-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaEstimating the Global Clinical Burden of Malaria in 2007
Background:
The epidemiology of malaria makes surveillance-based methods of estimating its disease burden problematic. Cartographic approaches have provided alternative malaria burden estimates, but there remains widespread misunderstanding about their derivation and fidelity. The aims of this study are to present a new cartographic technique and its application for deriving global clinical burden estimates of Plasmodium falciparum malaria for 2007, and to compare these estimates and their likely precision with those derived under existing surveillance-based approaches.Methods and Findings:
In seven of the 87 countries endemic for P. falciparum malaria, the health reporting infrastructure was deemed sufficiently rigorous for case reports to be used verbatim. In the remaining countries, the mapped extent of unstable and stable P. falciparum malaria transmission was first determined. Estimates of the plausible incidence range of clinical cases were then calculated within the spatial limits of unstable transmission. A modelled relationship between clinical incidence and prevalence was used, together with new maps of P. falciparum malaria endemicity, to estimate incidence in areas of stable transmission, and geostatistical joint simulation was used to quantify uncertainty in these estimates at national, regional, and global scales.
Combining these estimates for all areas of transmission risk resulted in 451 million (95% credible interval 349–552 million) clinical cases of P. falciparum malaria in 2007. Almost all of this burden of morbidity occurred in areas of stable transmission. More than half of all estimated P. falciparum clinical cases and associated uncertainty occurred in India, Nigeria, the Democratic Republic of the Congo (DRC), and Myanmar (Burma), where 1.405 billion people are at risk.
Recent surveillance-based methods of burden estimation were then reviewed and discrepancies in national estimates explored. When these cartographically derived national estimates were ranked according to their relative uncertainty and replaced by surveillance-based estimates in the least certain half, 98% of the global clinical burden continued to be estimated by cartographic techniques.Conclusions and Significance:
Cartographic approaches to burden estimation provide a globally consistent measure of malaria morbidity of known fidelity, and they represent the only plausible method in those malaria-endemic countries with nonfunctional national surveillance. Unacceptable uncertainty in the clinical burden of malaria in only four countries confounds our ability to evaluate needs and monitor progress toward international targets for malaria control at the global scale. National prevalence surveys in each nation would reduce this uncertainty profoundly. Opportunities for further reducing uncertainty in clinical burden estimates by hybridizing alternative burden estimation procedures are also evaluated.
: Please see later in the article for the Editors' Summary
Published in the journal: . PLoS Med 7(6): e32767. doi:10.1371/journal.pmed.1000290
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1000290Summary
Background:
The epidemiology of malaria makes surveillance-based methods of estimating its disease burden problematic. Cartographic approaches have provided alternative malaria burden estimates, but there remains widespread misunderstanding about their derivation and fidelity. The aims of this study are to present a new cartographic technique and its application for deriving global clinical burden estimates of Plasmodium falciparum malaria for 2007, and to compare these estimates and their likely precision with those derived under existing surveillance-based approaches.Methods and Findings:
In seven of the 87 countries endemic for P. falciparum malaria, the health reporting infrastructure was deemed sufficiently rigorous for case reports to be used verbatim. In the remaining countries, the mapped extent of unstable and stable P. falciparum malaria transmission was first determined. Estimates of the plausible incidence range of clinical cases were then calculated within the spatial limits of unstable transmission. A modelled relationship between clinical incidence and prevalence was used, together with new maps of P. falciparum malaria endemicity, to estimate incidence in areas of stable transmission, and geostatistical joint simulation was used to quantify uncertainty in these estimates at national, regional, and global scales.
Combining these estimates for all areas of transmission risk resulted in 451 million (95% credible interval 349–552 million) clinical cases of P. falciparum malaria in 2007. Almost all of this burden of morbidity occurred in areas of stable transmission. More than half of all estimated P. falciparum clinical cases and associated uncertainty occurred in India, Nigeria, the Democratic Republic of the Congo (DRC), and Myanmar (Burma), where 1.405 billion people are at risk.
Recent surveillance-based methods of burden estimation were then reviewed and discrepancies in national estimates explored. When these cartographically derived national estimates were ranked according to their relative uncertainty and replaced by surveillance-based estimates in the least certain half, 98% of the global clinical burden continued to be estimated by cartographic techniques.Conclusions and Significance:
Cartographic approaches to burden estimation provide a globally consistent measure of malaria morbidity of known fidelity, and they represent the only plausible method in those malaria-endemic countries with nonfunctional national surveillance. Unacceptable uncertainty in the clinical burden of malaria in only four countries confounds our ability to evaluate needs and monitor progress toward international targets for malaria control at the global scale. National prevalence surveys in each nation would reduce this uncertainty profoundly. Opportunities for further reducing uncertainty in clinical burden estimates by hybridizing alternative burden estimation procedures are also evaluated.
: Please see later in the article for the Editors' SummaryIntroduction
Estimating the disease burden posed by malaria is an important public health challenge [1]–[9]. The clinical consequences of Plasmodium falciparum infection have several features that confound traditional approaches to disease burden and disability measurement [10],[11]. First, not all infections result in progression to disease, notably in areas of stable transmission [12], where populations have acquired clinical immunity [13]. The overall risk of clinical disease has a curvilinear and uncertain association with the risk of infection as a combined function of age at first infection and immunity [13]–[18]. Second, the dominant symptom of fever, or other symptoms, does not distinguish malaria from other locally prevalent infections [19]–[23]. As a consequence, the routine reporting of “malaria” can overestimate disease rates by assuming that most fevers are malaria [24],[25] and that fevers associated with an infection are causally linked to that infection [20],[26]. Third, with few exceptions across malaria-endemic countries, fevers or other malaria-like syndromes are often self-medicated and may resolve regardless of cause before reaching formal health systems [27]. Fourth, inaccurate diagnoses [21],[25],[28] might be used to report disease rates, and these errors may be compounded through inadequate and incomplete national reporting systems [29]–[38].
To circumvent some of the clinical, treatment, and reporting problems inherent in malaria burden estimation, we previously computed the global incidence of P. falciparum clinical disease [5] for 2002, using assemblies of epidemiological data and a modified categorical map of historical malaria endemicity [39]. The publication of (i) the revised global spatial limits of P. falciparum transmission [40], (ii) a contemporary geostatistical description of P. falciparum malaria endemicity within these limits [41], and (iii) updates of the modelled relationship between clinical incidence and prevalence [42] have resulted in a substantially improved evidence base from which to revisit estimates of the clinical burden of P. falciparum, defined as the primary acute clinical event resulting from malaria infection at all ages. Most significantly, a geostatistical space–time joint simulation framework [43] is combined with these improved cartographic and epidemiological data sources to quantify uncertainty in the mapped outputs and to propagate it appropriately into the derived burden estimates. Using these joint simulation procedures we have built upon previous approaches to produce the first continuous map of global clinical P. falciparum incidence, and we use this to estimate the global clinical burden of P. falciparum malaria in 2007. These estimates are then compared with those available from surveillance, and the opportunity for the further hybridization of these techniques is discussed.
Methods
Analysis Outline
A schematic overview of the analysis procedures is provided in Figure 1. In brief, of the 87 countries classified as endemic for P. falciparum malaria [40], seven had sufficiently reliable health information systems for case report data to be used directly to enumerate clinical burden for 2007. We divided the population at risk (PAR) in the remaining 80 countries into regions of unstable and stable risk of transmission [40] (Figure 2). In unstable regions, a uniform clinical incidence rate was adopted of 0.1 case per 1,000 per annum (PA). This rate was multiplied by a population surface [44] for 2007 (Figure 3) and aggregated to obtain country and regional case estimates for these unstable areas. Upper and lower bounds were defined using uniform rates of zero and one case, respectively, per 1,000 PA. In stable regions, we used a previously defined Bayesian geostatistical model that took an assembly of space–time distributed P. falciparum parasite rate (PfPR) surveys and generated realisations of continuous age-standardized prevalence within the limits of stable transmission [41]. We then used a Bayesian nonparametric model [42] of a collection of all-age active case detection studies, to describe the uncertain relationship between the clinical incidence rate and the underlying age-standardized parasite prevalence. These two models were integrated in a geostatistical space–time joint simulation framework to generate joint realisations of clinical attack rate for every pixel as a function of the predicted underlying prevalence [43] (Protocol S1). These attack rates were then multiplied by the corresponding pixel population totals to yield joint realisations of a clinical burden surface (Figures 4 and 5). This joint simulation framework supported the aggregation of per-pixel burden estimates into defined spatial units, whilst preserving a space–time uncertainty structure, allowing country and regional estimates of burden to be made with appropriate credible intervals (Table 1, Protocol S2). Each of these analytical components are now discussed in more detail.
Fig. 1. Schematic diagram showing the procedure for burden estimation. 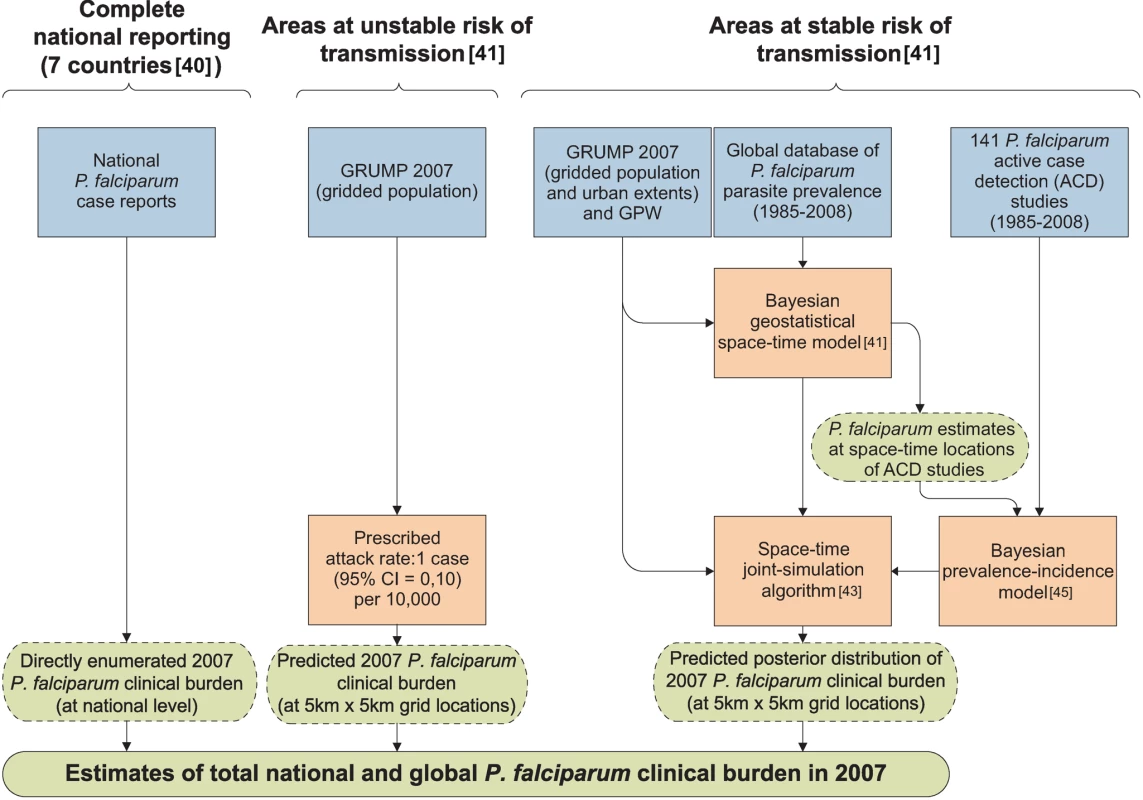
Blue boxes describe input data, orange boxes models and experimental procedures, dashed green rods intermediate output, and solid green rods the final output. The seven countries with reliable national reporting were Belize, Iran, Kyrgyzstan, Panama, Saudi Arabia, South Africa, and Tajikistan. The areas of unstable and stable transmission are defined as having less or more than one case per 10,000 PA, respectively [40],[41]. Fig. 2. Global limits and endemicity of P. falciparum in 2007. 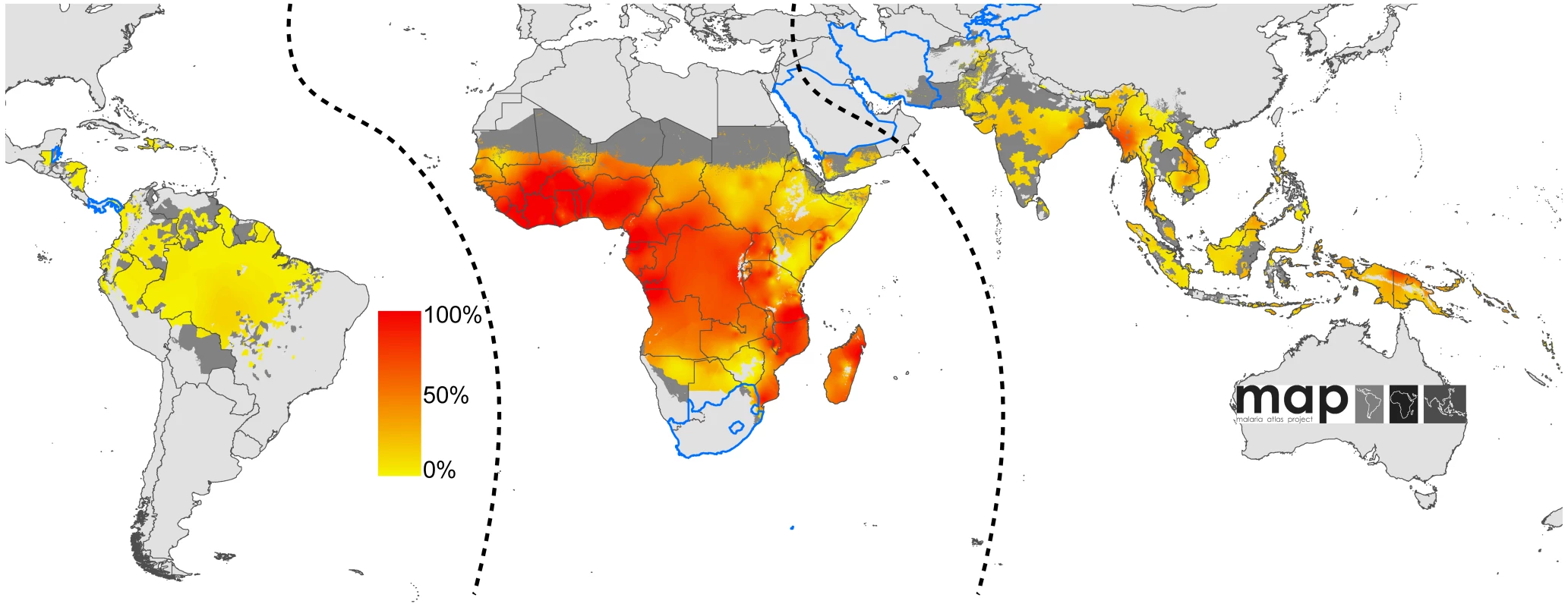
The land area was defined as no risk (light grey), unstable risk (medium grey areas, where PfAPI <0.1‰ PA), and stable risk (where PfAPI >0.1‰ PA) [40] with endemicity (PfPR in the 2- up to 10-year age group, PfPR2–10) displayed as a continuum of yellow to red between 0% and 100%. The dashed lines separate the Americas, Africa+, and the CSE Asia region, respectively, from left to right. The seven countries with thick blue borders have very low P. falciparum burden and reliable national health information systems. Fig. 3. Global human population density in 2007. 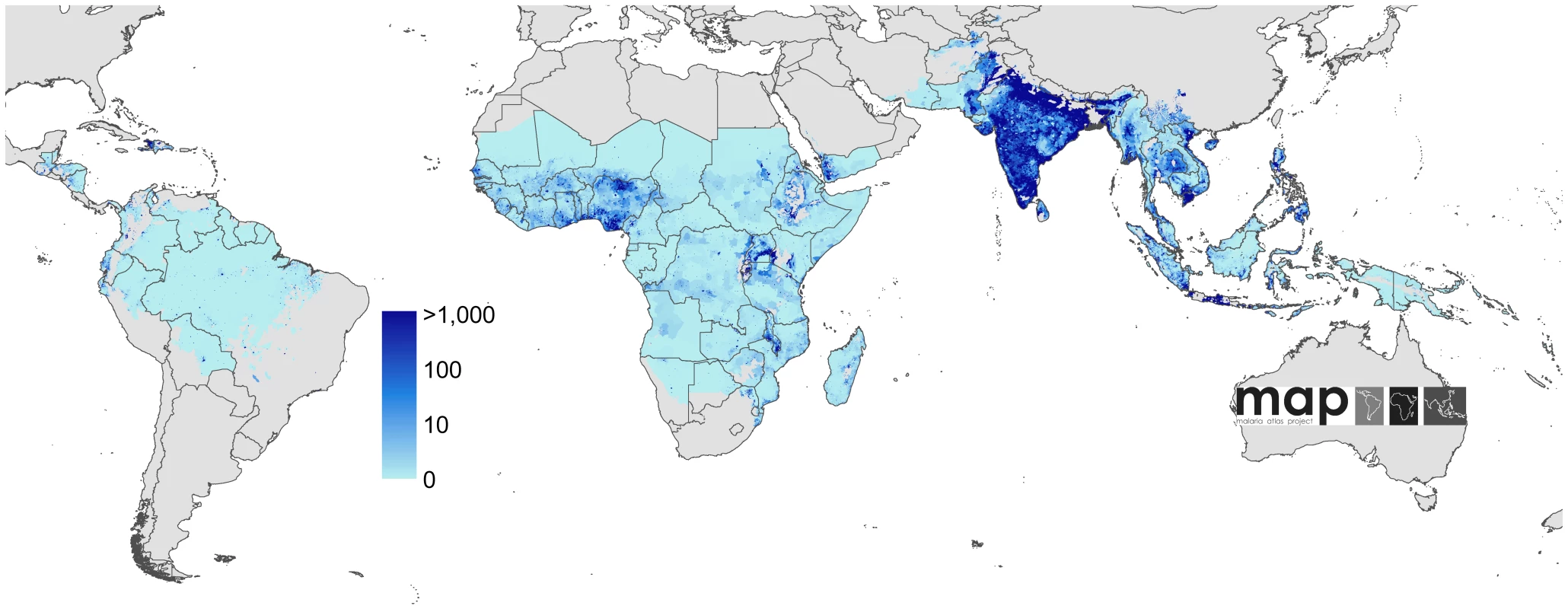
Human population density [44] in persons per km2 is displayed on a logarithmic colour scale within the limits of P. falciparum transmission. No malaria risk is shown in light grey. Fig. 4. Global clinical burden of P. falciparum in 2007. 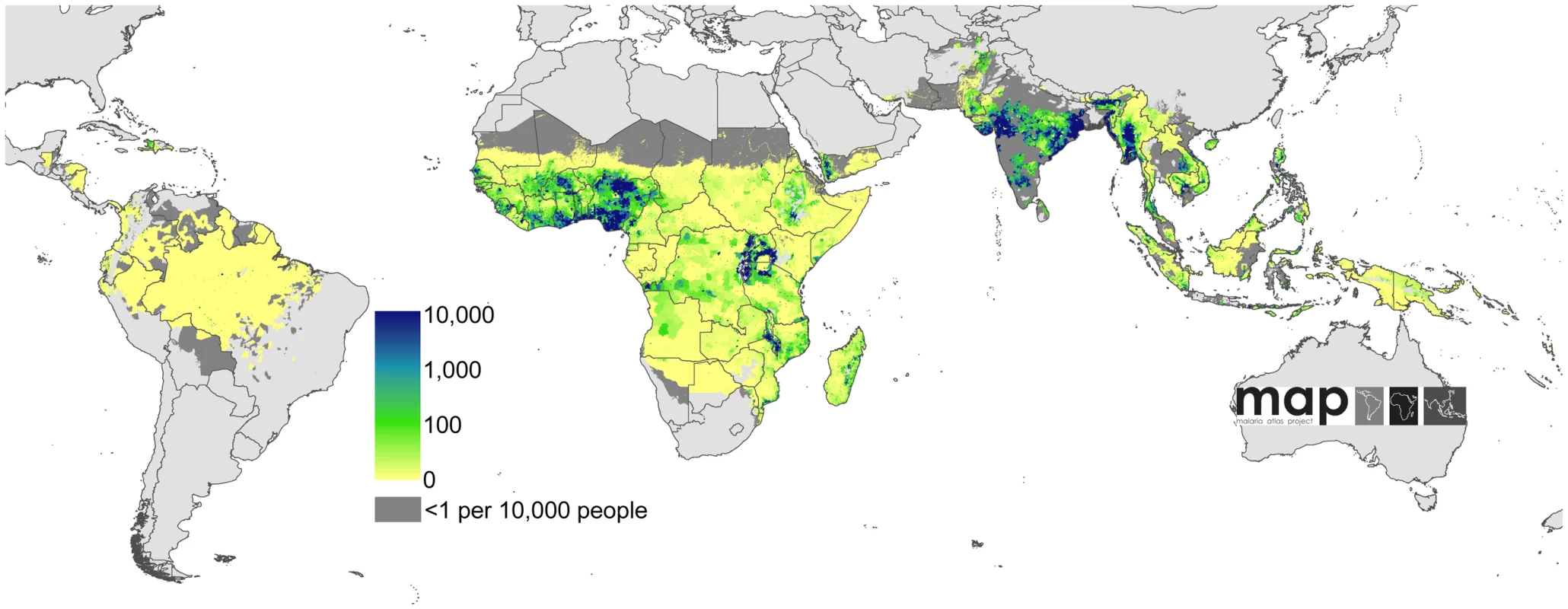
Bayesian geostatistical estimates (posterior means) of the number of all-age clinical cases per 5×5 km pixel displayed on a logarithmic colour scale between 0 and 10,000 cases, within the stable limits of P. falciparum transmission. Dark and light grey areas are as described in Figure 2. Fig. 5. Uncertainty in the global clinical burden of P. falciparum in 2007. 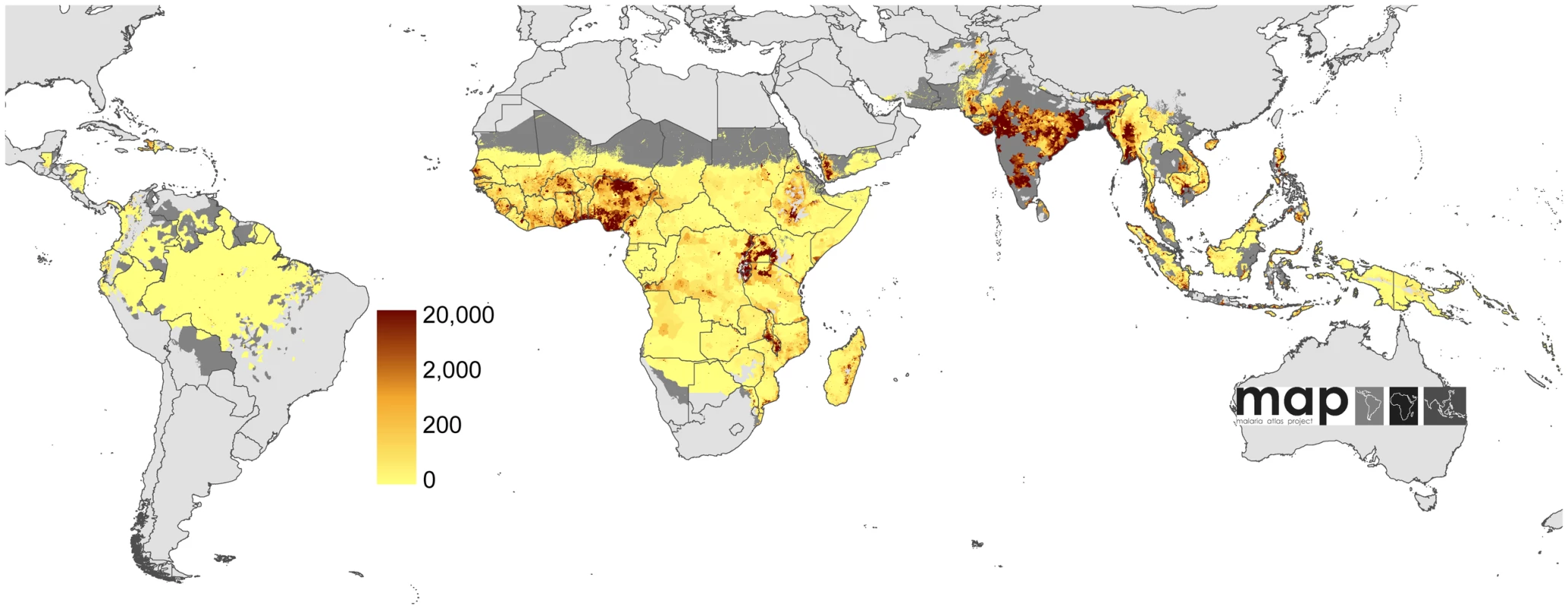
Bayesian geostatistical model-based prediction uncertainty (posterior standard deviations) on a logarithmic colour scale between 0 and 20,000 cases, within the stable limits of P. falciparum transmission. No model-based uncertainty metrics were produced for areas of unstable transmission. Dark and light grey areas are as described in Figure 2. Tab. 1. Numbers of Plasmodium falciparum clinical attacks by region globally in 2007. 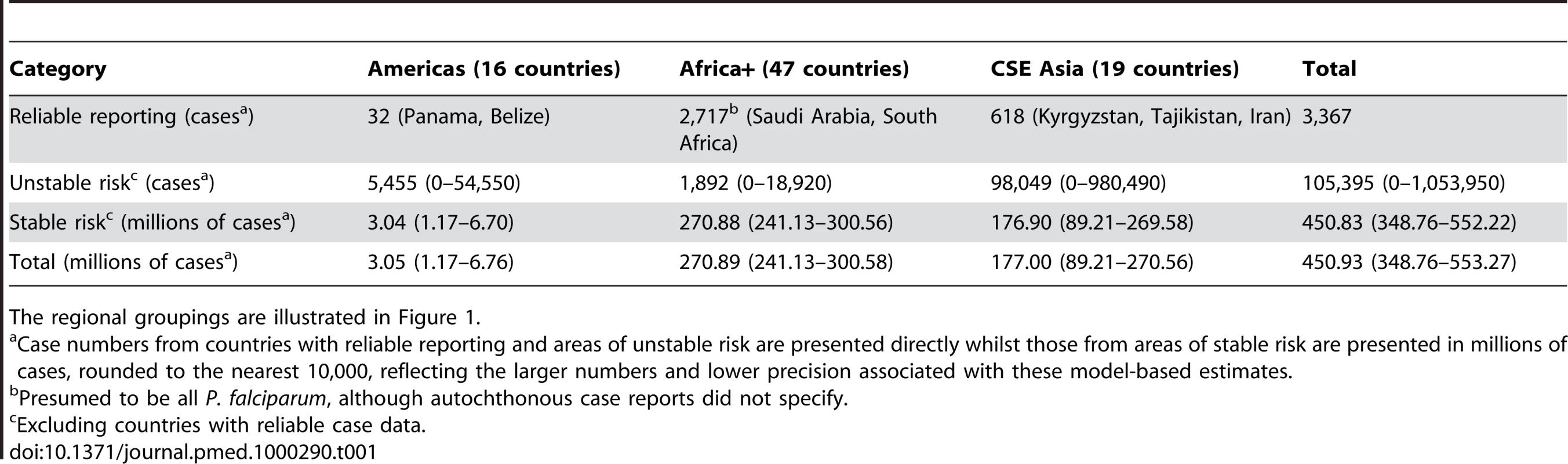
The regional groupings are illustrated in Figure 1. Defining Populations and Global Regions
The Global Rural Urban Mapping Project (GRUMP) alpha version [44] provides gridded population counts and population density estimates for the years 1990, 1995, and 2000, adjusted to the United Nations' national population estimates. Population counts for the year 2000 were projected to 2007 by applying national, medium variant, intercensal growth rates [45] by country using methods previously described [46] (Figure 3).
We have modified the World Health Organization (WHO) regional country groupings, recognizing that these geopolitical boundaries do not conform to the biogeographical determinants of malaria risk and thus disease burden [41],[47],[48]. For the purposes of disease risk estimation we have used three malaria regional groupings: Africa+ (including Yemen and Saudi Arabia, which share the same dominant Anopheles vectors as mainland Africa [49]), the Americas, and the combined regions of Near East, Asia, and the Pacific that we refer to as Central and South East (CSE) Asia (Figure 2). To facilitate comparison with other estimates, however, we have also shown the results aggregated by the regional groupings of the WHO (Protocol S2).
Defining the Limits of Stable and Unstable P. falciparum Transmission
To define the global spatial limits of P. falciparum transmission, we previously assembled confirmed P. falciparum clinical case data for 41 P. falciparum malaria-endemic countries (PfMECs) outside of Africa [40]. National case reported data were expressed as P. falciparum annual parasite incidence (PfAPI) derived from various combinations of active case detection (fever surveys in communities where every person presenting with a fever is tested for parasite infection) and passive case detection (reports from febrile patients attending the local health services) and usually expressed together as the number infected per 1,000 PA [50]–[52]. These data were provided by malaria coordinating officers in the WHO regional offices of the Eastern Mediterranean (EMRO), Europe (EURO), South East Asia (SEARO), and the Western Pacific (WPRO) at the highest available administrative level unit between 2002 and 2007. Among the countries in the American Regional Office (AMRO), PfAPI data from national surveillance systems in Brazil, Colombia, Peru, and Honduras were obtained directly from personal communication with national malaria specialists.
The PfAPI data were mapped to first, second, or third administrative level units and used to classify areas as no risk (zero cases) and either unstable or stable risk if the number of confirmed cases was lower or higher than 0.1 case per 1,000 PA, respectively [40]. The unstable/stable classification was based on a review of the statistical, logistical, and programmatic reasons underpinning the PfAPI levels used to define phases and action points during the Global Malaria Eradication Program [12],[53]–[55]. In addition, no transmission was assumed where medical intelligence from international travel advisories or national malaria control programmes stated no malaria risk or where the temperature was too low for sporogony to complete within the average lifespan of the local dominant vector species [49]. Measures of aridity were used to define areas in which transmission is biologically plausible in isolated manmade breeding sites, but overall transmission in surrounding areas is limited by its effects on anopheline survival, and the clinical incidence is likely to be less than 0.1 case per 1,000 PA. The spatial extents of stable and unstable risk defined using these inputs are shown (Figure 2).
Defining P. falciparum Clinical Incidence in Areas of Reliable Case Detection
Paradoxically, where the incidence of clinical malaria events are rare, their rapid detection and notification becomes increasingly important as part of national malaria control strategies, demanding more sophisticated surveillance [51],[55]–[57]. This is particularly true for countries aiming to attain or maintain WHO accredited elimination status [58]–[60]. Of the 87 PfMECs, we have identified seven countries that are relatively wealthy and have specified a goal of P. falciparum elimination where case-detection systems are an integral part of the control strategies [58]–[60]: Panama, Belize, Tajikistan, Kyrgyzstan, Iran, Saudi Arabia, and South Africa (Figure 2). For these seven countries, we have used the national reports for 2007 of all notified, locally acquired infections submitted to regional WHO offices (see Acknowledgments) as the definitive estimate of case burden. These countries are characterised by having a small number of annual cases, with a large proportion of the population living in areas of no risk or unstable transmission and are therefore likely to represent a very small proportion of the global P. falciparum malaria burden [40].
Defining Malaria Incidence in Areas of Unstable P. falciparum Malaria Transmission
We estimate that almost one billion people were living in areas where P. falciparum transmission was unstable in 2007 [40] (Figure 2). Defining annualized disease risk in these areas from empirical data is difficult, as epidemiological investigations for research or survey purposes are rare. Nevertheless, in computing disease burdens it is important to impute some measure of completeness of formal malaria reporting within these marginal, unstable transmission areas. A number of malaria treatment-seeking behaviour studies and qualitative examinations of routine malaria reporting frequency suggest large inadequacies in a range of national reporting systems from a variety of causes that can act multiplicatively: Cambodia (actual number of cases 2.7× greater than reported) [35], India (9–50×) [28],[61]–[65], Mozambique (2.7×) [32], Pakistan (5.9×) [30], Peru (4.3×) [34], Solomon Islands (4.7×) [38], Sri Lanka (1.9×) [29], and Syria (4.5×) [31].
There are remarkably few specific investigations of the completeness of malaria case notification systems in different settings. Only four reports provide an estimate of the numbers of cases likely to be missed by routine health system surveillance compared to more aggressive, active case detection methods in the same communities over the same time period. In the Yanomami area of Brazil, approximately 1.25 more events were detected by active detection than were reported to the routine health system [57]. Across different years at different sites the ratio of active to routine, passive detection varied from 4.5 to 42.1 in Vietnam [66], with similar under-reporting rates documented in Cambodia [67]. A 5-fold difference in survey-to-passive rates of case detection has been reported in Yunnan Province in China [68]. It is not possible to provide an evidence-based under-reporting correction factor that is specific for every national malaria information system. We have therefore elected to use a single worst-case rate of 10-fold under-reporting across all countries. We hence assume for all unstable areas a uniform incidence of 0.1 case per 1,000 PA, with a lower confidence bound of zero and an upper confidence bound assuming a 10-fold under-reporting rate; equating to one case per 1,000 PA.
Defining Malaria Incidence in Stable Endemic Areas
We estimated that in 2007, approximately 1.4 billion people lived in areas of stable P. falciparum transmission [40] (Figure 2). In these areas, we considered that case-reporting through routine health information systems was too unreliable for the calculation of incidence due to inadequate reporting coverage (see above), widespread self-medication [27], and poor diagnosis [21],[25]. Instead, we developed a model-based cartographic method for deriving estimates in the areas of stable transmission in which clinical incidence was modelled as a function of the underlying endemicity (parasite prevalence). This procedure required: (i) a spatially continuous model for endemicity; (ii) a further model to predict incidence as a function of endemicity; (iii) reliable data on 2007 population distribution; and (iv) a technique for combining these components so that the uncertainty inherent in the component models was propagated into the resulting burden estimates. These components are now outlined in turn, with additional statistical details provided in Protocol S1.
To estimate stable transmission intensity, a Bayesian space-time geostatistical modelling framework was developed to interpolate empirical estimates of age-corrected parasite prevalence derived from 7,953 community surveys undertaken between 1985 and 2008 across 83 malaria-endemic countries. This model has been described in detail elsewhere [41] and its output allows for a continuous, urban-adjusted, contemporary estimate of parasite prevalence in children aged from 2 up to 10 years (PfPR2–10) at a pixel spatial resolution of 5×5 km for the year 2007 (Figure 2).
To estimate clinical incidence, formal literature searches were conducted for P. falciparum malaria incidence surveys undertaken prospectively through active case detection at least every 14 days [42]. The incidence surveys were time–space matched with estimates of parasite prevalence derived from the geostatistical model described above [41]. Potential relationships between all-age clinical incidence and age-standardized parasite prevalence were then specified in a nonparametric Gaussian process model with minimal, biologically informed, prior constraints. A temporal volatility model was incorporated to describe the variance in the observed data and Bayesian inference was used to choose between the candidate models [42]. Separate relationships were preferred for each of the three regions defined globally (Figure 2) to accommodate regional-specific differences in the dominant vector species [47],[49],[69], the impact of drug resistance on recrudescent clinical attacks [70], the possible modification of P. falciparum clinical outcomes in areas of P. vivax co-infection [71],[72], and the genetic contribution to disease risk of inherited haemoglobin disorders [73]. Due to the sparse data in the Americas, however, this region was combined with CSE Asia. In the Africa+ region and the combined Americas and CSE Asia region, clinical incidence increased slowly and smoothly as a function of infection prevalence (Figures 6, 7, 8, and 9). In the Africa+ region, when infection prevalence exceeded 40%, clinical incidence reached a maximum of 500 cases per 1,000 PA (Figure 6). In the combined Americas and CSE Asia regions this maximum was reached at 250 cases per 1,000 PA (Figure 7).
Fig. 6. The posterior distribution of the prevalence-incidence relationship (, see Methods) in the Africa+ region. 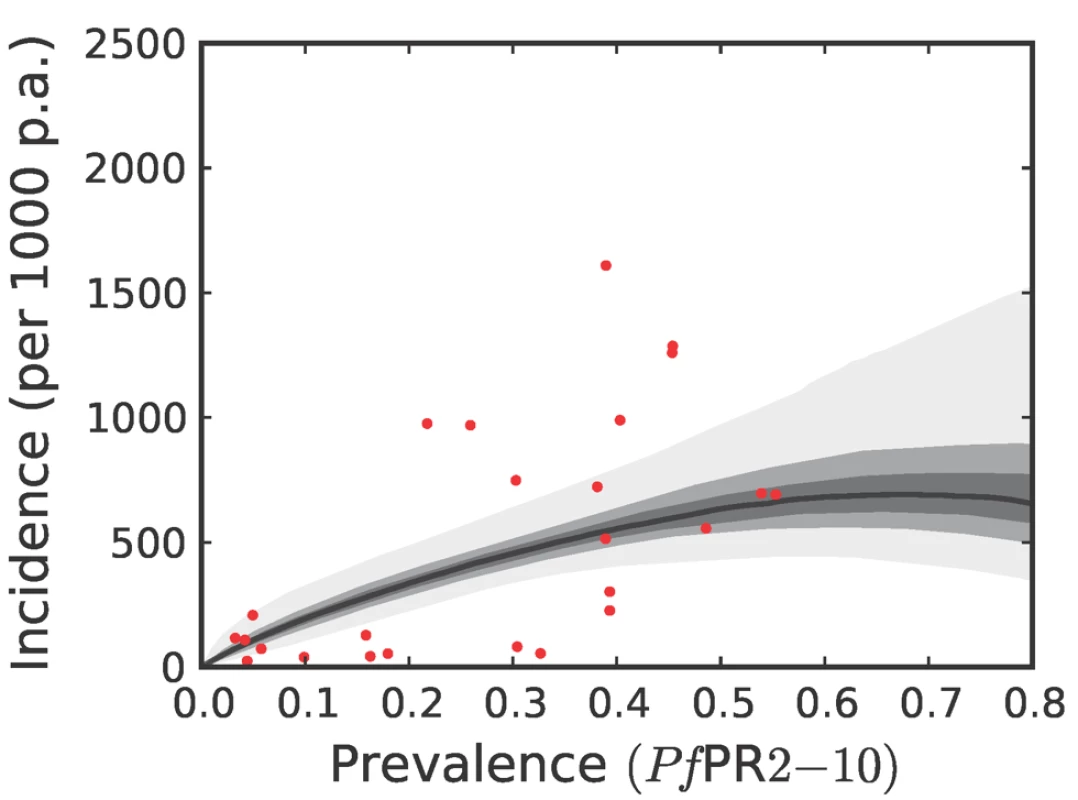
The relationship is plotted between malaria endemicity (PfPR in the 2- up to 10-year age group, PfPR2–10) and all-age incidence (clinical cases per thousand of the population PA) [42]. Please see reference [42] for a full description of the data, methods, and techniques used to define this relationship. The light grey, medium grey and dark grey regions define the 95%, 50%, and 25% credible intervals, respectively. The solid black line is the median and the data are shown as red dots. Fig. 7. The posterior distribution of the prevalence-incidence relationship (, see Methods) in the combined CSE Asia region and the Americas. 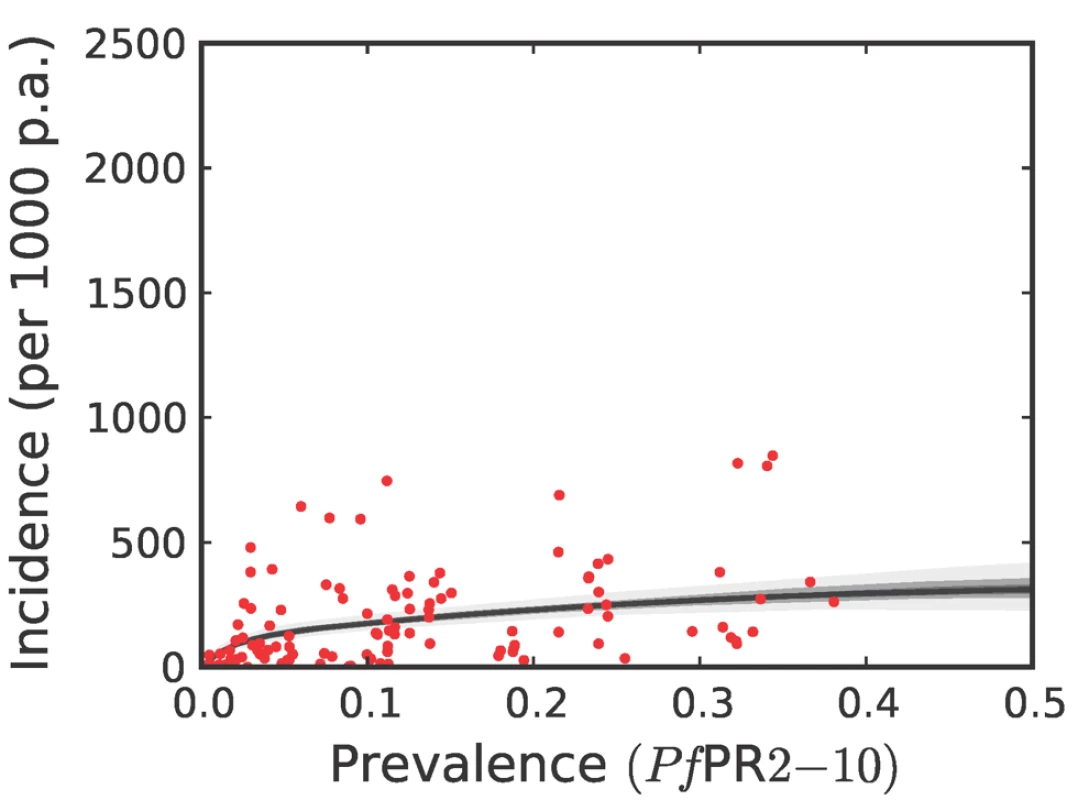
The techniques and colours used are identical to Figure 6. Fig. 8. The predictive distribution of the incidence that would actually be observed by weekly surveillance over a two-year period in the Africa+ region. 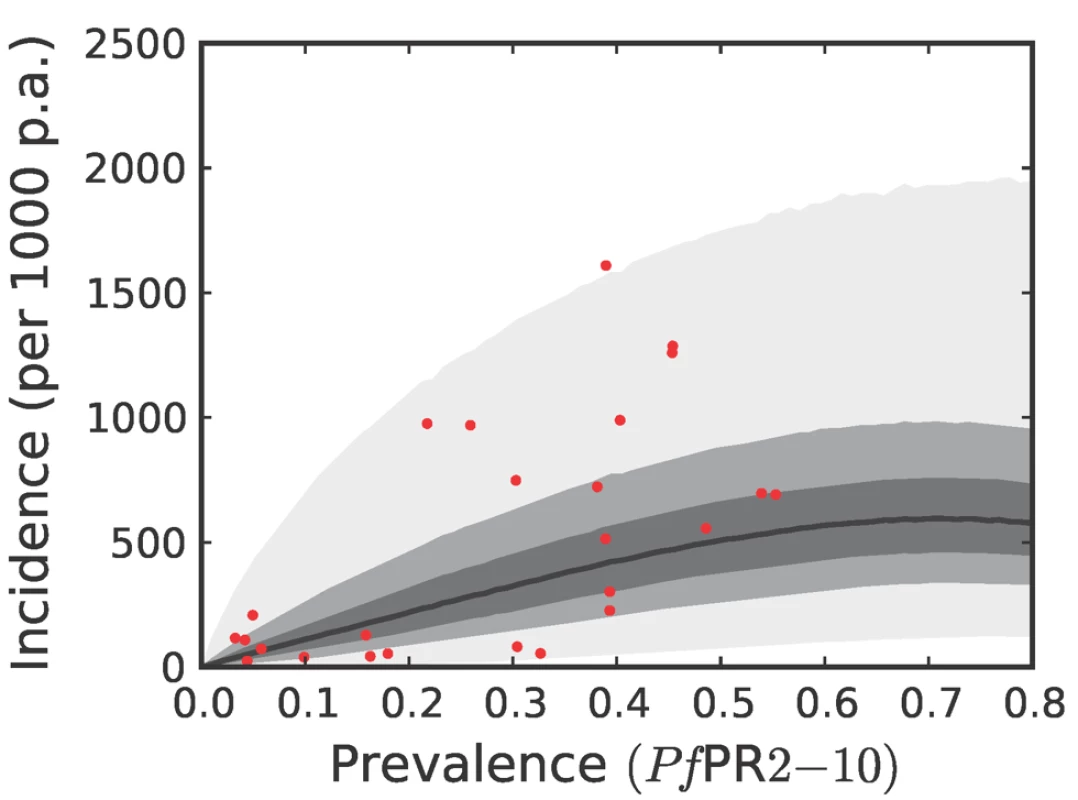
Please see reference [42] for a full description of the data, methods, and techniques used to define this relationship. The light grey, medium grey, and dark grey regions define the 95%, 50%, and 25% credible intervals, respectively. The solid black line is the median and the data are shown as red dots. Note that the data points were collected using different surveillance intervals over different time periods, and therefore should not be expected to follow the distribution predicted by the model exactly. The observed incidences are included in the figure as a visual aid only. Fig. 9. The predictive distribution of the incidence that would actually be observed by weekly surveillance over a two-year period in the combined CSE Asia region and the Americas. 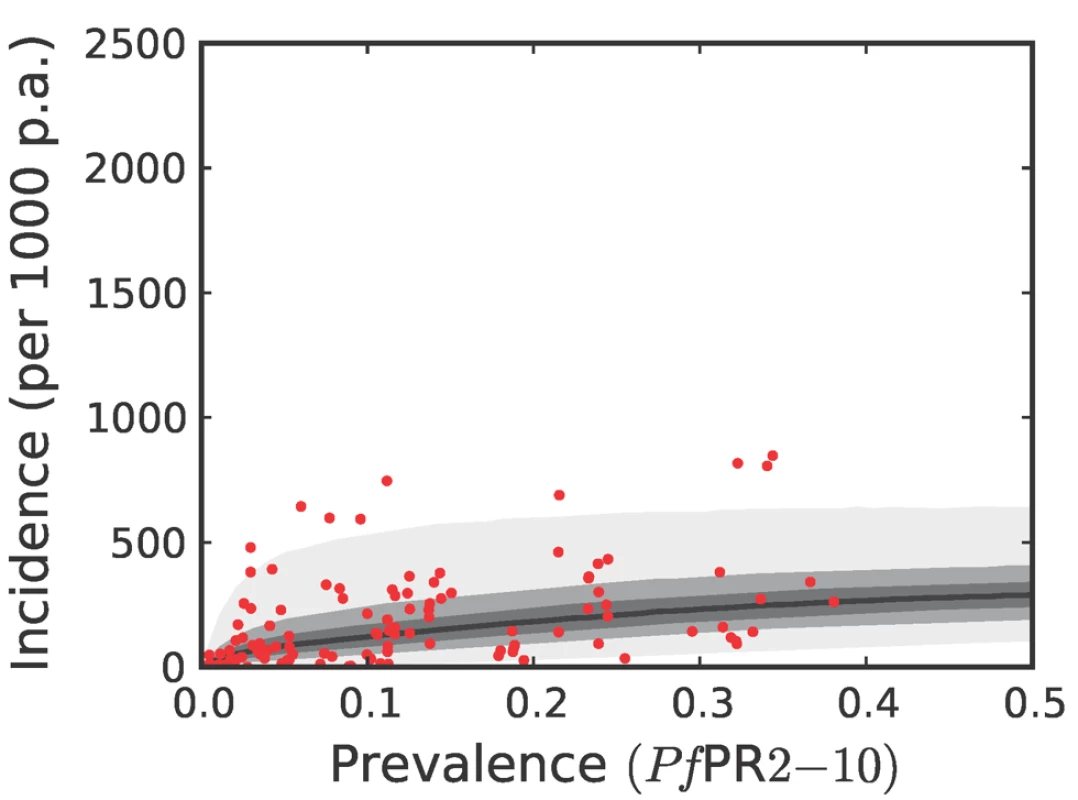
The techniques and colours used are identical to Figure 8. Both the geostatistical endemicity and the endemicity–incidence models were specified in a fully Bayesian framework. The output of the former was a large set of realisations (n = 250,000): possible maps that, together, represented the modelled uncertainty in endemicity at each location. Similarly, the output of the endemicity–incidence model was a large set (n = 250,000) of possible forms of the endemicity-incidence curve that encompassed the modelled uncertainty in this relationship (Figures 6, 7, 8, and 9). To combine the uncertainty from both models, each realisation of the uncertainty map was used as input into a realisation of the endemicity–incidence model to obtain a realisation of a 5×5 km resolution incidence map. This was downscaled to 1×1 km resolution and multiplied with the 2007 population surface to obtain, for every grid square, a realisation of the number of clinical cases in 2007. By repeating this procedure for every model realisation, a set of 250,000 burden values was generated for every grid square, approximating a complete posterior distribution for the estimates. Because each realisation of the endemicity map was jointly simulated, rather than calculated on a pixel-by-pixel basis, each realisation of burden could be aggregated spatially or temporally, whilst maintaining the correct variance structure. This allowed burden realisations at each pixel to be combined spatially to generate estimates of national and regional burdens with appropriate credible intervals. Joint simulation at this scale is enormously computationally intensive and a bespoke algorithm was developed to implement this stage of the analysis. The algorithm is presented elsewhere [43] and the statistical details are summarised in Protocol S1.
Results
The combined clinical burden of the seven nations with comprehensive reporting was 3,367 cases in 2007 (Table 1, Protocol S2). Multiplying the population surface (Figure 3) by the assumed incidence rate in unstable areas (see Methods) produced an estimate of 105,395 clinical cases of P. falciparum malaria in areas of unstable transmission (Table 1, Protocol S2), with a plausible range between zero and 1,053,950. The modelling procedures in the stable areas generated an estimate of 451 million cases (lower 95% credible interval 349 million and upper 95% credible interval 552 million) of P. falciparum malaria in areas of stable transmission in 2007, of which 271 (241–301) million were estimated to have occurred in the Africa+ region, 177 (89–270) million in the CSE Asia region and 3 (1–7) million in the Americas (Table 1).
Combining our estimates from the seven countries with comprehensive case reporting with those from areas of unstable and stable transmission in the remaining 80 PfMECs, we estimate that in 2007 there were 451 (349–553) million clinical cases of P. falciparum malaria. A continuous map of these incidence predictions is provided (Figure 4), with an additional map of the pixel-specific uncertainty (Figure 5). In addition to the regional summaries presented (Table 1), estimates of clinical burden are summarized for each country and for each of the WHO global regions (Figure 10 and Protocol S2). It is notable that more than half (51%) of the world's estimated P. falciparum clinical cases derive from just four countries: India, Nigeria, DRC, and Myanmar (Burma) (Figure 4 and Protocol S2) and that, in addition, these nations contribute 48% of the uncertainty (Figure 5) in the global incidence estimates.
Fig. 10. Pie chart of P. falciparum clinical cases in 2007. 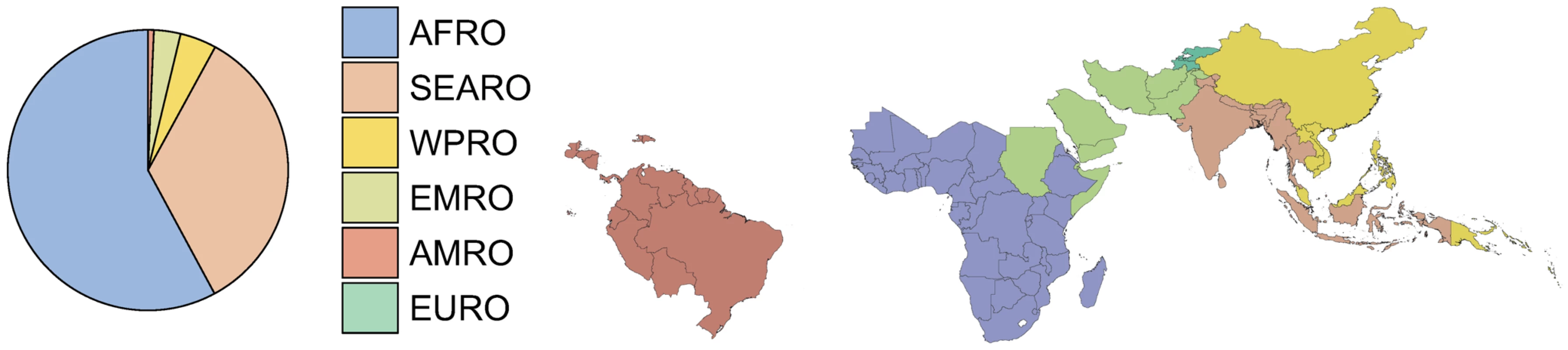
The pie chart shows the fraction of the 451 million cases of total clinical burden in each of the World Health Organization regions (Protocol S2). In the pie the regions are ordered counterclockwise starting at the top, from highest to lowest burden. The plotted area representing the EURO region is too thin to be visible. The thumbnail map shows the country composition of the WHO regions for all 87 P. falciparum endemic countries. Regional summary estimates of P. falciparum malaria cases in unstable and stable transmission areas are summarized in Table 1 and are also shown for the WHO regions in Figure 10. It is clear that African populations suffered the largest proportion (60%) of the 451 million clinical cases of P. falciparum estimated globally in 2007 (Figure 10, Table 1 and Protocol S2). The highest-burden countries in Africa are Nigeria and DRC, both countries with extensive regions of high endemicity (Figure 2) and large populations (Figure 3). These two countries account for 23% of the world's P. falciparum disease burden (Protocol S2). Less than 1% of the global P. falciparum burden occurred in the Americas, where transmission intensity is almost universally low or unstable (Figure 2). We estimate that the remaining 39% of global burden in 2007 occurred in the CSE Asia region (Table 1). In this region, the immense population living at risk of P. falciparum malaria means that, despite a low prevalence [41] (Figure 2) and the lower endemicity–incidence relationship [42] (Figure 7), cases in CSE Asia add substantially to the global disease burden (Table 1). At a country level, India and Myanmar contribute 22.6% and 5.8%, respectively, of the total number of clinical cases due to P. falciparum worldwide (Protocol S2).
Discussion
We have used a combination of methods, including a joint simulation of incidence in areas of stable transmission, to estimate 451 (349–552) million clinical cases of P. falciparum malaria in 2007 : 3 (1–7) million in the Americas, 271 (241–301) in the Africa+ region, and 177 (89–270) in the CSE Asia region.
Morbidity in Areas of Unstable Transmission
We have accepted as accurate the surveillance reports of seven relatively high income and low burden PfMECs, all nations with credible plans for malaria elimination [59],[60],[74]–[76]. We have further attempted to describe clinical disease incidence in areas of the world that we classify as unstable risk [40], which were home to almost a billion people in 2007. We know relatively little about the epidemiology of P. falciparum in the 40% of the global PAR of P. falciparum malaria living in unstable transmission areas. These areas are notoriously difficult to define in terms of potential disease outcomes; they may go several years without a single autochthonous case, transmission is extremely focal and, importantly, investigation of the clinical epidemiology is prohibitively expensive because of the rarity of the disease [77]. We have, therefore, defaulted to national reporting systems as an entry point to the definition of risk and have used surveys of under-reporting rates to define plausible ranges of the disease burden in these marginal transmission zones. We estimate that there were 105,395 (0–1,053,950) cases of P. falciparum in unstable transmission areas in 2007. Despite being relatively crudely defined, these sums represent only 0.02% of the global clinical P. falciparum burden. Therefore, while these cases are of significant concern to those nations with large populations at unstable risk and to those considering elimination [59],[60],[74]–[76], they make a very small contribution to the estimation of the global P. falciparum burden.
Morbidity in Stable Areas
We have improved upon a P. falciparum disease burden estimation rubric that has been used several times previously for Africa [1],[3],[4],[6],[7] and once before globally [5]. This method requires an understanding of the basic clinical epidemiology of P. falciparum malaria, its relationship to transmission intensity and the use of empirical, longitudinal observations in populations exposed to different conditions of transmission. However, these empirical studies of clinical incidence are not without their own caveats [42]. Longitudinal surveillance over a complete annual malaria transmission cycle within the same cohort is likely to underestimate the “natural” risk of disease given the ethical need to treat effectively all detected infections or clinical events. These studies are also conducted throughout a range of region-specific co-species infection [78], HIV/AIDS prevalence [79], and drug resistance [80] conditions. The number of studies meeting our inclusion criteria remains low, so these covariate determinants of clinical risk cannot be adequately modelled or controlled for in this series [42]. We have considered all infections that are associated with a reported or measured febrile event as clinical malaria. This seems appropriate under conditions of low transmission intensity, but as transmission intensity increases, the proportion of fevers that can be causally linked to malaria infection declines [26],[81]. Consequently, our estimates of clinical attack rates at the highest levels of transmission are likely to be overestimates of true P. falciparum clinical incidence. Locally derived age - and transmission-dependent aetiological fraction estimates were not available for the majority of studies in order to allow the application of meaningful corrections. Conversely, the use of fever and any level of peripheral infection to define a malaria case corresponds closely to the criteria recommended for case treatment across the world [82],[83] and thus has congruence with disease burdens that should be managed with appropriate medicines. Finally, we have not considered the impact of scaled or partial coverage of interventions aimed at preventing infection, because we feel this is reflected in the parasite prevalence surface [41]. The one exception is the use of failing monotherapy because recrudescent cases will not be reflected in our endemicity–incidence relationship based on active case detection with effective treatment and thus, where this poses a significant threat, our estimates will be even greater underestimates. Despite the caveats, we believe that this approach to P. falciparum disease burden estimation provides an alternative and, in nations with inadequate surveillance, the only existing approach to estimating the true global risk of malaria.
Robust Estimates of Uncertainty
We have used joint simulations from an established Bayesian geostatistical model for P. falciparum parasite prevalence in the 2 - up to 10-year age group (PfPR2–10) (Figure 2), integrated with a second Bayesian model for the endemicity-incidence relationship (Figures 6 and 7), to generate spatially distributed estimates of the clinical burden of P. falciparum malaria worldwide with associated uncertainty. This reflects the uncertainty in measures of risk that results in a range of possible estimates globally from 349 to 553 million cases in 2007; similar to the range size in other malaria burden estimations [1],[3],[5],[7],[84]. This elaborate modelling framework has allowed the incorporation of uncertainty in our knowledge of the intensity of transmission at any given location with uncertainty in our knowledge of how this intensity influences the rate of clinical episodes at that location, allowing the net uncertainty to be propagated into final estimates of clinical burden. Crucially, the joint simulation framework allows modelled uncertainty to be aggregated across regions to provide our final credible intervals for country and region-specific burden estimates, a procedure that is not possible using the per-pixel prediction approaches currently pervasive in disease mapping.
The WHO has recently used surveillance-based techniques to estimate the combined burden of P. falciparum and P. vivax to be 247 million cases in 2006 (189–287) [8]. The WHO placed greater reliance on data reported routinely through national health management information systems (HMIS), which were subjected to a range of evidence-based adjustments for nonattendance, reporting rates, and diagnostic practices. These HMIS data were used for national estimates in 77 of 107 countries considered worldwide (Protocol S2). The fidelity of these estimates and their sensitivity to assumptions underlying the suite of adjustment factors was dependent on the quality and completeness of the HMIS data from each country. In the 30 countries with the least reliable national data, a predecessor of the prevalence-based modelling protocol presented in this study was used [8],[85]. The results are shown for individual countries in Protocol S2. These estimates were revised in 2009 but data have not been made available for all countries [9].
Uncertainty in India
India is a country of considerable diversity in its current and historic malaria ecology, a country which suffered in excess of a million deaths PA during the colonial era [86]. Since its independence in 1947, India has achieved remarkable malaria control gains, reducing morbidity to 100,000 cases and mortality to zero in 1965 [87] at the peak of the Global Malaria Eradication Programme [53]. Since this time malaria resurgence has been widely reported in the country [87]–[89]. The contemporary burden is unknown [90]–[97] and is probably exacerbated by the unique problem of urban malaria, maintained by Anopheles stephensi [49],[88],[98].
India remains a massive source of uncertainty in our cartography-based estimates (Results and Protocol S2), contributing over three-quarters (76%) of the uncertainty range in the global incidence estimates. It is therefore important to explore ancillary evidence for the plausibility of these cartographic estimates of 102 (31–187) million compared to the much smaller estimate derived from surveillance-based techniques: 10.65 (9.00–12.41) million [8].
A wide range of factors can reduce the accuracy of surveillance data. Low rates of care-seeking for malaria in the formal health sector, unreliable diagnoses, poor record keeping, and inefficient data transfer and collation systems can all combine to make the number of cases formally reported a small fraction of the true number of cases in a population. To mitigate these substantial sources of bias in raw surveillance data, the approach taken by WHO is to modify the raw data using a number of adjustment parameters, which can include the proportion of people with fever seeking formal-sector care, the reporting rate by facilities, and the likely positivity rates amongst non-attending and non-slide–confirmed cases of fever [8],[85]. Such adjustments are essential, but the validity of the final estimate is entirely dependent on the values used for each parameter, which are drawn from a mixture of health-system reported figures, secondary data of varying fidelity, and ad-hoc decision rules. A key weakness of this approach is that, in many cases, the true uncertainty around key parameter values is not captured adequately.
In the case of India, raw surveillance data for 2006 reported 1.8 million malaria cases. Adjustments were made for care-seeking behaviour and reporting rate by health facilities, which combined to increase the estimate by a factor of 5.0–6.9, to the final figure of 10.65 (9.00–12.41) million [8], with the confidence range primarily reflecting differing assumptions for positivity rate amongst nonpresenting fevers. Assessing the validity of either the individual adjustment parameters or the final estimate is difficult since, by definition, gold-standard values for comparison do not exist. However, numerous studies in India have compared case numbers detected via routine surveillance with parallel community-based longitudinal surveys and found disparities much larger than the factor of approximately six used by the WHO. For example, malaria incidence in the Kichha Primary Health Centre (PHC) and Kharkhoda PHC were 23.5 and 38.9 times under-reported, respectively [61]. Large discrepancies were also reported in Gadarpur PHC (53.5×) [62], Nichlaul PHC (20.3×) [64] and Ahmedabad City (9×) [65]. For India, the WHO estimate makes no allowance for misdiagnosis within the formal health sector, although studies have shown that this can be substantial. In the PHCs of ten districts in Uttar Pradesh, 75% of slide-confirmed infections were missed when the slides were checked by a reference centre [28], and an estimated 58% were missed in Bisra PHC when fortnightly rather than weekly surveillance was used [63].
In completely independent work, the final estimate for malaria mortality in India in 2006, taken from the “million deaths” verbal autopsy study was approximately 200,000 deaths (Dhingra N, et al., unpublished data). Assuming a conservative case fatality rate of only one per 1,000 [99],[100], this would lead to a morbidity estimate much closer to those retrieved using cartographic techniques—somewhere in the region of 200 million cases. Similar arguments of plausible morbidity totals can be made using other recent mortality estimates of 50,000 deaths in 1998 in 15 of 38 States and Union Territories [90],[93]. In sum, we find that cartography-based estimates are supported by, and resonate most closely with, the findings in the recent literature [90]–[96], although it should be acknowledged that there is likely to be a publication bias in reports of problems over progress.
There is no perfect post-hoc correction to compensate for poor malaria surveillance. Both methods using routine HMIS adjusted for nonattendance, poor reporting, and inadequate diagnostics, and those presented here, have limitations with respect to coverage and quality of the input data for each model, and with respect to underlying modelling assumptions. Both approaches to burden estimation result in wide margins of confidence and the inevitable plea from any such analysis is for accurate national reporting systems or more empirical epidemiological data. It can be seen clearly from these analyses that improvements in basic malariometric information in only four countries would radically reduce uncertainty in the global estimates of the malaria burden. Additionally, the approach presented does provide a standardized method across all malaria-endemic countries, using a set of transparent epidemiological rules allowing countries to be compared without concerns about differences in national health information quality or coverage.
A Hybrid Approach?
To allay some of the concerns about the use of cartographic techniques in low-endemicity settings [101], we have also investigated the possibility of combining the two burden estimation processes for the 87 PfMECs.
Seven countries have “gold-standard” reporting systems requiring no adjustment by either technique. These are in the African Regional Office (AFRO): South Africa; in AMRO: Belize and Panama; EMRO: Iran and Saudi Arabia; and EURO: Kyrgyzstan and Tajikistan (7/87). In many PfMECs in the Africa+ region, an outdated cartographic technique was used by WHO [8]. Since the new methods outlined here are an unambiguous improvement, these were adopted for the following PfMECs: in AFRO: Angola, Burkina Faso, Cameroon, Central African Republic, Chad, Congo, Côte d'Ivoire, DRC, Equatorial Guinea, The Gambia, Ghana, Guinea, Guinea-Bissau, Liberia, Malawi, Mali, Mauritania, Mozambique, Niger, Nigeria, Sierra Leone, Togo, Uganda, and Zimbabwe; and in EMRO: Yemen (25/87). In addition, Mayotte in AFRO and French Guiana in AMRO have no WHO estimates, so we default to the cartographic approach (2/87). Conversely there are two small island nations in AFRO (Cape Verde and the Comoros) for which we had no contemporary PfPR data and the spatial resolution of mapping was not ideal, so the WHO estimates were used (2/87).
We then calculated, for all countries, the ratio of the width of the 95% credible interval to the point estimate obtained using the cartographic method and ranked this relative uncertainty metric by nation (Protocol S2). For those countries where this cartography-based uncertainty ranked in the bottom half (i.e., the least uncertain, corresponding to a ratio of <40), we adopted our cartographic-based estimates. They were in AFRO: Benin, Burundi, Ethiopia, Gabon, Kenya, Madagascar, Rwanda, Senegal, United Republic of Tanzania, and Zambia; in EMRO: Somalia and Sudan; in SEARO: India, Indonesia, and Myanmar; and in WPRO: Papua New Guinea (16/87). Conversely, in countries where cartography-based uncertainty was ranked in the top half (ratio ≥40) we defaulted to the WHO estimate. They were in AFRO: Botswana, Eritrea, Namibia, São Tomé and Príncipe, and Swaziland; in AMRO: Bolivia, Brazil, Colombia, Dominican Republic, Ecuador, Guatemala, Guyana, Haiti, Honduras, Nicaragua, Peru, Suriname, and Venezuela; in EMRO: Afghanistan, Djibouti, and Pakistan; in SEARO: Bangladesh, Bhutan, Nepal, Sri Lanka, Thailand, and Timor-Leste; and in WPRO: Cambodia, China, Lao People's Democratic Republic, Malaysia, Philippines, Solomon islands, Vanuatu, and Viet Nam (35/87).
This hybrid approach resulted in seven countries using gold standard national reports, 43 nations using cartographic techniques and 37 using the surveillance-based methods of WHO. The percentage of the global burden estimated by each technique was 0.001%, 97.722%, and 2.277%, respectively. Using a hybrid approach therefore makes very little difference to the global clinical burden estimate for 2007, although it has a significant impact on the absolute number of cases estimated for each country (Protocol S2).
Interpreting Estimates
These estimates improve upon previous efforts, which used epidemiological approaches to estimate the global burden of P. falciparum clinical attacks in 2002 (515 million, interquartile range 300–660 million) [5], and more recent efforts to estimate paediatric clinical events due to high parasite densities of P. falciparum in Africa in 2000 (116 million, uncertainty interval 91–258 million) [7]. The differences between these results and previous efforts are not primarily due to differences in the base year of analysis or definitions of a clinical attack, but stem largely from differences in estimation of the endemicity-structured PARs. In our previous global estimates [5], we adapted a historical, categorical description of malaria endemicity, whilst in Africa we [1],[3],[4] and others [6],[7] have previously used a climate suitability model of the likelihood of stable transmission as an index of differences in transmission intensity [102],[103]. The single largest difference between previous work and the present iteration of P. falciparum disease burden estimation is that neither previous approach was based upon an empirically defined risk map of malaria transmission [41]. Comparing estimates derived using these different techniques, over various time periods, is not a sound basis for investigating trends and should be avoided.
It is clear that investing in radically improved surveillance and/or nationally representative malariometric surveys would substantially increase the fidelity of national and, by extension, global burden estimates. Because there are regional differences in the uncertain relationship between transmission intensity and disease outcome [42], more information derived from active case detection studies would improve the precision in our estimates of disease incidence within these transmission ranges. This information, while welcome, is likely to make only small differences to the computed risk in most scenarios of malaria transmission defined here. As a consequence, we believe that until there is a universally reliable reporting system for malaria cases worldwide to support comprehensive surveillance-based estimates, a concerted effort to map the changing spatial extents and intensity of transmission will remain a valuable contribution to the future estimations of a changing disease burden worldwide. In the short term, measuring how the “denominator” changes with time is clearly easier and cheaper than improving the global state of health information systems.
Future Directions
Many improvements will be possible with further work. We have not stratified incidence by age nor considered any of the consequential morbid events, sequelae, or mortality. Systematic biases in the identification of the extent of stable and unstable transmission would clearly impact estimates, and developing the datasets and techniques to address this problem is an important avenue for future work. Nor have we modelled uncertainty in HMIS reporting in unstable and low-stable transmission zones, and this might be possible with a methodological hybrid combining higher spatial resolution HMIS facility data with geostatistical techniques [37]. Moreover, we have not been able to consider some sources of uncertainty in the current framework; for example, those concerning the enumeration of the underlying population, based on collated census data; urban extent maps; and UN population projections. Finally, we have not considered the morbid burden posed by P. vivax. There are important differences in the biology of P. vivax [104] which make its control [105], and thus cartography-based burden estimation, problematic: its tendency to cause relapses [106], the routine reliability of parasite diagnosis when coincidentally prevalent with P. falciparum [107],[108] and the less well-defined relationship between transmission intensity and disease outcome. These all make an informed cartography of P. vivax distribution and estimations of disease burden considerably more complex than for P. falciparum. We do not underestimate the likely disease burden of P. vivax malaria [109]–[112], but new, innovative approaches based on an understanding of the clinical epidemiology and better cartography are required to improve upon current efforts to define the burden due to P. vivax.
It is worth reiterating that if the international community wishes to demonstrate progress in malaria control, then the quantity and timeliness of prevalence information and parasite-specific surveillance records must dramatically improve. This is true for all countries but is particularly important in India, Nigeria, DRC, and Myanmar because of the large populations at risk and the paucity of existing malariometric information. These improvements in information collection and provision are as important across space (to be geographically representative of all transmission settings and intervention scenarios) as they are through time, so that impact can be evaluated in a timely manner. Conceptually, we also envisage that significant progress will be made in improving the accuracy of these estimates by hybridising cartographic and surveillance-based approaches. This would be best achieved by combining geopositioned HMIS facility data with geostatistical model outputs [37], so that the relative uncertainty of each can be compared and complementary information from both sources combined in a single coherent spatial framework. Globally, this is likely to be of particular utility in those areas of low and unstable transmission where surveillance capabilities are often more robust and correspondingly where prevalence data are often rare as the number of people needed to be sampled to find infections is prohibitive [12].
The malaria clinical burden estimates presented in this paper are driven by the underlying model of global prevalence [41]. This global malaria map is, to our knowledge, the first evidence-based attempt to define populations at risk of different levels of parasite transmission. It is needed in order to define the ranges of disease outcomes at a global scale and can serve as the benchmark for malaria disease burden estimations. The map will inevitably change with time as new information on the spatial extents of transmission and new PfPR2–10 data become increasingly available with the scale-up of interventions. The time–space functionality of the geostatistical model will increasingly capture the effects of scaled intervention efforts to reduce transmission, causing the size of the PfPR used to compute disease burden to change. Revising the limits and endemicity maps from this baseline and propagating these changes through to revised enumerations of clinical burden thus represents a useful complementary technique to assessing the impact of financing [113] on our progress towards international development targets for reducing malaria burden [59],[114].
Supporting Information
Zdroje
1. SnowRW
CraigM
DeichmannU
MarshK
1999 Estimating mortality, morbidity and disability due to malaria among Africa's non-pregnant population. Bull World Health Organ 77 624 640
2. CarterR
MendisKN
2002 Evolutionary and historical aspects of the burden of malaria. Clin Microbiol Rev 15 564 594
3. SnowRW
CraigMH
NewtonCRJC
SteketeeRW
2003 The public health burden of Plasmodium falciparum malaria in Africa: deriving the numbers. 1 71 Working Paper No. 11, Disease Control Priorities Project Bethesda, Maryland, U.S.A.: Fogarty International Centre, National Institutes of Health
4. HaySI
GuerraCA
TatemAJ
AtkinsonPM
SnowRW
2005 Urbanization, malaria transmission and disease burden in Africa. Nat Rev Microbiol 3 81 90
5. SnowRW
GuerraCA
NoorAM
MyintHY
HaySI
2005 The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature 434 214 217
6. RoweAK
RoweSY
SnowRW
KorenrompEL
SchellenbergJRA
2006 The burden of malaria mortality among African children in the year 2000. Int J Epidemiol 35 691 704
7. Roca-FeltrerA
CarneiroI
Armstrong SchellenbergJR
2008 Estimates of the burden of malaria morbidity in Africa in children under the age of 5 years. Trop Med Int Health 13 771 783
8. WHO 2008 World malaria report 2008. Geneva World Health Organization 215 WHO/HTM/GMP/2008.1
9. WHO 2009 World malaria report 2009. Geneva World Health Organization 202
10. MurrayCJ
LopezAD
1996 Global burden of disease and injury series. Geneva World Health Organization 320 327
11. MurrayCJL
LopezAD
1997 Mortality by cause for eight regions of the world: Global Burden of Disease Study. Lancet 349 1269 1276
12. HaySI
SmithDL
SnowRW
2008 Measuring malaria endemicity from intense to interrupted transmission. Lancet Infect Dis 8 369 378
13. MarshK
SnowRW
1999 Malaria transmission and morbidity. Parassitologia 41 241 246
14. TrapeJF
RogierC
1996 Combating malaria morbidity and mortality by reducing transmission. Parasitol Today 12 236 240
15. SnowRW
MarshK
1998 New insights into the epidemiology of malaria relevant for disease control. Br Med Bull 54 293 309
16. SmithTA
LeuenbergerR
LengelerC
2001 Child mortality and malaria transmission intensity in Africa. Trends Parasitol 17 145 149
17. SnowRW
MarshK
2002 The consequences of reducing transmission of Plasmodium falciparum in Africa. Adv Parasitol 52 235 264
18. SmithT
KilleenG
LengelerC
TannerM
2004 Relationships between the outcome of Plasmodium falciparum infection and the intensity of transmission in Africa. Am J Trop Med Hyg 71 80 86
19. GentonB
SmithT
BaeaK
NararaA
al-YamanF
1994 Malaria: how useful are clinical criteria for improving the diagnosis in a highly endemic area? Trans R Soc Trop Med Hyg 88 537 541
20. SmithT
HurtN
TeuscherT
TannerM
1995 Is fever a good sign for clinical malaria in surveys of endemic communities? Am J Trop Med Hyg 52 306 310
21. ChandramohanD
JaffarS
GreenwoodB
2002 Use of clinical algorithms for diagnosing malaria. Trop Med Int Health 7 45 52
22. da Silva-NunesM
MRS
LBA
SouzaEA
MartinsLC
2006 The Acre Project: the epidemiology of malaria and arthropod-borne virus infections in a rural Amazonian population. Cad Saude Publica 22 1325 1334
23. KoramKA
MolyneuxME
2007 When is “malaria” malaria? The different burdens of malaria infection, malaria disease, and malaria-like illnesses. Am J Trop Med Hyg 77 1 5
24. AmexoM
TolhurstR
BarnishG
BatesI
2004 Malaria misdiagnosis: effects on the poor and vulnerable. Lancet 364 1896 1898
25. ReyburnH
MbatiaR
DrakeleyC
CarneiroI
MwakasungulaE
2004 Overdiagnosis of malaria in patients with severe febrile illness in Tanzania: a prospective study. Br Med J 329 1212
26. SmithT
SchellenbergJA
HayesR
1994 Attributable fraction estimates and case definitions for malaria in endemic areas. Stat Med 13 2345 2358
27. ChaturvediHK
MahantaJ
PandeyA
2009 Treatment-seeking for febrile illness in north-east India: an epidemiological study in the malaria endemic zone. Malar J 8 301
28. ChoudhuryDS
SharmaVP
BhallaSC
AggarwalSS
DasSK
1987 Malaria prevalence in patients attending primary health centres in ten districts of Uttar Pradesh. Indian J Malariol 24 79 83
29. AbeysekeraT
WickremasingheAR
GunawardenaDM
MendisKN
1997 Optimizing the malaria data recording system through a study of case detection and treatment in Sri Lanka. Trop Med Int Health 2 1057 1067
30. DonnellyMJ
KonradsenF
BirleyMH
1997 Malaria-treatment-seeking behaviour in the southern Punjab, Pakistan. Ann Trop Med Parasitol 91 665 667
31. Al-LahamH
KhouryR
BashourH
2001 Reasons for underreporting of notifiable diseases by Syrian paediatricians. East Mediterr Health J 7 590 596
32. ChilundoB
SundbyJ
AanestadM
2004 Analysing the quality of routine malaria data in Mozambique. Malar J 3 3
33. MurrayCJ
LopezAD
WibulpolprasertS
2004 Monitoring global health: time for new solutions. Br Med J 329 1096 1100
34. BranchO
CasapiaWM
GamboaDV
HernandezJN
AlavaFF
2005 Clustered local transmission and asymptomatic Plasmodium falciparum and Plasmodium vivax malaria infections in a recently emerged, hypoendemic Peruvian Amazon community. Malar J 4 27
35. OumS
ChandramohanD
CairncrossS
2005 Community-based surveillance: a pilot study from rural Cambodia. Trop Med Int Health 10 689 697
36. StansfieldS
2005 Structuring information and incentives to improve health. Bull World Health Organ 83 562
37. GethingPW
NoorAM
GikandiPW
OgaraEAA
HaySI
2006 Improving imperfect data from health management information systems in Africa using space-time geostatistics. PLoS Med 3 e271 doi:10.1371/journal.pmed.0030271
38. KunimitsuA
2009 The accuracy of clinical malaria case reporting at primary health care facilities in Honiara, Solomon Islands. Malar J 8 80
39. LysenkoAJ
SemashkoIN
1968 Geography of malaria. A medico-geographic profile of an ancient disease [in Russian].
LebedewAW
Itogi Nauki: Medicinskaja Geografija Moscow Academy of Sciences, USSR 25 146
40. GuerraCA
GikandiPW
TatemAJ
NoorAM
SmithDL
2008 The limits and intensity of Plasmodium falciparum transmission: implications for malaria control and elimination worldwide. PLoS Med 5 e38 doi:10.1371/journal.pmed.0050038
41. HaySI
GuerraCA
GethingPW
PatilAP
TatemAJ
2009 A world malaria map: Plasmodium falciparum endemicity in 2007. PLoS Med 6 e1000048 doi:10.1371/journal.pmed.1000048
42. PatilAP
OkiroEA
GethingPW
GuerraCA
SharmaSK
2009 Defining the relationship between Plasmodium falciparum parasite rate and clinical disease: statistical models for disease burden estimation. Malar J 8 186
43. GethingPW
PatilAP
HaySI
2010 Quantifying aggregated uncertainty in Plasmodium falciparum malaria prevalence and populations at risk via efficient space-time geostatistical joint simulation. PLoS Comput Biol 6 e1000724 doi:10.1371/journal.pcbi.1000724
44. BalkDL
DeichmannU
YetmanG
PozziF
HaySI
2006 Determining global population distribution: methods, applications and data. Adv Parasitol 62 119 156
45. U.N.P.D 2006 New York United Nations Population Division (U.N.D.P) World population prospects: the 2006 revision population database. http://esa.un.org/unpp/
46. HaySI
NoorAM
NelsonA
TatemAJ
2005 The accuracy of human population maps for public health application. Trop Med Int Health 10 1073 1086
47. MacdonaldG
1957 Local features of malaria. London Oxford University Press 63 99 The epidemiology and control of malaria
48. MouchetJ
CarnevaleP
CoosemansM
JulvezJ
ManguinS
2004 Paludisme et grandes régions biogéographiques. Montrouge, France John Libbey Eurotext Biodiversité du paludisme dans le monde
49. HaySI
SinkaME
OkaraRM
KabariaCK
MbithiPM
2010 Developing global maps of the dominant Anopheles vectors of human malaria. PLoS Med 7 e1000209 doi:10.1371/journal.pmed.1000209
50. PullJH
1972 Malaria surveillance methods, their uses and limitations. Am J Trop Med Hyg 21 651 657
51. RayAP
BeljaevAE
1984 Epidemiological surveillance: a tool for assessment of malaria and its control. J Commun Dis 16 197 207
52. MolineauxL
MuirDA
SpencerHC
WernsdorferWH
1988 The epidemiology of malaria and its measurement.
WernsdorferWH
McGregorI
Malaria: principles and practice of malariology Edinburgh Churchill Livingstone 999 1089
53. PampanaE
1969 A textbook of malaria eradication. London Oxford University Press
54. SwaroopS
GilroyAB
UemuraK
1966 Statistical methods in malaria eradication. Geneva World Health Organization 164
55. YekutielP
1960 Problems of epidemiology in malaria eradication. Bull World Health Organ 22 669 683
56. WHO 1963 Terminology of malaria and of malaria eradication. Report of a drafting committee Geneva World Health Organization 127
57. MacauleyC
2005 Aggressive active case detection: a malaria control strategy based on the Brazilian model. Soc Sci Med 60 563 573
58. WHO 2007 Malaria elimination: a field manual for low and moderate endemic countries. Geneva World Health Organization 85
59. R.B.M.P 2008 The global malaria action plan for a malaria free world. Geneva, Switzerland Roll Back Malaria Partnership (R.B.M.P), World Health Organization
60. on behalf of the Malaria Elimination Group 2009 Shrinking the Malaria Map: a Prospectus on Malaria Elimination.
FeachemRGA
PhillipsAA
TargettGA
San Francisco, U.S.A. The Global Health Group, University of California - Santa Cruz Global Health Sciences 187
61. SharmaVP
ChoudhuryDS
AnsariMA
MalhotraMS
MenonPKB
1983 Studies on the true incidence of malaria in Kharkhoda (District Sonepat, Haryana) and Kichha (District Nainital, U.P.) Primary Health Centres. Indian J Malariol 20 21 34
62. MalhotraMS
ShuklaRP
SharmaVP
1985 Studies on the incidence of malaria in Gadarpur town of Terai, distt.Nainital U.P. Indian J Malariol 22 57 60
63. GhoshSK
KumarA
ChandSK
ChoudhuryDS
1989 A preliminary malaria survey in Bisra PHC, district Sundergarh, Orissa. Indian J Malariol 26 167 170
64. JoshiPL
ChandraR
BhattacharyaM
1999 An outbreak of malaria in district Maharajganj: an outcome of neglected surveillance. J Commun Dis 31 63 64
65. YadavRS
BhattRM
KohliVK
SharmaVP
2003 The burden of malaria in Ahmedabad city, India: a retrospective analysis of reported cases and deaths. Ann Trop Med Parasitol 97 793 802
66. ErhartA
ThangND
XaNX
ThieuNQ
HungLX
2007 Accuracy of the health information system on malaria surveillance in Vietnam. Trans R Soc Trop Med Hyg 101 216 225
67. IncardonaS
VongS
ChivL
LimP
NhemS
2007 Large-scale malaria survey in Cambodia: novel insights on species distribution and risk factors. Malar J 6 37
68. KidsonC
IndaratnaK
1998 Ecology, economics and political will: the vicissitudes of malaria strategies in Asia. Parassitologia 40 39 46
69. MouchetJ
CarnevaleP
CoosemansM
JulvezJ
ManguinS
2004 Biodiversité du paludisme dans le monde. Montrouge, France John Libbey Eurotext 428
70. TalisunaAO
BlolandP
D'AlessandroU
2004 History, dynamics, and public health importance of malaria parasite resistance. Clin Microbiol Rev 17 235 254
71. GunewardenaDM
CarterR
MendisKN
1994 Patterns of acquired anti-malarial immunity in Sri Lanka. Mem Inst Oswaldo Cruz 89 63 65
72. MaitlandK
WilliamsTN
NewboldCI
1997 Plasmodium vivax and P. falciparum: biological interactions and the possibility of cross-species immunity. Parasitol Today 13 227 231
73. WeatherallD
AkinyanjuO
FucharoenS
OlivieriN
MusgroveP
2006 Inherited disorders of hemoglobin. Disease control priorities in developing countries (2nd Edition).
JamisonDT
BremanJG
MeashamAR
AlleyneG
ClaesonM
New York Oxford University Press 663 680
74. WHO/PAHO 2006 Regional strategic plan for malaria in the Americas 2006-2010. Washington D.C. Pan American Health Organization, Regional Office for the Americas 71
75. WHO/Regional Office for Europe 2008 WHO meeting on progress achieved with malaria elimination in the WHO European Region. Copenhagen, Denmark World Health Organization Regional Office for Europe 41 WHO-EUR/08/5061325
76. WHO/Regional Office for the Eastern Mediterranean 2007 Strategic plan for malaria control and elimination in the WHO Eastern Mediterranean Region 2006–2010. Cairo World Health Organization Regional Office for the Eastern Mediterranean 41 WHO-EM/MAL/340/E
77. WHO/Regional Office for the Eastern Mediterranean 2007 Guidelines on the elimination of residual foci of malaria transmission. Cairo World Health Organization Regional Office for the Eastern Mediterranean 47 EMRO Technical Publications Series, 33
78. BrookerS
ClementsACA
HotezPJ
HaySI
TatemAJ
2006 The co-distribution of Plasmodium falciparum and hookworm among African schoolchildren. Malar J 5 99
79. Abu-RaddadLJ
PatnaikP
KublinJG
2006 Dual infection with HIV and malaria fuels the spread of both diseases in sub-Saharan Africa. Science 314 1603 1606
80. PearceRJ
PotaH
EveheMS
Ba elH
Mombo-NgomaG
2009 Multiple origins and regional dispersal of resistant dhps in African Plasmodium falciparum malaria. PLoS Med 6 e1000055
81. BejonP
MwangiT
LoweB
PeshuN
HillAV
2007 Clearing asymptomatic parasitaemia increases the specificity of the definition of mild febrile malaria. Vaccine 25 8198 8202
82. WHO 2006 Guidelines for the treatment of malaria. Geneva, Switzerland World Health Organization 253 WHO/HTM/MAL/2006.1108
83. WHO 2010 Guidelines for the treatment of malaria. Second edition. Geneva, Switzerland World Health Organization 194
84. KorenrompEL
2004 Malaria incidence estimates at country level for the year 2004-proposed estimates and draft report. Geneva World Health Organization
85. CibulskisRE
BellD
ChristophelEM
HiiJ
DelacolletteC
2007 Estimating trends in the burden of malaria at country level. Am J Trop Med Hyg 77 133 137
86. HehirP
1927 Malaria in India. London and New York Oxford University Press
87. AkhtarR
LearmonthA
1977 The resurgence of malaria in India 1965-76. GeoJournal 1 69 80
88. AkhtarR
DuttAK
WadhwaV
2010 Malaria resurgence in urban India: lessons from health planning strategies.
AkhtarR
DuttAK
WadhwaV
Malaria in South Asia Eradication and Resurgence During the Second Half of the Twentieth Century Netherlands Springer 141 155
89. SharmaVP
1996 Re-emergence of malaria in India. Indian J Med Res 103 26 45
90. KumarA
ValechaN
JainT
DashAP
2007 Burden of malaria in India: retrospective and prospective view. Am J Trop Med Hyg 77 69 78
91. SharmaVP
2007 Battling the malaria iceberg with chloroquine in India. Malar J 6 105
92. DashAP
ValechaN
AnvikarAR
KumarA
2008 Malaria in India: challenges and opportunities. J Biosci 33 583 592
93. DashAP
2009 Estimation of true malaria burden in India. New Delhi, India National Institute of Malaria Research 91 99 A Profile of National Institute of Malaria Research. 2nd ed
94. Diamond-SmithN
SinghN
GuptaRD
DashA
ThimasarnK
2009 Estimating the burden of malaria in pregnancy: a case study from rural Madhya Pradesh, India. Malar J 8 24
95. SharmaVP
2009 Hidden burden of malaria in Indian women. Malar J 8 281
96. SinghN
DashA
ThimasarnK
2009 Fighting malaria in Madhya Pradesh (Central India): Are we loosing the battle? Malar J 8 93
97. CohenAA
DhingraN
JotkarRM
RodriguezPS
SharmaVP
2010 The Summary Index of Malaria Surveillance (SIMS): a stable index of malaria within India. Popul Health Metr 8 1
98. WadhwaV
AkhtarR
DuttAK
2010 The dynamics of urban malaria in India: an update.
AkhtarR
DuttAK
WadhwaV
Malaria in South Asia Eradication and Resurgence During the Second Half of the Twentieth Century Netherlands Springer 157 178
99. LuxemburgerC
RicciF
NostenF
RaimondD
BathetS
1997 The epidemiology of severe malaria in an area of low transmission in Thailand. Trans R Soc Trop Med Hyg 91 256 262
100. SharmaPK
RamanchandranR
HutinYJ
SharmaR
GupteMD
2009 A malaria outbreak in Naxalbari, Darjeeling district, West Bengal, India, 2005: weaknesses in disease control, important risk factors. Malar J 8 288
101. SnowRW
GuerraCA
NoorAM
MyintHY
HaySI
2005 Malaria risk: Snow et al. reply. Nature 437 E4 E5
102. CraigMH
SnowRW
le SueurD
1999 A climate-based distribution model of malaria transmission in sub-Saharan Africa. Parasitol Today 15 105 111
103. SmallJ
GoetzSJ
HaySI
2003 Climatic suitability for malaria transmission in Africa, 1911-1995. Proc Natl Acad Sci U S A 100 15341 15345
104. CoatneyGR
CollinsWE
WarrenM
ContacosPG
2003 The primate malarias. Atlanta, Georgia Centers for Disease Control and Prevention [CD-ROM; original book published 1971]. Version 1.0
105. SattabongkotJ
TsuboiT
ZollnerGE
SirichaisinthopJ
CuiL
2004 Plasmodium vivax transmission: chances for control? Trends Parasitol 20 192 198
106. GarnhamPCC
1988 Malaria parasites of man: life-cycles and morphology (excluding ultrastructure).
WernsdorferWH
McGregorI
Malaria: principles and practice of malariology Edinburgh Churchill Livingstone 61 96
107. MayxayM
PukrittayakameeS
NewtonPN
WhiteNJ
2004 Mixed-species malaria infections in humans. Trends Parasitol 20 233 240
108. RosenbergR
2007 Plasmodium vivax in Africa: hidden in plain sight? Trends Parasitol 23 193 196
109. MendisK
SinaBJ
MarchesiniP
CarterR
2001 The neglected burden of Plasmodium vivax malaria. Am J Trop Med Hyg 64 97 106
110. BairdJK
2007 Neglect of Plasmodium vivax malaria. Trends Parasitol 23 533 539
111. PriceRN
TjitraE
GuerraCA
YeungS
WhiteNJ
2007 Vivax malaria: neglected and not benign. Am J Trop Med Hyg 77 79 87
112. MuellerI
GalinskiMR
BairdJK
CarltonJM
KocharDK
2009 Key gaps in the knowledge of Plasmodium vivax, a neglected human malaria parasite. Lancet Infect Dis 9 555 566
113. SnowRW
GuerraCA
MutheuJJ
HaySI
2008 International funding for malaria control in relation to populations at risk of stable Plasmodium falciparum transmission. PLoS Med 5 e142 doi:10.1371/journal.pmed.0050142
114. AttaranA
2005 An immeasurable crisis? A criticism of the millennium development goals and why they cannot be measured. PLoS Med 2 e318 doi:10.1371/journal.pmed.0020318
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2010 Číslo 6- Není statin jako statin aneb praktický přehled rozdílů jednotlivých molekul
- S prof. Vladimírem Paličkou o racionální suplementaci kalcia a vitaminu D v každodenní praxi
- Moje zkušenosti s Magnosolvem podávaným pacientům jako profylaxe migrény a u pacientů s diagnostikovanou spazmofilní tetanií i při normomagnezémii - MUDr. Dana Pecharová, neurolog
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
- Ferinject: správně indikovat, správně podat, správně vykázat
-
Všechny články tohoto čísla
- Studies Needed to Address Public Health Challenges of the 2009 H1N1 Influenza Pandemic: Insights from Modeling
- The Association of Factor V Leiden and Prothrombin Gene Mutation and Placenta-Mediated Pregnancy Complications: A Systematic Review and Meta-analysis of Prospective Cohort Studies
- Evaluating the Quality of Research into a Single Prognostic Biomarker: A Systematic Review and Meta-analysis of 83 Studies of C-Reactive Protein in Stable Coronary Artery Disease
- Gestational Age at Delivery and Special Educational Need: Retrospective Cohort Study of 407,503 Schoolchildren
- Closing the Gaps: From Science to Action in Maternal, Newborn, and Child Health in Africa
- Sub-Saharan Africa's Mothers, Newborns, and Children: How Many Lives Could Be Saved with Targeted Health Interventions?
- Secondary Prevention of Suicide
- The Prevalence and Drug Sensitivity of Tuberculosis among Patients Dying in Hospital in KwaZulu-Natal, South Africa: A Postmortem Study
- Estimating the Global Clinical Burden of Malaria in 2007
- Long-Term Biological and Behavioural Impact of an Adolescent Sexual Health Intervention in Tanzania: Follow-up Survey of the Community-Based MEMA kwa Vijana Trial
- Where to for Sexual Health Education for Adolescents in Sub-Saharan Africa?
- Incidence and Reproduction Numbers of Pertussis: Estimates from Serological and Social Contact Data in Five European Countries
- Hospital Performance, the Local Economy, and the Local Workforce: Findings from a US National Longitudinal Study
- Sub-Saharan Africa's Mothers, Newborns, and Children: Where and Why Do They Die?
- Revised STandards for Reporting Interventions in Clinical Trials of Acupuncture (STRICTA): Extending the CONSORT Statement
- Developing ANDI: A Novel Approach to Health Product R&D in Africa
- Maternal Health: Time to Deliver
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Gestational Age at Delivery and Special Educational Need: Retrospective Cohort Study of 407,503 Schoolchildren
- Evaluating the Quality of Research into a Single Prognostic Biomarker: A Systematic Review and Meta-analysis of 83 Studies of C-Reactive Protein in Stable Coronary Artery Disease
- Closing the Gaps: From Science to Action in Maternal, Newborn, and Child Health in Africa
- Secondary Prevention of Suicide
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



