-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaThe Clock Is Ticking: Countdown to Metastases
article has not abstract
Published in the journal: . PLoS Genet 12(9): e32767. doi:10.1371/journal.pgen.1006299
Category: Perspective
doi: https://doi.org/10.1371/journal.pgen.1006299Summary
article has not abstract
Metastases cause more than 90% of the morbidity and mortality associated with human cancers. Gene expression signatures associated with cancer progression and metastasis serve as unique tools to assist in patient diagnosis, prognosis, and treatment. Various types of signatures have been identified, ranging from those that are tumor-intrinsic or specific to a particular cancer subtype [1] to genes associated with a specific clinical outcome (e.g., “poor prognosis gene signature”) [2], as well as to genes associated with the development of metastatic lesions [3]. Of those genes that drive cancer processes, some may function by acting on the primary tumor—causing rise of metastatic lesions—or at the metastatic site to promote colonization, survival, and incorporation of surrounding stroma. Identification of metastasis susceptibility genes is thus key for prediction of cancer risk and metastatic relapse.
A Mouse Model for Breast Cancer Tumorigenesis
The process of metastasis is complex, and debate has ensued concerning the role of host gene variation in contributing to metastatic potential. Although evaluation and sequencing of human tumors is revealing insights, much work still needs to be done to achieve the goal of personalized medicine, in which sequence variants dictate a patient’s course of treatment. To understand how genetic background influences primary cancer development and subsequent metastases, Hunter and colleagues chose an elegant model system [4]—namely, a transgenic mouse in which the polyoma virus middle T antigen is under the control of the mouse mammary tumor virus (MMTV-PyMT) promoter on the FVB/NJ inbred strain background. MMTV-PyMT mice develop mammary cancers that metastasize to the lung [5].
Using the MMTV-PyMT mice, Hunter and colleagues screened inbred strains to identify those that were susceptible or resistant to mammary tumor growth and metastases. By comparing the PAM50 gene signatures of primary mammary tumors, they demonstrated that tumor subtype is significantly impacted by the host genome [6]. Crossing MMTV-PyMT males with females from the genetically divergent MOLF/EiJ inbred strain, followed by crossing hybrid MMTV-PyMT males to FVB/NJ females, predisposed MMTV-PyMT N2 offspring to mammary tumors with gene signatures resembling estrogen receptor negative (ER-) breast cancers, thus tilting the model towards an aggressive form of breast cancer [6]. In this issue, the authors use quantitative trait loci (QTL) analyses to identify a region on mouse chromosome 6 that contains candidate genes for lung metastasis, including the Aryl hydrocarbon receptor nuclear translocator (Arntl2) gene (a member of the circadian clock; the Arntl2 gene has also been called Bmal2, Mop9, and bHLHe6). Using an Arntl2 knockout mouse, the authors demonstrate that absence of Arntl2 increases the number of lung metastases but not metastatic latency or burden. Furthermore, the authors use CRISPR/Cas9 gene editing to recapitulate specific FVB/NJ polymorphisms in the MOLF/EiJ genetic background and show that sequence variants in the promoter region of Arntl2 alter transcript levels, leading to changes in the metastatic potential of primary mammary tumors ([7]; this issue). Finally, they translate these findings to human cancers by evaluating sequence variants affecting ARNTL2 expression and their impact on disease-free survival. This study demonstrates the power of mammalian model systems coupled with unbiased screens to identify understudied genes, test hypotheses using gene editing technology, and decipher mechanisms involved in metastatic spread (Fig 1).
Fig. 1. Identification of the Arntl2 gene and its relationship to cancer metastases. 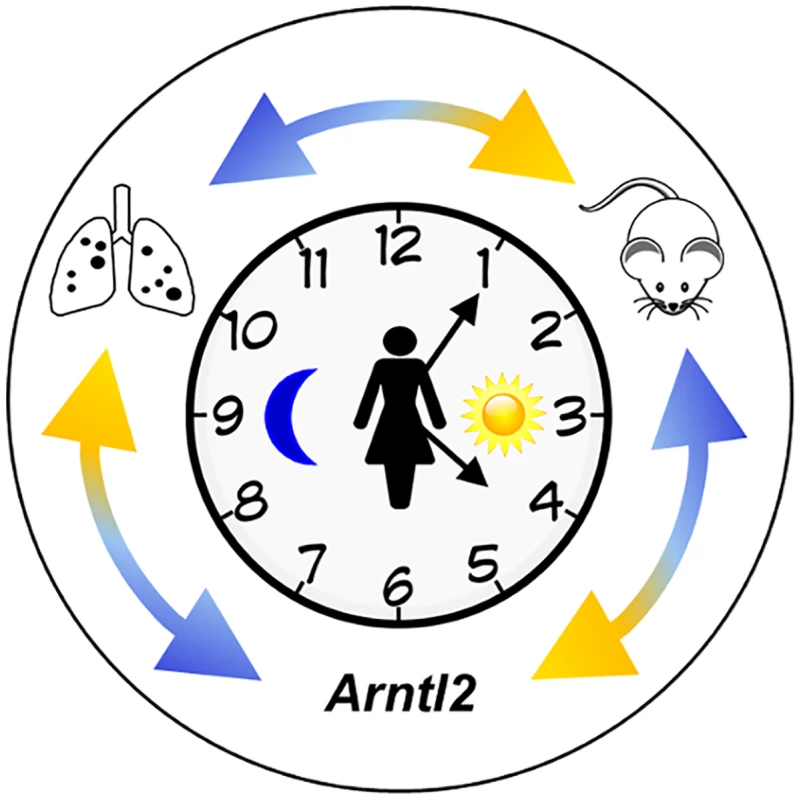
The clock represents circadian rhythms. The external double arrows indicate how genetic studies using mouse models inform studies of human cancer, leading to the discovery of polymorphisms in the Arntl2 gene that influence mammary cancer metastases to the lungs [7]. Black dots on the lung diagram represent metastatic lesions. The Arntl2 Gene Acts in a Tumor Cell–Autonomous Manner
The discovery of Arntl2 was based on its sequence similarity to the Arntl and Drosophila Cycle (cyc) genes; Arntl2 arose following duplication and divergence of the Arntl gene in vertebrates (the Arntl gene has also been called Bmal1, Mop3, and bHLHe5) and is linked to modulation of circadian rhythms [8]. Interestingly, Ha et al. established that alteration of Arntl2 solely impacts cells of the tumor, with no impact on supporting stromal cells [7]; when hybrid Arntl2 knockout mice were compared with wildtype Arntl2 mice after orthotopic injection of syngeneic 4T1 mammary cells, there were no differences in the number of lung metastases, suggesting that Arntl2 acts in a tumor cell–autonomous manner. Similar studies by Hunter and colleagues indicate that these properties are gene-specific, with other metastasis susceptibility genes (specifically Cadm1) impacting signaling and subsequent tumor progression in CD8+ T lymphocytes [9].
Most recently, Brady et al. showed a supportive role for Arntl2 in driving lung adenocarcinoma and subsequent metastatic outgrowths [10]. ARNTL2 expression was also shown to be up-regulated in colorectal cancers and correlated with tumor invasiveness [11]. Importantly, the relationship of ARNTL2 to lung metastases may not be limited to breast cancer, as its paralog, the ARNTL gene, has been implicated in colorectal, hepatic, and other cancers [12,13].
Specificity of Metastasis Susceptibility Genes to Tumor Subtype
It is now accepted that a family history of cancer correlates with increased risk and potential development of metastatic lesions. With the discovery of high-risk penetrant mutations in the BRCA1 and BRCA2 genes, it became evident that some breast cancers are inherited diseases [14,15]. Hunter and colleagues previously used the MMTV-PyMT mouse model to identify genes that influence metastatic progression [9,16]; however, these prior studies identified genes influencing metastasis of ER+ breast tumors, such as the RRP1B and SIPA1 genes [17]. Their current study illustrates a link between metastasis susceptibility genes and breast cancer subtypes.
Future Prospects
The current challenge is to identify genes and/or sequence variants predisposing individuals to cancer metastases. While in vitro assays allow for the investigation of proliferation, motility, and invasion, and in vivo models illustrate specific portions of the metastatic cascade, both systems do not fully recapitulate human cancer progression and the metastatic microenvironment. The vast genetic diversity present in inbred mouse strains, coupled with the ability to produce novel genetically engineered alleles with surgical precision, provide for mammalian model systems that closely mimic human cancer subtypes and enable identification of genes associated with inherited metastasis susceptibility. The report by Ha et al. describes a clever mating scheme that converted a predominantly ER+, luminal phenotype into a system that more closely resembles ER - tumors. Similarly, Lee et al. recently utilized QTL analysis of (TRAMP x PWK/PhJ)F2 mice to identify two novel candidate genes for prostate cancer metastasis susceptibility [18]. These types of studies illustrate the importance of designing creative model systems capable of recapitulating early stages of cancer development through progression and metastases to answer provocative questions pertaining to human disease.
Data collected from mammalian models, in concert with data generated by profiling human tissues, will facilitate the development of algorithms useful for predicting an individual’s specific risk of cancer and/or metastatic relapse, given their personalized gene signature. More importantly, the interchange of information between these systems may help solve important questions such as:
Are there specific genes that govern whether and how long disseminated tumor cells of a given cancer type remain in a quiescent state?
Are there specific metastatic genes that cause dormant cancer cell reawakening?
Of the cancers that preferentially metastasize to the same organ, are there common genes causing directional metastasis?
Mammalian model systems are perfectly poised to test these and other questions, including ones regarding the emerging associations between dysregulation of clock genes and cancer (reviewed in [19]). Further intriguing issues revolve around the timing of cancer treatments, which may be adjusted to each patient’s circadian rhythms to result in better outcomes (reviewed in [20]). The answers will enhance our understanding of metastases as well as provide for better clinical management and development of preventive strategies.
Zdroje
1. Perou C. M., Sorlie T., Eisen M. B., van de Rijn M. Jeffrey S. S., Rees C. A., Pollack J. R., Ross D. T., Johnsen H., Akslen L. A., Fluge O., Pergamenschikov A., Williams C., Zhu S. X., Lonning P. E., Borresen-Dale A. L., Brown P. O., Botstein D. Molecular portraits of human breast tumours. Nature. 2000;406 : 747–52. 10963602
2. Varmbally S., Yu J., Laxman B., Rhodes D. R., Mehra R., Tomlins S. A., Shah R. B., Chandran U., Monzon F. A., Becich M. J., Wei J. T., Pienta K. J., Ghosh D., Rubin M. A., Chinnaiyan A. M. Integrative genomic and proteomic analysis of prostate cancer reveals signatures of metastatic progression. Cancer Cell. 2005;8 : 393–406. 16286247
3. Paik S., Shak S., Tang G., Kim C., Baker J., Cronin M., Baehner F. L., Walker M. G., Watson D. Park T., Hiller W., Fisher E. R., Wickerman D. L., Bryant J., Wolmark N. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;35 : 2817–26.
4. Hunter K. W., Broman K. W., Voyer T. L., Lukes L., Cozma D., Debies M. T., Rouse J., Welch D. R. Predisposition to efficient mammary tumor metastatic progression is linked to the breast cancer metastasis suppressor gene Brms1. Cancer Res. 2001;61 : 8866–72. 11751410
5. Guy C. T., Cardiff R. D., Muller W. J. Induction of mammary tumors by expression of polyomavirus middle T oncogene: a transgenic mouse model for metastatic disease. Mol Cell Biol. 1992;12 : 954–61. 1312220
6. Hu Y., Bai L., Geiger T., Goldberger N. Walker R. C., Green J. E., Wakefield L. M., Hunter K. W. Genetic background may contribute to PAM50 gene expression breast cancer subtype assignments. PLoS ONE. 2013;8:e72287. doi: 10.1371/journal.pone.0072287 24015230
7. Ha N-H., Long J., Cai Q., Shu X. O., Hunter K. W. The circadian rhythm gene Arntl2 is a metastasis susceptibility gene for estrogen receptor-negative breast cancer. PLoS Genet. 2016; 12(9): e1006267.
8. Takahashi J. S. Molecular components of the circadian clock in mammals. Diabetes Obes Metab. 2015;17 Suppl:6–11.
9. Faraji F., Pang Y., Walker R. C., Borges R. N., Yang L, Hunter K. W. Cadm1 is a metastasis susceptibility gene that suppressed metastasis by modifying tumor interaction with the cell-mediated immunity. PLoS Genet. 2012;8:e1002926. doi: 10.1371/journal.pgen.1002926 23028344
10. Brady J. J., Chuang C-H., Greenside P. G., Rogers Z. N., Murray C. W., Caswell D. R., Hartmann U., Connolly A. J., Sweet-Cordero E. A., Kundaje A., Winslow M. M.,. An Arntl2-driven secretome enables lung adenocarcinoma metastatic self-sufficiency. Cancer Cell. 2016;29 : 697–710. doi: 10.1016/j.ccell.2016.03.003 27150038
11. Mazzoccoli G., Pazienza V., Panza A., Valvano M. R., Benegiao G., Vinciguerra M., Andriulli A., Piepoli A. ARNTL2 and SERPINE1: potential biomarkers for tumor aggressiveness in colorectal cancer. J Cancer Res Clin Oncol. 2012;138 : 501–11. doi: 10.1007/s00432-011-1126-6 22198637
12. Karantanos T., Theodoropoulus G., Pektasides D., Gazouli M. Clock genes: their role in colorectal cancer. World J Gastroenterol. 2014;20 : 1986–92. doi: 10.3748/wjg.v20.i8.1986 24587674
13. Valenzuela F. J., Vera J., Venegas C., Munoz S., Oyarce S., Munoz K., Lagunas C. Evidences of polymorphism associated with cricadian system and risk of pathologies: a review of the literature. Int J Endo. 2016;2016 : 2746909.
14. Tung N., Domchek S. M., Stadler Z., Nathanson K. L., Couch F., Garber J. E., Offit K., Robson M. E. Counselling framework for moderate-penetrance cancer-susceptibility mutations. Nat Rev Clin Oncol. 2016;13(9):581–8. doi: 10.1038/nrclinonc.2016.90 27296296
15. de Jong M. M., Nolte I., te Meerman G. J., van der Graaf W. T. A., Oosterwijk J., Kleibeuker J., Schaapveld M., de Vries E. G. E. Genes other than BRCA1 and BRCA2 involved in breast cancer susceptibility. J Med Genet. 2002;39 : 225–42. 11950848
16. Faraji F., Hu Y., Wu G., Goldberger N. E., Walker R. C., Zhang J., Hunter K. W. An integrated systems genetics screen reveals the transcriptional structure of inherited predisposition to metastatic disease. Genome Res. 2014;24 : 227–40. doi: 10.1101/gr.166223.113 24322557
17. Hsiesh S.-M., Look M. P., Sieuwerts A. M., Foekens J. A., Hunter K. W. Distinct inherited metastasis susceptibility exists for different breast cancer subtypes: a prognosis study. Breast Cancer Res. 2009;11:R75. doi: 10.1186/bcr2412 19825179
18. Lee M., Williams K. A., Hu Y., Andreas J., Patel S. J., Zhang S., Crawford N. P. GNL3 and SKA3 are novel prostate cancer metastasis susceptibility genes. Clin Exp Mets. 2015;32 : 769–82.
19. Kelleher F. C., Rao A., Maguire A. Circadian molecular clocks and cancer. Cancer Lett. 2014;342 : 9–18. doi: 10.1016/j.canlet.2013.09.040 24099911
20. Ortiz-Tudela E., Mteyrek A., Ballesta A., Innominato P. F., Levi F. Cancer chronotherapeutics: experimental, theoretical, and clinical aspects. Handb Exp Pharmacol. 2013;217 : 261–88. doi: 10.1007/978-3-642-25950-0_11 23604483
Štítky
Genetika Reprodukční medicína
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2016 Číslo 9- Akutní intermitentní porfyrie
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Růst a vývoj dětí narozených pomocí IVF
- IVF a rakovina prsu – zvyšují hormony riziko vzniku rakoviny?
- Pilotní studie: stres a úzkost v průběhu IVF cyklu
Nejčtenější v tomto čísle- Reactive Oxygen Species: From Harmful Molecules to Fine-Tuning Regulators of Stem Cell Niche Maintenance
- The Clock Is Ticking: Countdown to Metastases
- Whole Exome Sequencing in Atrial Fibrillation
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



