-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaNanofiber scent carrier
Authors: Radek Divín; Bruno Sopko; Jiří Ščučka; Evžen Amler
Published in the journal: Lékař a technika - Clinician and Technology No. 4, 2018, 48, 123-130
Category: Original research
Summary
The nanofiber scent carriers prepared by electrospinning from poly-vinyl-butyral (PVB), poly-ε-polycaprolactone (PCL), poly-vinyl - alcohol (PVA) and nylon 6/6 were tested as materials for collecting and preserving cigarette tobacco olfactory trace. Nanofiber material can include polar groups on its surface that influence wettability and also attracting of specific molecules creating olfactory trace. Nanofiber material and Aratex were also morphologicaly compared by scanning electron microscope (SEM) where nanofiber carrier had from twenty-two times to thirty-six times smaller fibre diameter than Aratex fibres. Olfactory testing of nanofiber scent carriers were compared to Aratex as traditionally used material for collecting of olfactory traces in criminology. Olfactory tests were carried out by dogs with special training and by mass spectrometer. Olfactory tests carried out by dogs clearly proved that nanofiber scent carriers were able to collect and preserve olfactory trace of lower concentration despite of their lower weight compared to samples of Aratex material. Olfactory tests carried out by mass spectrometer affirmed better olfactory properties of nanofiber scent carriers compared to Aratex, when nanofiber scent carriers were able to preserve 9 of 14 specific molecules characteristic for cigarette tobacco compared to Aratex that was able to preserve only 5 of 14 specific molecules characteristic for cigarette tobacco. The experiments with olfactory trace detection with materials with a comparable mass are in progress.
Keywords:
nanofiber – olfactory trace – scent
Introduction
Odour sensing is fundamentally different from molecule detection by gas sensors. While typical gas sensors detect a single or only a few targets, the human nose can detect and discriminate thousands of odorant molecules at concentrations in the parts-per-trillion (ppt) [1, 2]. This capability is thought to originate from the presence of more than 350 olfactory receptor (OR) genes in humans [3, 4]. However, this alone does not explain the high sensitivity and selectivity of human or animal odours perception. It is also thought to stem from the ability of OR proteins to display differential affinity for a range of molecules, which allows for a single odorant molecule to bind to several different receptor proteins [5]. The combinatorial interaction between odorants and ORs results in “odorant map”, enabling discrimination of a large number of odours. Interest-ingly, it has also been speculated that ORs might be able to sense various vibrational energy levels of a molecule, rather than structural motifs, via quantum coherence mechanisms [6, 7]. All of this evidence suggests that human odours perception is a unique and complex process. A man is capable to detect and identify more than 107 of different odours. However, several animal species are able to identify significantly higher number odours. As an example, the olfactory ability of dogs is better than a man’s ones 800 thousand times [8].
Odours analysis is carried out for different reasons. One of the reasons is the analysis of patient’s breath which can give the insight into physiological and patho-physiological processes in the body with possible association with some diseases [9]. The analysis of exhaled breath has been proposed as a convenient and safe complementary method to blood and urine sam-pling [10]. Breath analysis has a number of advantages compared to the traditional diagnostic techniques. This is a non-invasive and painlessness procedure and its sampling does not require skilled medical staff [11]. Even though up to 3000 compounds can be detected in different persons’ breath [12, 13], the matrix of exhaled air is less complex than that of blood or other body fluids. Considering all these facts the breath analysis is a lot simpler to perform [14].
The odours bare studied by odorology and forensic odorology. Forensic odorology is a separate section of forensic technology and olfactory studies are widely used by law enforcement in the criminal justice system [15]. Technical means of collecting and preservation of olfactory traces include devices and instruments for detecting and confiscating olfactory traces and objects for preservation and conservation of smell. Olfactory traces are collected by cotton napkin (Aratex) and then olfactory trace is preserved in the cotton napkin conserved in a glass cans with a glass or metal lids.
Olfactory traces are human smell, drugs and other odours/scents. In order to collect as much olfactory traces as possible the optimized material (scent carrier) is needed, mainly when characteristic odours are at low concentrations. Optimal material according to the basic knowledge carried out by chemistry and physics should have the highest possible ratio of a material surface to material volume, and material should have optimal chemical and physical properties for to binding mole-cules, which create characteristic scent.
When the diameters of polymer fibre materials shrank from micrometers (e.g. 10–100 μm) to submicrons or nanometers (e.g. 10×10−3–100×10−3μm), new amazing characteristics appear, such as a very large surface area to volume ratio (this ratio for a nanofiber can be thousand times higher than in the case of microfiber), flexibility in surface functionalities, and superior mechanical performance (e.g. stiffness and tensile strength) compared to any other known form of the material. These outstanding properties make the poly-mer nanofiber to be optimal candidates for many important applications. A number of processing tech-niques such as drawing [16], template synthesis [17, 18], phase separation [19], self-assembly [20], electro-spinning [21], etc. have been used to prepare polymer nanofiber in recent years.
Polymeric nanofiber scaffold can be prepared from different types of polymers creating different fibre surface chemistry. Moreover, surface of polymeric nanofiber scaffold could be modified by specific anti-bodies to detect selective molecules.
The goal of this study is to prove that nanofiber scent carriers with a large surface area and variable surface chemistry exhibit better properties as scent carrier than now day used Aratex material.
Material and methods
Material preparation
Nonwoven textile Aratex® (CHLUM-TEX s.r.o.) and four types of polymeric nanofiber scaffolds were used as scent carrier. Aratex® was composed of 74% cotton, 16% viscose, 6% polyester and 4% of polyamide and its grammage was 273 ± 4 g·m-2. Four different polymeric nanofiber scent carriers (Nanuntio s.r.o.) were prepared from different polymers PCL, PVA, PVB and nylon 6/6 PCL nanofiber scent carrier grammage was 43 ± 2g·m -2. PVB nanofiber scent carrier grammage was 31 ± 3 g·m-2. PVA nanofiber scent carrier grammage was 35 ± 3 g·m-2. Nylon 6/6 nanofiber scent carrier grammage was 38 ± 3 g·m-2.
SEM imaging
Scent carriers were sputtered (Sputter Coater Q150R, Quorum Technologies Ltd) by conductive 15 nm layer of golden particles in order to provide SEM imaging. Scent carriers were visualized by scanning electron microscopy (SEM) Vega3 SB (TESCAN a.s.) and images were analyzed by ImageJ software from five different areas of a scent carrier. Average diameter size its standard deviation and fibre diameter size distri-bution was made from sets of 500 different fibres measured for each type of scent carrier.
Measurement of wettability
Measurements of scent carrier wettability (defined as hydrophilic/hydrophobic ratio) were carried out by measuring of the contact angle of deionised water drop in contact with scent carrier surface. An equilibrium of vector forces determines the contact angle α (Fig. 1) at the contact line of a deposited drop. The surface energy of the solid σS acts along the solid surface. The solid-liquid interfacial energy σSL acts in the opposite direction and the surface tension σL of the liquid acts tangential to the drop surface. This can be calculated using a simple scalar equation. Contact angle is defined as the angle between σSL and σL. The drop was viewed in profile during the contact angle measurement and tangential to the drop surface from the contact line was measured by fitting mathematical function to the drop surface. Contact angle measurement was carried out by See system (Advex-Instruments s.r.o.). Hydrophobicity or hydrophilicity of the material carry out some important information. Air humidity could be even more than 70%, what is the reason for collecting of scent traces can be influenced by wettability of scent carrier surface.
Fig. 1. Illustrative picture of the contact angle during wettability testing, where: α—the contact angle, σS—surface energy of the solid, σSL—the solid-liquid interfacial energy, σL— the surface tension. 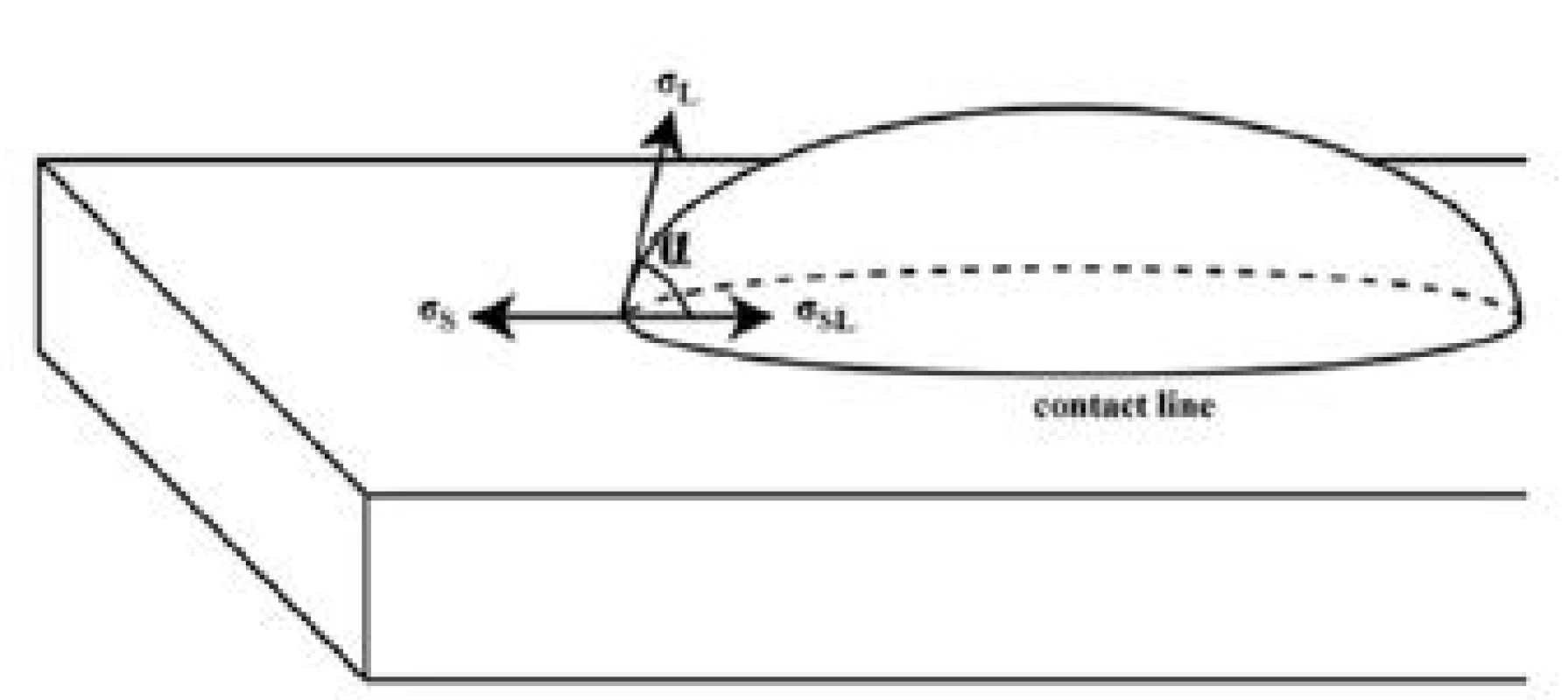
Wettability of the scent carrier gives an information about presents of polar group on the fibre surface and also information about roughness of the surface. Presents of polar or non-polar groups on the scent carrier surface can indicate attraction of specific molecules to the scent carrier as part of the odour trace. Wettability of the material indicates the usability of material in the wet environment, as well.
Olfactory traces
Samples for olfactory testing were divided into ten groups. Each group included samples A, B, C and D according to table 1. Samples D in each group were created by combination of PCL, PVB, PVA and nylon 6/6 nanofiber scent carrier.
Tab. 1. Average specification of samples in each group. 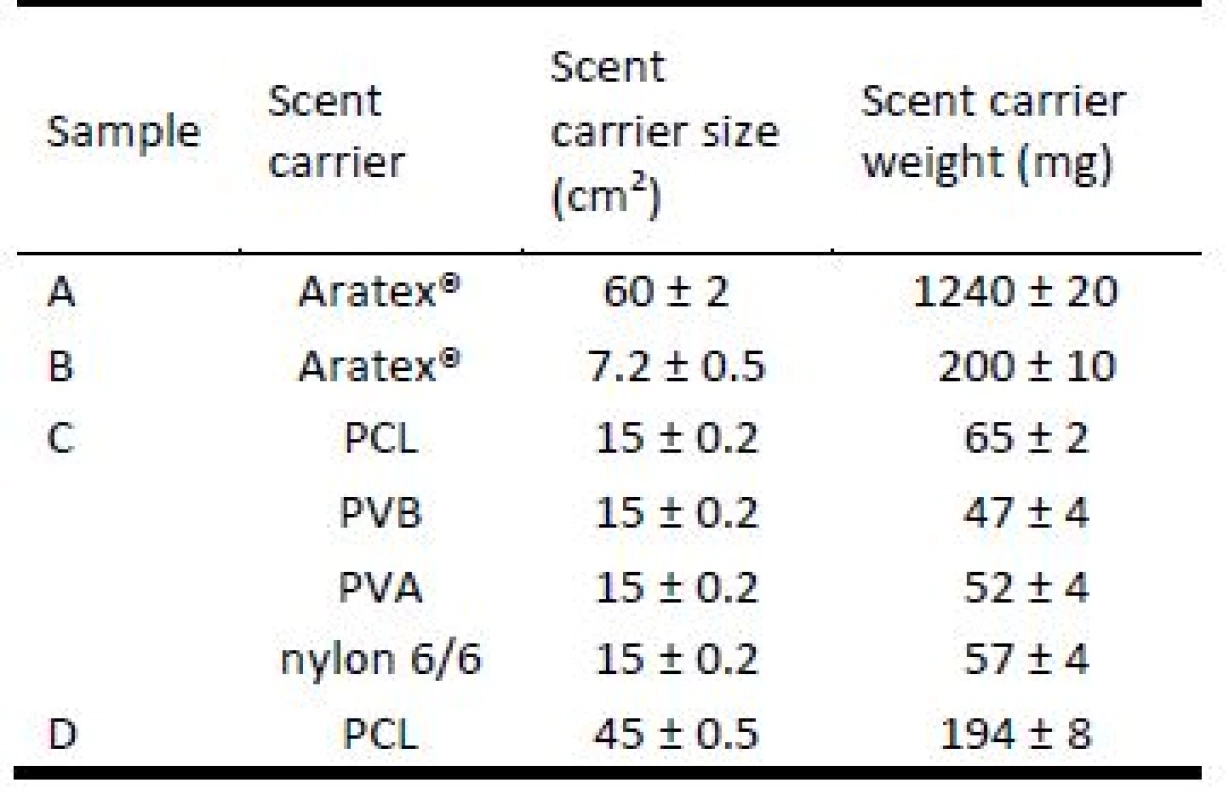
During collecting of olfactory traces each group of scent carriers was exposited to a olfactory trace of cigarette tobacco in controlled manner by system illustrated in the figure 1. An exicator 1a (SIMAX) ~ 17170 ml included a plate 6 with apertures. Two pieces of laboratory test sieves 4 with apertures 100 µm and 200 µm were placed on the plate 6 in the exicator 1a. Between two pieces of laboratory test sieves 4 was located a cigarette tobacco 6 as olfactory sing sample. The exicator 1a was connected to an identical desiccator 1b trough two valves 2a, 2b and a connecting cube 3. The desiccator 1b included scent carrier samples of one group and a manometer Greisinger GDH 200-14.
Every time before samples were exposed to the olfactory trace of tobacco pressure in the exicator 1a was established to 974 mbar for 1 hour. Basic accuracy of all pressure measurements was 2 mbar. After inserting a samples of one group to the desiccator 1b the desic-cator 1b was connected to a rotary vacuum pump (MD 1C, Maneko s.r.o.) trough the valve 2b and in the desiccator 1b was established initial pressure P1. After initial pressure P1 was established, the valve 2b was closed and the vacuum pump was disconnected from the desiccator 1b. After 5 minutes when initial pressure P1 was established in the desiccator 1b, the exicators 1a and 1b were connected together by the connecting tube 3 and the valve 2a was opened. After the valve 2b was opened and final pressure P2 in the desiccator 1b was established, then the valves 2a and 2b were closed and samples were exposed to tobacco scent for exposure time T1. After T1 lapsed, the exicator 1b was connected to vacuum pump and a pressure of 275 mbar was established in the desiccator 1b. The desiccator 1b was disconnected from the vacuum pump after described cycle and the desiccator 1b was relocated to another isolated room where the desiccator 1b was opened, the samples were removed and separately hermetically closed to glass jars.
Each sample group was exposed to tobacco olfactory trace separately. Scent intensity was varied by absolute value of difference between initial pressure P1 and final pressure P2. The exposure time T1 was also varied during a testing as it is illustrated in the Table 2.
Tab. 2. Schema of olfactory traces preparation for individual groups of samples. 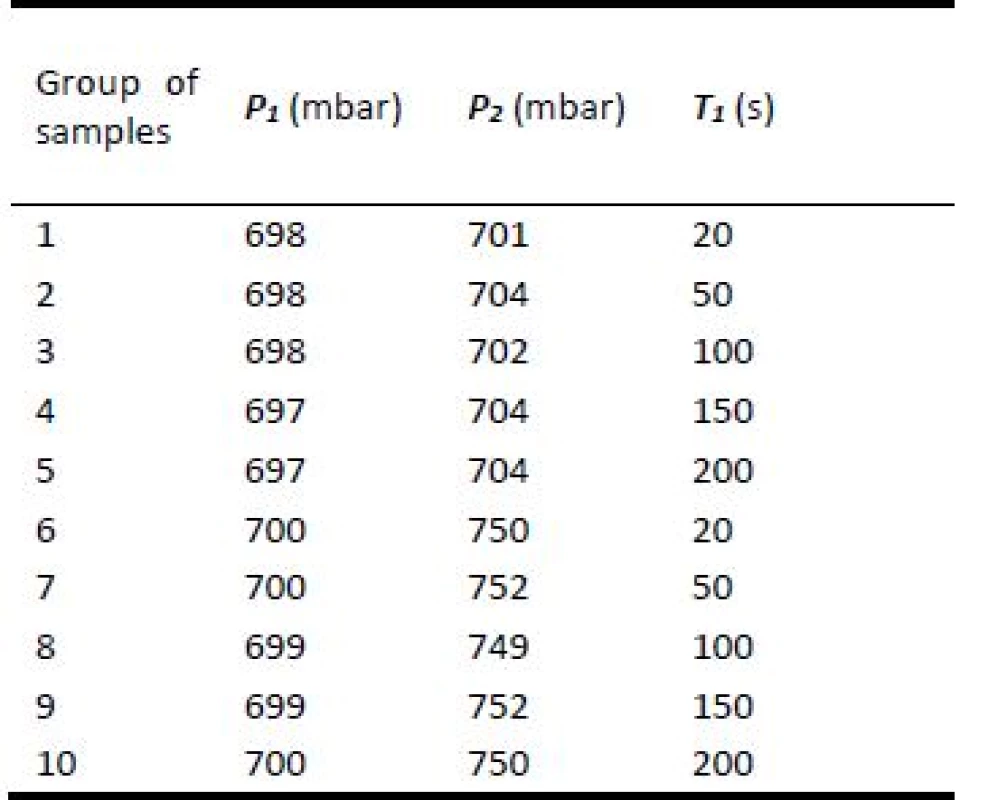
P1—initial pressure, P2—final pressure, T1—exposure time. Olfactory testing by dogs
Olfactory tests were carried out by specialized Animal Training Academy s.r.o. For animal tests were used two dogs (Belgian Shepherd and one German Shorthaired
Pointer) with special training. Dogs were trained to find and mark olfactory tobacco trace of the same batch as was used during creation of the olfactory traces of Aratex and nanofiber scent carriers.
The dogs had to find and mark sample prepared as olfactory tobacco trace between four others samples without tobacco olfactory trace. Olfactory testing by dogs was carried out without visual contact between dogs and samples. Samples were hidden in non-trans-parent boxes. Dogs used only their sense of smell during the test to mark sample with olfactory trace. Each sample was tested 20 times (five times by each dog in two series).
If a dog marked the sample with olfactory trace without hesitation it means logical value 1.
If a dog marked the sample with olfactory trace with hesitation it means logical value 2.
If a dog did not marked the sample with olfactory trace it means logical value 3.
Result of olfactory testing of individual samples with olfactory trace was average value of 20 logical values obtained by testing rounded to nearest whole number.
Olfactory testing by mass spectrometer
The studied olfactory sing captured in the samples have been extracted by 3 ml of methanol. After centri-fugation 100 µl of the extract have been diluted by 900 µl of water and analysed. The chromatographic separation of analytes has been achieved using Agilent 1290 Infinity UHPLC system with a C18 column (Zorbax Eclipse Plus C18 RRHD (2.1 mm x 100 mm; 1.8 µ), Agilent Technologies). 5mM ammonium formate with 0.01% formic acid (A) and methanol containing 0.01% formic acid (B), have been chosen as mobile phases and gradient elution has been used. A Q-TOF mass spectrometer (Agilent 6550 QTOF) with positive and negative electrospray ionisation has been used for the untargeted analysis. Using Agilent MassHunter software exact masses and probable summary formulas of detected specific molecules characteristic for olfac-tory tobacco trace have been created.
#540562
Results and discussion
Wettability
Wettability properties of nanofiber scent carriers were tested by measuring of contact angle by See system (Advex-Instruments s.r.o.). When contact angle is lower than 90° after 0,1 s when water drop was deposited on the nanofiber scent carrier the surface is hydrophilic, when contact angle is higher than 90° the nanofiber scent carrier surface is hydrophobic. Nylon 6/6 and PVA nanofiber scent carriers were determined as hydrophilic surfaces as it illustrated in Tab. 3. PVB nanofiber scent carrier had hydrophobic surface and PCL nanofiber scent carrier was on the edge between hydrophobic and hydrophilic surface.
Tab. 3. Contact angle of nylon 6/6, PVA, PVB, PCL nanofiber scent carrier. 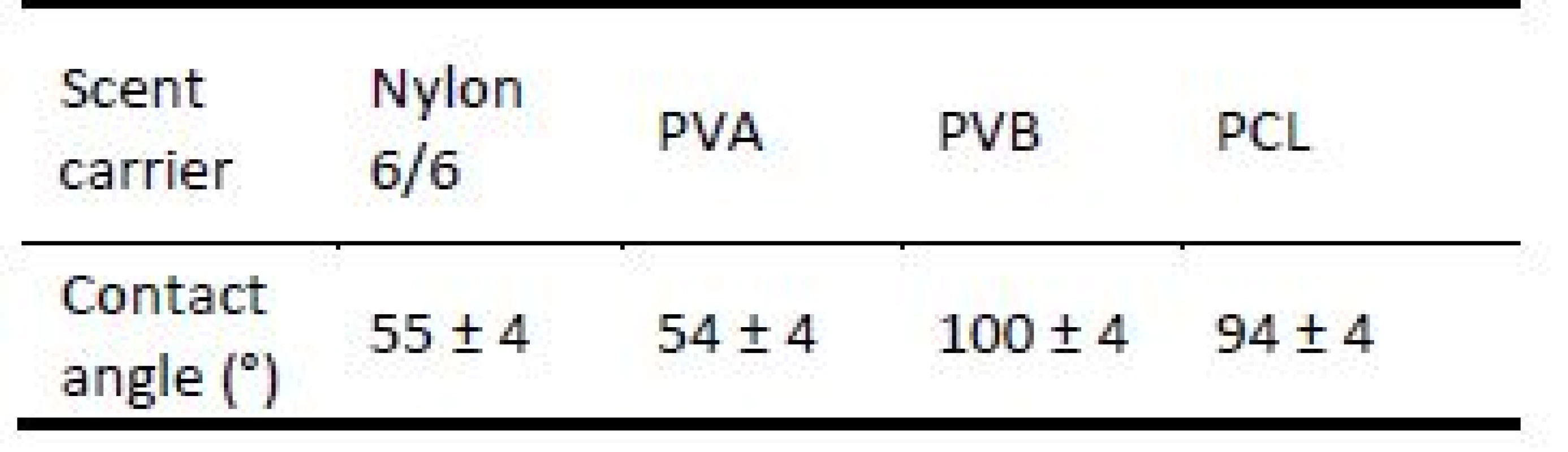
Measurement of contact angle was carried out 0.1 s after water drop contacted a nanofiber scaffold surface. Some hydrophilic porous material can absorb water drop as it illustrates contact angle characteristic of the PVA scent carrier during the time (Fig. 3). Error bars with longer time are greater due to the of problematic determination of the contact line of the water drop. Water absorbing properties can play important role during collecting of olfactory traces in an environment with higher humidity.
Fig. 2. Absorbance of water drop to PVA scent carrier described by contact angle during 12 s. 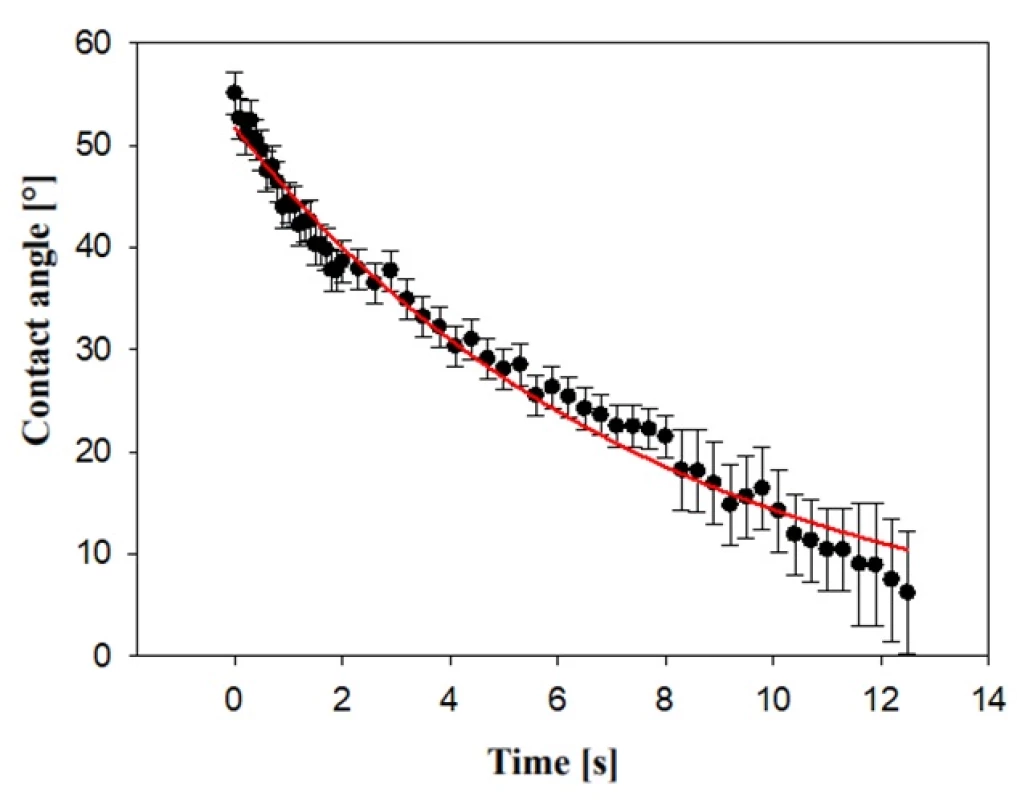
Wettability of the surface is influenced by chemistry and roughness of the tested surface. Roughness of the surface is influenced by diameter, planar density of fibres number and orientation of individual fibres of the scaffold creating scent carrier.
SEM analysis
Structure of individual scent carriers can be compared among themselves using SEM images in the Fig. 4. ARATEX structure was completely different from the structures of the other scent carriers. Aratex was created by fibres with larger fibre diameter and also shape of fibres cross section. Nearly none of ARATEX fibres had circular cross section. Average ARATEX fibres diameter was 12.5 µm with standard deviation 3.7 µm. Distribution of Aratex fibres diameter showed Gauss distribution (Fig. 5). According to Fig. 5 25% of all ARATEX fibres had diameter between 10 µm to 12 µm but 3% of the Aratex fibres were thicker than 20 µm.
Fig. 3. SEM images comparison of Aratex and PVB scent carrier morphology with the same magnification. 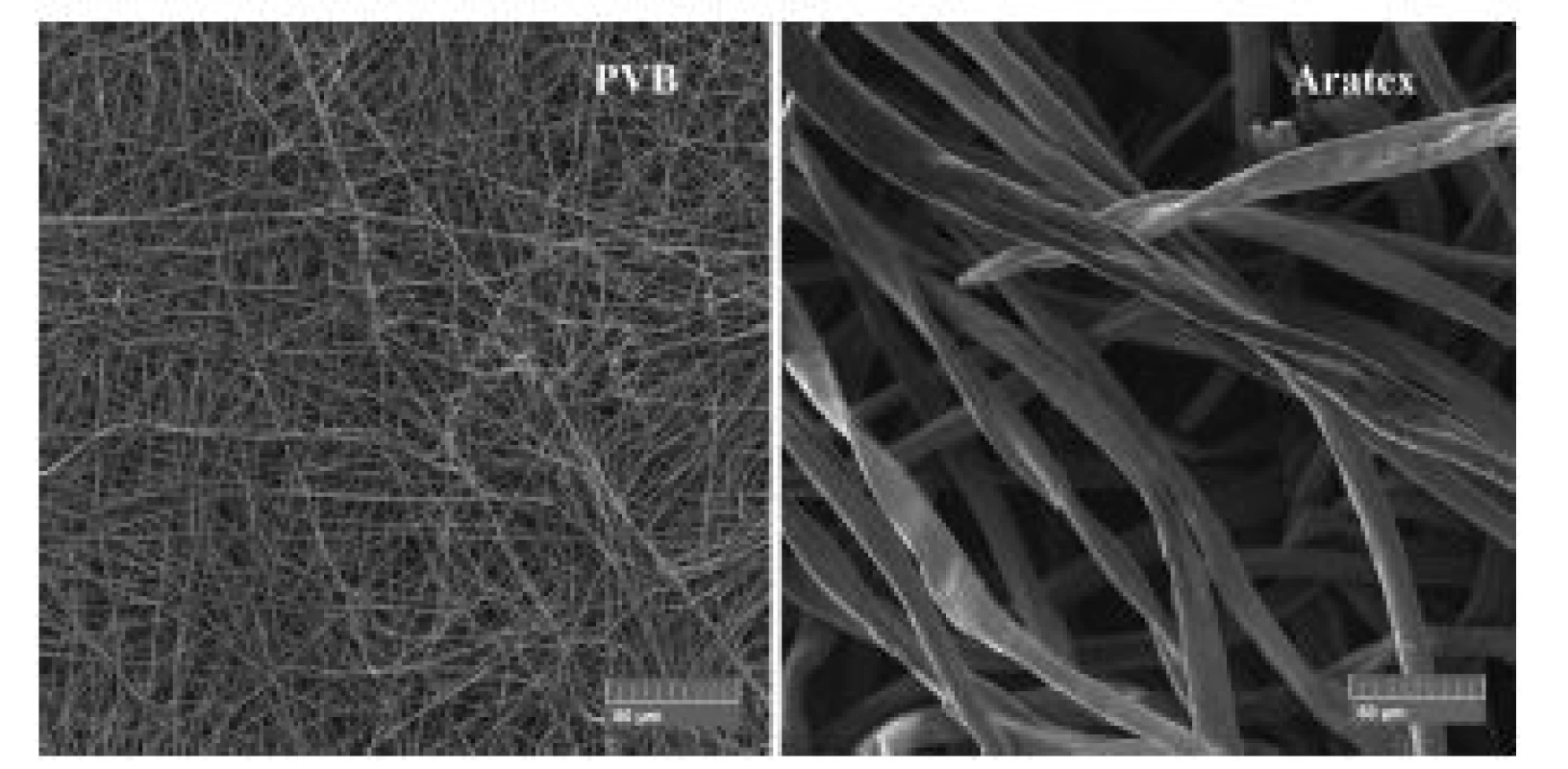
Fig. 4. Fibers diameter distribution for Aratex scent carrier. 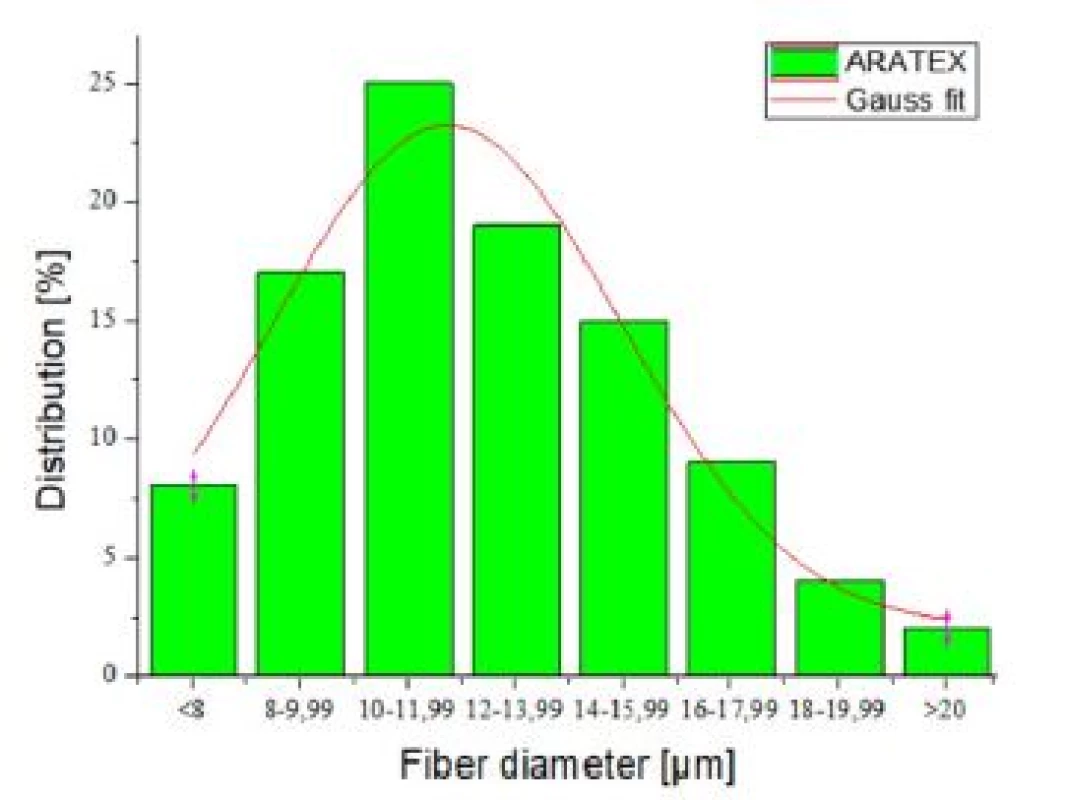
More detailed SEM images in the Fig. 6 illustrates nanofiber scant carriers. Nylon 6/6 and PCL scent carriers were characterized by a more disordered struc-ture than PVA and PVB scent carriers. Also structure of nylon 6/6 and PCL scent carriers were more chaotic with higher percentage of fibres with diameter larger than 1µm compared to PVA and PVB scent carriers. Average fibre diameter size and standard deviation of individual scent carriers are shown in the Tab. 4.
Tab. 4. SEM images of nylon 6/6, PCL, PVA and PVB nanofiber scent carriers. 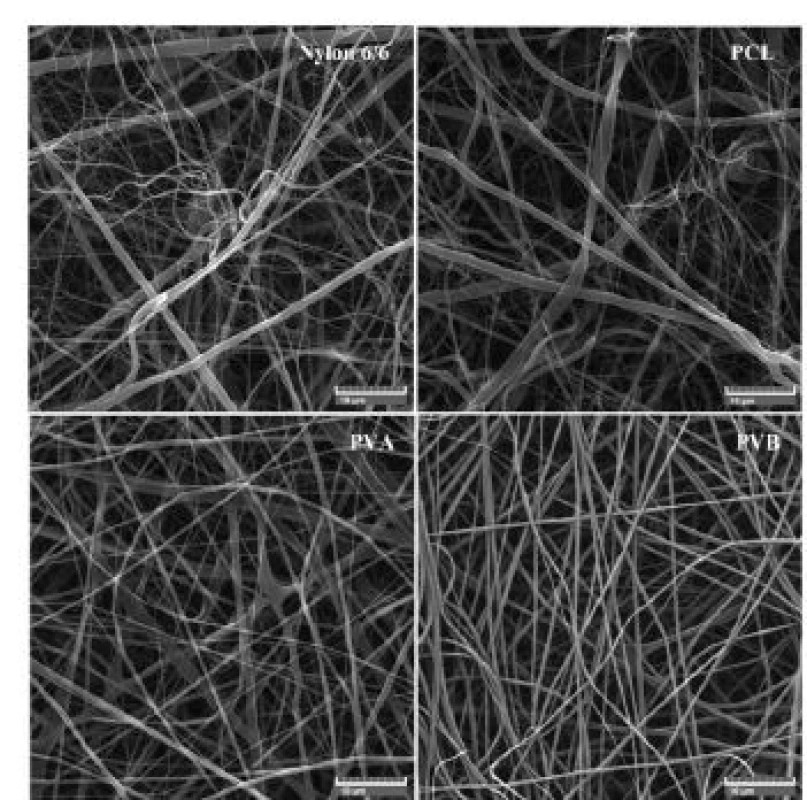
Fig. 5. Fiber diameter distribution of PCL, PVB, PVA and nylon 6/6 scent carriers. 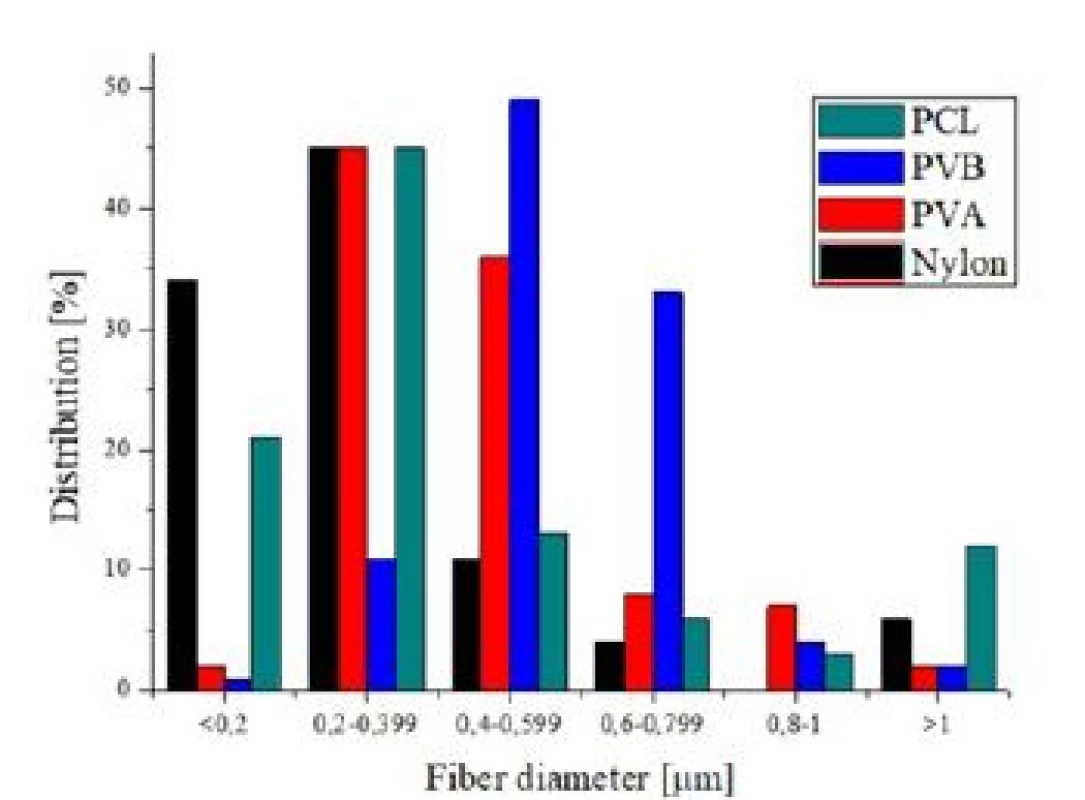
Tab. 5. Average fibre diameter size and standard deviation for individual scent carriers. 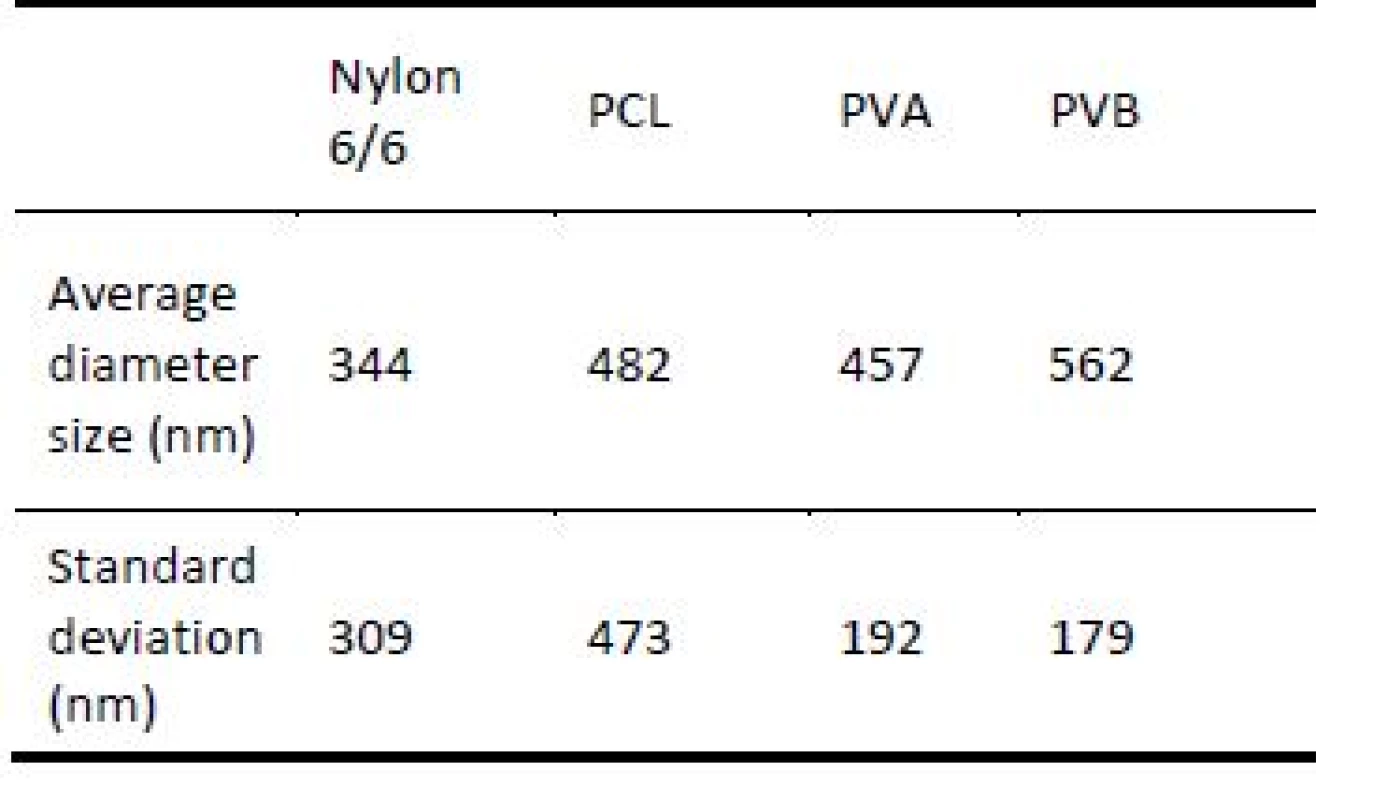
Olfactory testing by dogs
Olfactory tests were evaluated according to method described in the chapter Material and methods and results of tested samples were summarized in the Tab. 5. Groups 1, 2 and 3 of samples which were exposited to tobacco olfactory trace with lowest intensity and for the shortest time interval proved that nanofiber scent carriers are better for collection and preservation of olfactory trace/traces. Group number 1 of the samples includes samples exposited the lowest concentration of tobacco olfactory trace for the shortest time interval. Group 1 of the samples may not be the key repre-sentative of the test samples, because at such low concentration of scent/door particles for such short time interval inhomogeneity of the scent particles redistribution in the desiccator 1b could influence result. Group of samples 3 clearly proved that nanofiber scent carriers were able to collect and preserve olfactory trace better than Aratex material, even if the Aratex sample had 5.6 times higher weight. Groups 4 and 8 also showed that Aratex nonwoven textile had insufficient parameters to create olfactory traces compared to nanofiber scant carrier of the same weight which were able to create olfactory traces recognizable by dogs.
Tab. 6. Results of olfactory testing by dogs. 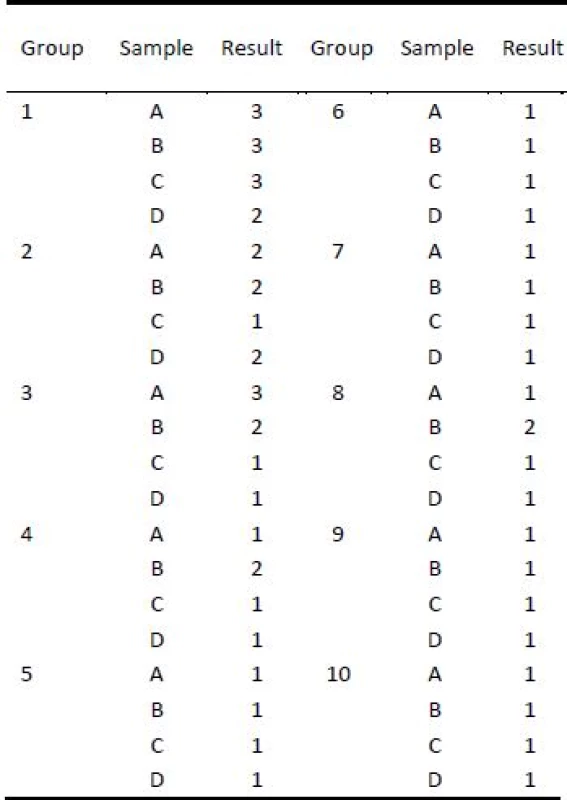
A, B, C and D are defined according to table 1. Results: 1—dogs marked the sample without hesitation, 2—dogs marked sample with hesitation, 3—dogs did not marked the sample. Olfactory testing by mass spectrometer
Reason why nanofiber scent carriers were more effective during collection and preservation of tobacco olfactory trace was given not only by SEM images in the Fig. 4 and 6, but also by A Q-TOF mass spectrometer (Agilent 6550 QTOF) measurement. Measurement by mass spectrometer is summarized in Tab. 6. Samples were tested of ability to captured 14 specific molecules characteristic for cigarettes that are presented in olfac-tory tobacco trace (1—C14 H30 O5 S; 2—C18 H33 N O6; 3—C22 H47 N O2; 4—C23 H46 N S; 5—C24 H33 N5 O S4; 6—C26 H45 N8 O2; 7—C29 H54 N12 O2; 8—C30 H60 O2; 9—C31 H26 Cl N8 O4; 10—C35 H69 N5 O5; 11—C36 H75 N4 O2; 12—C37 H73 N5 O6; 13—C38 H79 N4 O2; 14—C5 H11 N O2). Number of counts detected for specific molecules is not important in the view of the used method of odour extraction from the scent carriers. Important result of this test was if specific molecule was presented in a specific scent carrier after olfactory traces preparation. According to Tab. 6 Aratex captured 5 different molecules charac-teristic for cigarette tobacco. PVA nanofiber scent carrier captured 5, PVB 6, nylon 6/6 5 and PCL 4 different molecules characteristic for tobacco. More important was that the samples C were composed of PVA, PVB, nylon 6/6 and PCL nanofiber scents carriers. Samples C created from 4 different nanofiber scent carriers were able to detect 9 of 14 tested characteristic tobacco molecules that gives more complex information about olfactory trace/odour compared to olfactory trace gained from Aratex scent carrier (samples A and B).
Tab. 7. Counts of specific molecules characteristic for olfactory tobacco trace detected by mass spectroscopy measurement. 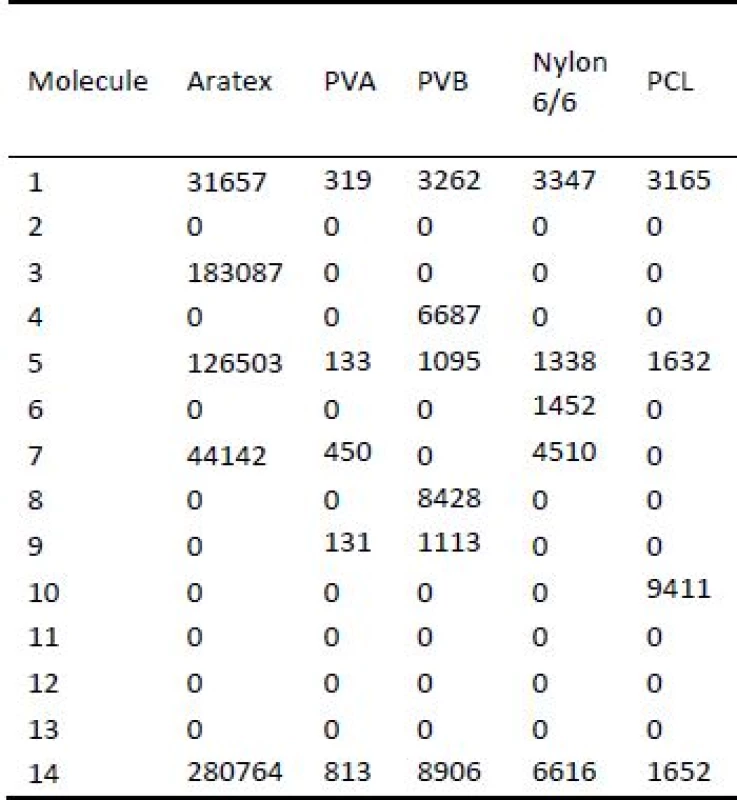
Characteristic molecule: 1—C14 H30 O5 S; 2—C18 H33 N O6; 3—C22 H47 N O2; 4—C23 H46 N S; 5—C24 H33 N5 O S4; 6—C26 H45 N8 O2; 7—C29 H54 N12 O2; 8—C30 H60 O2; 9—C31 H26 Cl N8 O4; 10—C35 H69 N5 O5; 11—C36 H75 N4 O2; 12—C37 H73 N5 O6; 13—C38 H79 N4 O2; 14—C5 H11 N O2. Conclusion
According to olfactory tests carried out by dogs (Tab. 5) and mass spectrometer (Tab. 6) polymeric nanofiber
scent carriers were proved as better material for capturing and preservation of cigarette tobacco olfactory trace. Samples C composed of PVA, PVB, PCL and nylon 6/6 polymeric nanofiber scent carriers were able to capture and preserve 9 of 14 different characteristic molecules compared to 5 of 14 different characteristic molecules captured by Aratex according to mass spec-trometer measurement (Tab. 6). Olfactory tests carried out by dogs clearly proved that nanofiber scent carriers were able to collect and preserve olfactory trace of lower concentration (Tab. 5) despite of their lower weight compared to the samples from Aratex material (Tab. 1). Higher efficiencies to capture and preserve odour of nanofiber scent carriers compared to Aratex can be caused by smaller diameter and higher number of nano-fiber to one milometer square (Fig. 4). The nanofiber scent carriers compared to Aratex had higher active surface which can increase probability to capture mole-cules involved in olfactory trace and also higher number of nanofiber to one cubic millimeter which created creates better filtration of the flowing air that can increase probability to capture particles involved in olfactory trace as a result.
Nanofiber scent carriers can have another great feature as olfactory trace collector compared to tradi-tionally used Aratex. Nanofiber scaffolds can include fibres of multiple different polymers that are charac-terized by different polar or non-polar groups binding different molecules. Polymeric nanofiber surface can also be functionalized by specific antibody to selectively detect specific molecules and so nanofiber scaffolds can create sensor for selective detection [22] of potentially dangerous substances. Creating of sensor for selective molecule detection can be used across wide fields of human activities in the future.
Acknowledgement
This work has been supported by the Ministry of the Interior within, project No. VI20152018010.
Conflicts of Interest
The authors declare no conflict of interest that could influence the results of any test. Olfactory tests were provided by entities without any link to the sample supplier and the entities have no financial or personal relationships or affiliations that could influence or bias presented results.
The entities performed the tests without knowledge of the characteristics of the individual samples that support the objectivity of the presented results.
Mgr. Radek Divín
Czech Technical University in Prague, University
Centre for Energy Efficient Buildings, Třinecká 1024,
273 43 Buštěhrad, Czech Republic
E-mail: divin.r@seznam.cz
Phone: +420 773 068 162
Zdroje
- Buck L, Axel R. A novel multigene family can encode odorant receptors: A molecular basis for odor recognition. Cell 1991 Apr 5;65 : 175–187.
- Keller A, Vosshall LB. Human olfactory psychophysics. Curr Biol 2004 Oct 26;14 : 875–8.
- Mombaerts P. Genes and ligands for odorant, vomeronasal and taste receptors. Nat Rev Neurosci 2004 Apr 1;5 : 263–78.
- Niimura Y, Nei M. Evolution of olfactory receptor genes in the human genome. Proc Natl Acad Sci 2003 Oct 14;100 : 12235–40.
- Buck LB. Olfactory receptors and odor coding in mammals. Nutr Rev 2004 Nov;62 : 184–8.
- Brookes JC, Hartoutsiou F, Horsfield AP, Stoneham AM. Could humans recognize odor by phonon assisted tunneling? Phys Rev Lett 2007 Jan 19;98 : 038101.
- Franco MI, Turin L, Mershin A, Skoulakis EM. Molecular vibration-sensing component in Drosophila melanogaster olfaction. Proc Natl Acad Sci 2011 Feb 14;108 : 3797–802.
- Komarov IM, Markhgeim V, Novikova AE, Tonkov EE. Micro-biological Method for Forensic Identification of a Man: Problem Setting, Res.J.Med.Sci 2015;9(4):214–217.
- Ma W, Liu X and Pawliszyn J. Analysis of human breath with micro extraction techniques and continuous monitoring of carbon dioxide concentration. Analytical and Bioanalytical Chemistry 2006 Jul 18;385 : 1398–1408.
- Amann A, Smith D. Breath Analysis for Clinical Diagnosis and Therapeutic Monitoring. World Scientific: Singapore, 2005 May.
- Spinhirne JP, Koziel JA and Chirase NK. A device for non-invasive on-site sampling of cattle breath with solid-phase microextraction. Biosystems Engineering 2003 Feb;84(2):239–246.
- Phillips M, Herrera J, Krishnan S, Zain M, Greenberg J and Cataneo RN. Variation in volatile organic compounds in the breath of normal humans. Journal of Chromatography B 1999 Jun 11;729(1–2):75–88.
- Phillips M, Glesson K, Hughes JMB, Greenberg J, Cataneo RN, Baker L and McVay WP. Volatile organic compounds in breath as markers of lung cancer: a cross-sectional study. Lancet 1999 Jun 5;353 : 1930–1933.
- Prado C, Marín P and Periago JF. Application of solid-phase microextraction and gas chromatography–mass spectrometry to the determination of volatile organic compounds in end-exhaled breath samples. Journal of Chromatography A 2003 Sept 5;1011:
125–134. - Jezierski T, Ensminger J, Papet L, CanineOlfaction Science and Law. Boca Raton: CRC Press 2016.
- Ondarcuhu T, Joachim C. Drawing a single nanofiber over hundreds of microns. Europhys Lett 1998 Apr 15;42(2):215–20.
- Feng L, Li S, Li H, Zhai J, Song Y, Jiang L, et al. Super-Hydrophobic Surface of Aligned Polyacrylonitrile Nanofibers. Angew Chem Int Ed 2002 Mar 27;41(7):1221–3.
- Martin CR. Membrane-based synthesis of nanomaterials. Chem Mater 1996 Aug 14;8 : 1739–46.
- Ma PX, Zhang R. Synthetic nano-scale fibrous extracellular matrix. J Biomed Mat Res 1999 June 26;46 : 60–72.
- Liu GJ, Ding JF, Qiao LJ, Guo A, Dymov BP, Gleeson JT, et al. Polystyrene-block-poly (2-cinnamoylethyl methacrylate) nano-fibers-Preparation, characterization, and liquid crystalline properties. Chem-A European J 1999 Aug 30;5 : 2740–9.
- Deitzel JM, Kleinmeyer J, Hirvonen JK, BeckTNC. Controlled deposition of electrospun poly(ethylene oxide) fibers. Polymer 2001 May 7;42 : 8163–70.
- Wang D, Sun G, Xiang B, Chiou BS. Controllable biotinylated poly(ethylene-co-glycidyl methacrylate) (PE-co-GMA) nano-fibers to bind streptavidin–horseradish peroxidase (HRP) for potential biosensor applications,European Polymer Journal, 2008 May 7;44 : 72032–2039.
Štítky
Biomedicína
Článek vyšel v časopiseLékař a technika

2018 Číslo 4-
Všechny články tohoto čísla
- Computer-aided modeling and additive manufacturing fabrication of patient-specific mandibular implant
- Immediate effect of physical on blood flow velocity in radial artery in young adults
- Nanofiber scent carrier
- NANOFIBERS FROM POLYVINYL ALCOHOL AND CAN ENLARGE THE SET OF TRAPPED ODOROLOGICAL TRACES
- GLOBAL CENTERS OF MEDICAL DEVICE TECHNOLOGY: UNITED STATES, EUROPE AND CHINA
- Lékař a technika
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- GLOBAL CENTERS OF MEDICAL DEVICE TECHNOLOGY: UNITED STATES, EUROPE AND CHINA
- Computer-aided modeling and additive manufacturing fabrication of patient-specific mandibular implant
- Nanofiber scent carrier
- Immediate effect of physical on blood flow velocity in radial artery in young adults
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



