline demographic variables correlated with the outcome at the year 2 follow-up determined according to a backward stepwise selection procedure, and baseline value of the outcome (not available for BSID-III outcomes). Caregiver–child interactions
At baseline, no differences were found in reports of caregiver–child interactions (Table 4). The intervention had a positive impact on reported interactions at year 1 follow-up (0.70 SD [95% CI 0.51 to 0.89]; p < 0.001). At the re-consent visit, at the end of a 5-month interruption in the delivery of the intervention, reported interactions remained significantly higher in the intervention group (0.71 SD [95% CI 0.52 to 0.90]; p < 0.001). Significant but somewhat smaller differences were found at year 2 follow-up (0.35 SD [95% CI 0.12 to 0.59]; p = 0.005). Caregiver–child interactions at each survey round are summarized in S3 Table.
Tab. 4. Caregiver–child interaction at each period of data collection. 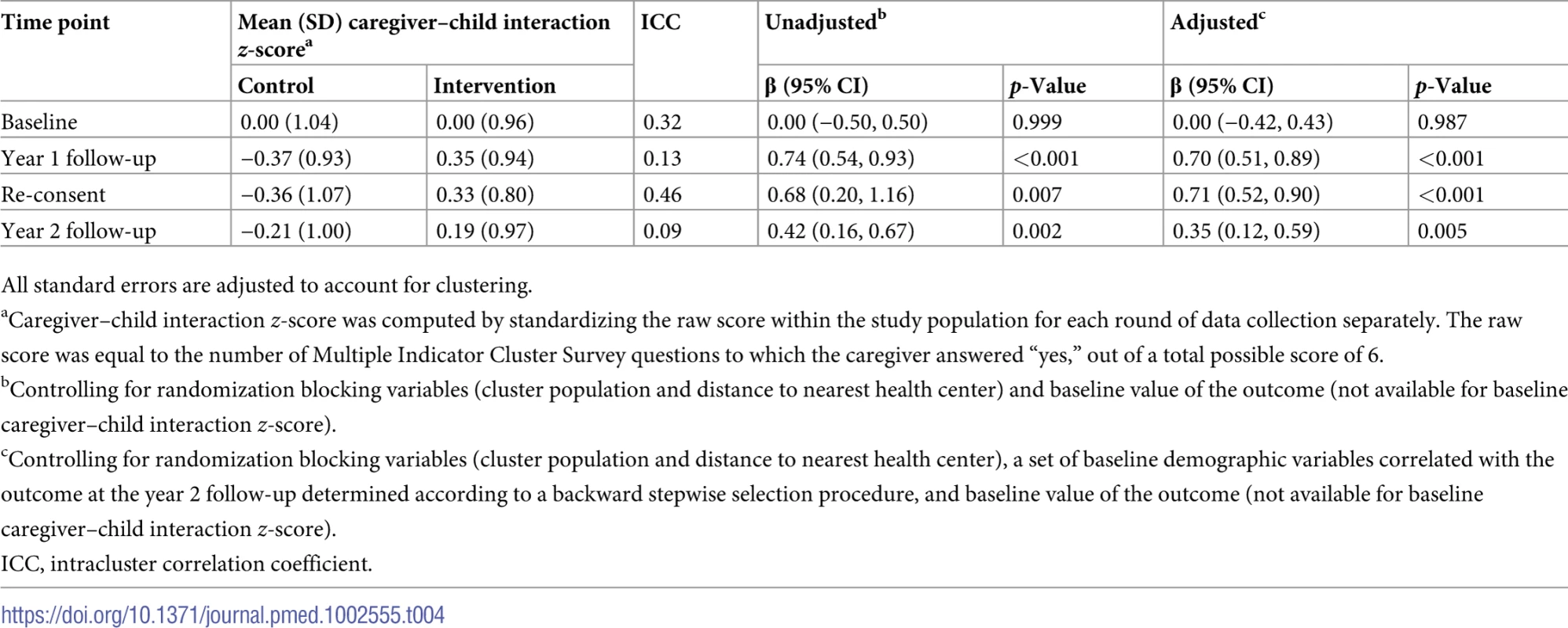
All standard errors are adjusted to account for clustering. Impact of the intervention on other secondary outcomes
Caregivers in the intervention group had slightly lower odds of depression (OR 0.63 [95% CI 0.31 to 1.26]; p = 0.193) at the year 2 follow-up, though these results were nonsignificant (Table 5). The intervention did not significantly impact HAZ (0.15 SD [95% CI −0.12 to 0.41]; p = 0.266) or child diet diversity score (0.20 [95% CI −0.13 to 0.53]; p = 0.218). Child diet data are summarized in S3 Table.
Tab. 5. Impact of the intervention on secondary outcomes at year 2 follow-up. 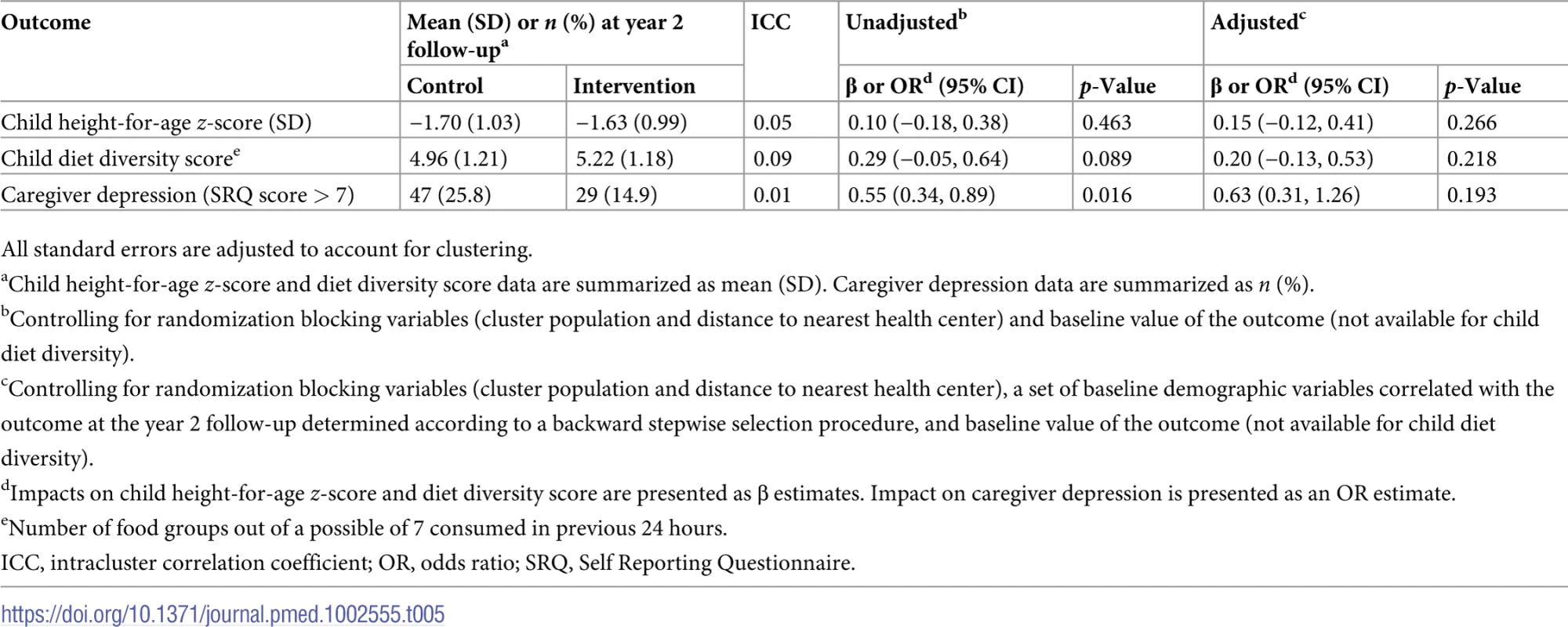
All standard errors are adjusted to account for clustering. Discussion
We investigated the impact of community-based parenting groups on child development in Southern Province, Zambia. After 2 years, the intervention substantially reduced the odds of stunting and improved child language development. The intervention had a positive impact on caregiver–child interaction, which appears to have persisted during a 5-month interruption in delivery of the intervention.
The large reductions in stunting are somewhat surprising given the low intensity of the intervention and also the minimal changes in diet observed. The small differences in diet may be partly explained by the seasonal timing of the year 2 follow-up assessment, which was conducted in December, in the middle of what is known in Zambia as the “hungry season,” when food reserves from the most recent harvest are mostly exhausted and households tend to reduce their overall nutritional intake and diet diversity. Significant impacts on diet diversity were observed at the year 1 follow-up, which was conducted in October, when food reserves are slightly more abundant [21]. While seasonal impacts on diet would affect both treatment groups, intervention impact estimates are likely smaller in settings where external constraints reduce food diversity to a minimum. The fact that a high density of study children was found to have HAZ near the stunting threshold may have contributed to the large impact on stunting; relatively modest and nonsignificant improvements in HAZ appear to have moved a significant number of children to just above the threshold.
While changes in parenting behavior were observed at the year 1 follow-up, only small, nonsignificant impacts on child development were found at that time [21]. Child physical growth and neurocognitive development are the results of complex and cumulative processes, with differentials resulting from early adversity often becoming more pronounced over time. It stands to reason that positive impacts from child development interventions might also develop over time. The temporal aspect of child development is important to consider when designing future studies, as longer intervention periods and follow-up may be needed to realize potential impacts. In addition, the utilization of BSID-III as a more detailed tool (rather than the INTERGROWTH-21st Neurodevelopment Assessment [INTER-NDA] [35] used at the year 1 follow-up) undoubtedly also increased our ability to detect developmental differences, which appear to have been domain-specific.
There are several key limitations to this work. First, the intervention package changed after the first year of the study, when household visits were stopped. We cannot say for certain that the impacts observed at the year 2 follow-up are entirely the result of parenting groups. However, the parenting group curriculum was the only platform for providing caregivers with nutrition and child stimulation information; therefore, it is very likely that this was the primary mechanism driving observed impacts. This conclusion is supported by the finding that in general larger impacts were found among children in complier households. Second, the delivery of the intervention relied on a cadre of CDAs operating parallel to the existing health system. Without significant additional public resources, scale-up efforts would likely require a delivery platform that is integrated into existing structures, such as Safe Motherhood Action Groups [36] or community health workers delivering integrated community case management [37]. Third, our measurement of parenting behavior was largely dependent on self-report, and parents may have had reasons to misreport their own behavior, introducing a source of potential bias in our behavior change estimates. This concern does not, however, apply to our primary outcomes, which were directly observed and measured by trained study staff. Fourth, we tested the impact of the intervention on 6 primary outcomes, raising concerns around multiple hypothesis testing. With Bonferroni corrections, only the estimated impact on stunting would be statistically significant, while the impact on language development would be nonsignificant. With the relatively small sample size used in this study, large effect sizes would be needed to yield p-values below the corrected significance threshold. Lastly, nearly 30% of households enrolled at baseline were lost to the study at the year 2 follow-up, which may have introduced some bias in our impact estimates. However, only 1 statistically significant difference was found between those retained and those lost to follow-up: in the control group, cough at baseline was more likely in the retained group (p = 0.03). Given the number of variables tested, 1 significant association is expected by chance. As in most low - and middle-income countries, migration from rural to urban areas in search of economic opportunities is common in Zambia and may explain the observed attrition rates [38].
This paper adds to a growing body of literature on the delivery of early childhood interventions in low-resource settings [17]. Community-based parenting groups appear to be a feasible and effective means of reaching a large number of households that may otherwise not be able to access center-based services. In settings like Zambia, where optimal child nutrition and stimulation are often lacking, parenting groups hold promise for improving child health and welfare. However, improvements in child development may not be immediate, and continued and sustained efforts are likely needed.
Supporting Information
Zdroje
1. Black MM, Walker SP, Fernald LC, Andersen CT, DiGirolamo AM, Chunling L, et al. Early childhood development coming of age: science through the life course. Lancet. 2017;389 : 77–90. doi: 10.1016/S0140-6736(16)31389-7 27717614
2. Walker SP, Wachs TD, Grantham-McGregor S, Black MM, Nelson CA, Huffman S, et al. Inequality in early childhood: risk and protective factors for early child development. Lancet. 2011;378 : 1325–38. doi: 10.1016/S0140-6736(11)60555-2 21944375
3. Zambia Central Statistical Office, Zambia Ministry of Health, ICF International. Zambia Demographic and Health Survey 2013–14. Rockville (MD): ICF International; 2014.
4. World Bank. World Development Indicators Database. Washington (DC): World Bank; 2017.
5. McCoy DC, Zuilkowski SS, Yoshikawa H, Fink G. Early childhood care and education and school readiness in Zambia. J Res Educ Eff. 2017;10 : 482–506.
6. Scaling Up Nutrition. Zambia. Geneva: SUN Movement; 2017 [cited 2017 Sep 26]. Available from: http://scalingupnutrition.org/sun-countries/zambia/
7. Walker SP, Wachs TD, Gardner JM, Lozoff B, Wasserman GA, Pollitt E, et al. Child development: risk factors for adverse outcomes in developing countries. Lancet. 2007;369 : 145–57. doi: 10.1016/S0140-6736(07)60076-2 17223478
8. Engle PL, Black MM, Behrman JR, de Mello MC, Gertler PJ, Kapriri L, et al. Strategies to avoid the loss of developmental potential in more than 200 million children in the developing world. Lancet. 2007;369 : 229–42. doi: 10.1016/S0140-6736(07)60112-3 17240290
9. Hess SY, Abbeddou S, Jimenez EY, Somé JW, Vosti SA, Ouédraogo ZP, et al. Small-quantity lipid-based nutrient supplements, regardless of their zinc content, increase growth and reduce the prevalence of stunting and wasting in young Burkinabe children: a cluster-randomized trial. PLoS ONE. 2015;10.3:e0122242.
10. Grantham-McGregor SM, Powell CA, Walker SP, Himes JH. Nutritional supplementation, psychosocial stimulation, and mental development of stunted children: the Jamaican study. Lancet. 1991;338(8758):1–5. 1676083
11. Powell C, Baker-Henningham H, Walker S, Gernay J, Grantham-McGregor S. Feasibility of integrating early stimulation into primary care for undernourished Jamaican children: cluster randomised controlled trial. BMJ. 2004;329 : 89. doi: 10.1136/bmj.38132.503472.7C 15217841
12. Yousafzai AK, Rasheed MA, Rizvi A, Armstrong R, Bhutta ZA. Effect of integrated responsive stimulation and nutrition interventions in the Lady Health Worker programme in Pakistan on child development, growth, and health outcomes: a cluster-randomised factorial effectiveness trial. Lancet. 2014;384 : 1282–93. doi: 10.1016/S0140-6736(14)60455-4 24947106
13. Lewycka S, Mwansambo C, Rosato M, Kazembe P, Phiri T, Mganga A, et al. Effect of women’s groups and volunteer peer counselling on rates of mortality, morbidity, and health behaviours in mothers and children in rural Malawi (MaiMwana): a factorial, cluster-randomised controlled trial. Lancet. 2013;381 : 1721–35. doi: 10.1016/S0140-6736(12)61959-X 23683639
14. Prost A, Colbourn T, Seward N, Azad K, Coomarasamy A, Copas A, et al. Women’s groups practising participatory learning and action to improve maternal and newborn health in low-resource settings: a systematic review and meta-analysis. Lancet. 2013;381 : 1736–46. doi: 10.1016/S0140-6736(13)60685-6 23683640
15. Aboud FE. Evaluation of an early childhood parenting programme in rural Bangladesh. J Health Popul Nutr. 2007;25 : 3–13. 17615899
16. Aboud FE, Singla DR, Nahil MI, Borisova I. Effectiveness of a parenting program in Bangladesh to address early childhood health, growth and development. Soc Sci Med. 2013;97 : 250–8. doi: 10.1016/j.socscimed.2013.06.020 23871435
17. Aboud FE, Yousafzai AK. Global health and development in early childhood. Annu Rev Psychol. 2015;66 : 433–57. doi: 10.1146/annurev-psych-010814-015128 25196276
18. Boivin M, Bangirana P, Nakasujja N, Page CF, Shohet C, Givon D, et al. A year-long caregiver training program to improve neurocognition in preschool Ugandan HIV-exposed children. J Dev Behav Pediatr. 2013;34 : 269–78. doi: 10.1097/DBP.0b013e318285fba9 23535340
19. Bass JK, Nakasujja N, Familiar-Lopez I, Sikorskii A, Murray SM, Opoka R, et al. Association of caregiver quality of care with neurocognitive outcomes in HIV-affected childrenaged 2–5 years in Uganda. AIDS Care. 2016;28(Suppl 1):76–83.
20. Signla DR, Kumbakumba E, Aboud FE. Effects of a parenting intervention to address maternal psychological wellbeing and child development and growth in rural Uganda: a community-based, cluster-randomised trial. Lancet Global Health. 2015;3:e458–69. doi: 10.1016/S2214-109X(15)00099-6 26144389
21. Rockers PC, Fink G, Zanolini A, Banda B, Biemba G, Sullivan C, et al. Impact of a community-based package of interventions on child development in Zambia: a cluster-randomised controlled trial. BMJ Global Health. 2016;1:e000104. doi: 10.1136/bmjgh-2016-000104 28588962
22. Semrau KEA, Herlihy J, Grogan C, Musokotwane K, Yeboah-Antwi K, Mbewe R, et al. Effectiveness of 4% chlorhexidine umbilical cord care on neonatal mortality in Southern Province, Zambia (ZamCAT): a cluster-randomised controlled trial. Lancet Global Health. 2016;4(11):e827–36. doi: 10.1016/S2214-109X(16)30215-7 27693439
23. World Health Organization, United Nations Children’s Fund. Care for child development: improving the care of young children. Geneva: World Health Organization; 2012.
24. Inter-Agency Taskforce on HIV and ECD. The essential package: holistically addressing the needs of young vulnerable children and their caregivers affected by HIV and AIDS. Atlanta: Care International; 2012.
25. World Health Organization, United Nations Children’s Fund. WHO child growth standards and the identification of severe acute malnutrition in infants and children. Geneva: World Health Organization; 2009.
26. Bann CM, Wallander JL, Do B, Thorsten V, Pasha O, Biasini FJ, et al. Home-based early intervention and the influence of family resources on cognitive development. Pediatrics. 2016;137:e20153766. doi: 10.1542/peds.2015-3766 26977079
27. Pearson Clinical Assessments. Bayley-III Scoring Assistant. Version 2.0.2. San Antonio: Pearson; 2012.
28. United Nations Children’s Fund. Multiple indicator cluster surveys (MICS). New York: United Nations Children’s Fund; 1995.
29. Steyn NP, Nel JH, Nantel G, Kennedy G, Labadarios D. Food variety and dietary diversity scores in children: are they good indicators of dietary adequacy? Public Health Nutr. 2006;9 : 644–50. 16923296
30. Beusenberg M, Orley J. A user’s guide to the Self Reporting Questionnaire (SRQ). Geneva: World Health Organization; 1994.
31. Harpham T, Reichenheim M, Oser R, Thomas E, Hamid N, Jaswal S, et al. Measuring mental health in a cost-effective manner. Health Policy Plan. 2003;18 : 344–9. 12917276
32. McCoy DC, Sudfeld CR, Bellinger DC, Muhihi A, Ashery G, Weary TE, et al. Development and validation of an early childhood development scale for use in low-resourced settings. Popul Health Metr. 2017;15 : 3. doi: 10.1186/s12963-017-0122-8 28183307
33. StataCorp. Stata Statistical Software. Release 14. College Station (TX): StataCorp; 2015.
34. Rockers PC, Zanolini A, Hamer DH, Fink G. Data from: Two-year impact of community-based health screening and parenting groups on child development in Zambia: follow-up to a cluster-randomized controlled trial. Dryad Digital Repository. Durham (NC): Dryad; 2018. Available from: https://doi.org/10.5061/dryad.3340hc4
35. Fernandes M, Stein A, Newton CR, Cheikh-Ismail L, Kihara M, Wulff K, et al. The INTERGROWTH-21st Project Neurodevelopment Package: a novel method for the multi-dimensional assessment of neurodevelopment in pre-school age children. PLoS ONE. 2014; 9(11):e113360. doi: 10.1371/journal.pone.0113360 25423589
36. Kruk ME, Vail D, Austin-Evelyn K, Atuyambe L, Greeson D, Grépin KA, et al. Evaluation of a maternal health program in Uganda and Zambia finds mixed results on quality of care and satisfaction. Health Aff (Millwood). 2016;35 : 510–9.
37. Seidenberg PD, Hamer DH, Iyer H, Pilingana P, Siazeele K, Hamainza B, et al. Impact of integrated community case management on health-seeking behavior in rural Zambia. Am J Trop Med Hyg. 2012;87 : 105–10. doi: 10.4269/ajtmh.2012.11-0799 23136285
38. Potts D. Debates about African urbanisation, migration and economic growth: what can we learn from Zimbabwe and Zambia? Geogr J. 2016;182 : 251–64.
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2018 Číslo 4- Není statin jako statin aneb praktický přehled rozdílů jednotlivých molekul
- Magnosolv a jeho využití v neurologii
- Moje zkušenosti s Magnosolvem podávaným pacientům jako profylaxe migrény a u pacientů s diagnostikovanou spazmofilní tetanií i při normomagnezémii - MUDr. Dana Pecharová, neurolog
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
- Antikoagulační léčba u pacientů před operačními výkony
-
Všechny články tohoto čísla
- Preprints in medical research: Progress and principles
- From surviving to thriving: What evidence is needed to move early child-development interventions to scale?
- Preprints: An underutilized mechanism to accelerate outbreak science
- Attacks on medical workers in Syria: Implications for conflict research
- Determining the scope of attacks on health in four governorates of Syria in 2016: Results of a field surveillance program
- Estimating the health and economic effects of the proposed US Food and Drug Administration voluntary sodium reformulation: Microsimulation cost-effectiveness analysis
- Maternal age and offspring developmental vulnerability at age five: A population-based cohort study of Australian children
- Two-year impact of community-based health screening and parenting groups on child development in Zambia: Follow-up to a cluster-randomized controlled trial
- Universal versus conditional day 3 follow-up for children with non-severe unclassified fever at the community level in Ethiopia: A cluster-randomised non-inferiority trial
- Universal versus conditional day 3 follow-up for children with non-severe unclassified fever at the community level in the Democratic Republic of the Congo: A cluster-randomized, community-based non-inferiority trial
- Breastfeeding during infancy and neurocognitive function in adolescence: 16-year follow-up of the PROBIT cluster-randomized trial
- Impacts 2 years after a scalable early childhood development intervention to increase psychosocial stimulation in the home: A follow-up of a cluster randomised controlled trial in Colombia
- Genetic scores to stratify risk of developing multiple islet autoantibodies and type 1 diabetes: A prospective study in children
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Breastfeeding during infancy and neurocognitive function in adolescence: 16-year follow-up of the PROBIT cluster-randomized trial
- Preprints in medical research: Progress and principles
- Determining the scope of attacks on health in four governorates of Syria in 2016: Results of a field surveillance program
- Estimating the health and economic effects of the proposed US Food and Drug Administration voluntary sodium reformulation: Microsimulation cost-effectiveness analysis
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání