-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaNew approach for detoxification of patients dependent on benzodiazepines and Z-drugs for reduction of psychogenic complications
Nový přístup k detoxikaci pacientů závislých na benzodiazepinech a Z-lécích pro snížení psychogenních komplikací
Benzodiazepiny (BZDs) a Z-hypnotika jsou silně návykové látky, které působí na identické GABA receptory. Detoxifikace by měla být dlouhodobá a postupná, obvykle s využitím dlouhodobě působícího BZD (diazepamu). Zatím však neexistuje žádný vhodný komerčně dostupný produkt s potřebným nízkým obsahem diazepamu. Tento přehledový článek popisuje specifické farmakologické aspekty a srovnání jednotlivých BZDs ve vztahu k jejich účinkům a návykovosti. Úspěch léčby souvisí s komfortem pacienta během procesu vysazování léku. Pacienti se obvykle obávají přechodu na vhodnější dlouhodobě působící BZD (diazepam) a během vysazování mají obavy z abstinenčních příznaků. Tyto překážky by bylo možné překonat individualizovanou detoxifikací podle již publikovaných léčebných režimů. K dispozici je třeba mít lékovou formu s velmi přesnými dávkami diazepamu, což by umožnilo dlouhodobé postupné snižování dávky s možnou přísadou adjuvantních léčiv. Snížení dávky přitom nemění vnější vzhled lékové formy a pacient by mohl být léčen až do fáze podávání placeba. Individuálně připravená léková forma s odlišným a přesným obsahem diazepamu může být použita pro pohodlnou detoxifikaci a může také eliminovat psychogenní stres při přechodu z původního léčiva na diazepam a při snižování dávky v průběhu vysazování.
Klíčová slova:
benzodiazepiny – Z-hypnotika – závislost – vysazování – snižování dávky – detoxifikace
Authors: Kateřina Kubová; Aleš Franc; Jakub Vysloužil; Jan Šaloun; David Vetchý
Published in the journal: Čes. slov. Farm., 2019; 68, 139-147
Category: Přehledy a odborná sdělení
Summary
Benzodiazepines (BZDs) and Z-drugs are strongly addictive substances, acting on identical GABA receptors. Detoxification should be long-term and gradual, usually by tapering a long-acting BZD (diazepam) but no suitable commercial pharmaceutic product exists with the necessary low drug content. This review describes the specific pharmacological aspects and comparisons of individual BZDs in relation to their effects and addictiveness. The success of the treatment relates to the patient’s comfort during this process. Patients are typically afraid of switching to a more suitable long-acting BZD (diazepam), and become stressed during the tapering and anxious from withdrawal symptoms. These obstacles could be overcome through individualized detoxification according to already published withdrawal schedules based on the administration of very precise diazepam doses in a long-term gradual tapering with possible addition of adjuvant drugs. Dose reduction does not change external appearance of the dosage form, and the patient could be treated until the placebo phase. Individually prepared pharmaceutics with different and precise diazepam contents can be used for comfortable detoxification and also may eliminate psychogenic stress during switching, tapering, and the withdrawal period.
Keywords:
benzodiazepines – Z-drugs – addiction – tapering – withdrawal – Detoxification
Introduction
According to the International Classification of Diseases, dependence on a drug is characterized by a strong desire to obtain the substance (craving), problems in controlling a dosage regimen, favouring its consumption over other activities and obligations, an increased tolerance, and sometimes a physical withdrawal state with subsequent social pathology1). (Long-term dependence on some addictive substances, including benzodiazepines (BZDs), may even result in a reduced ability to work or in permanent disability2).
Along with BZDs, Z-drugs (zolpidem, zopiclone, eszopiclone, zaleplon) are also among currently emerging drug abuse cases and addictions, acting on identical brain receptors3). This is compounded by the fact that these drugs are widely prescribed by doctors and used by millions of people worldwide, particularly in relation to the treatment of anxiety and sleep disorders, which afflict up to a third of the current population4). Despite now being under international control, along with nicotine addiction and alcoholism they represent the most common drug addiction, primarily in the elderly5). The discovery of BZDs was met with great enthusiasm because of their safety for the human body6). Due to their addictiveness unknown7), to a large extent they replaced highly hazardous barbiturates8), which were heavily prescribed until that time.
From the beginning of their use, BZDs were indicated as anxiolytics, sedatives, and muscle relaxants8, 9). To this day they are used to treat anxiety as well as generalized anxiety and panic disorders10), short-term insomnia11) and epilepsy, particularly seizure stages including status epilepticus12). They are also used in the treatment of alcohol dependence and its associated complications, including delirium tremens13). Psychiatric indications, however, have recently begun to be questioned in connection with the formation of dependence during long-term therapy14).
The first information about possible addictive potential began to appear as early as the 1980s, when patients using long half-life BZDs, and later the Z-drugs, began to realize that they had developed a tolerance to the medication and had difficulties discontinuing its use. Although mental and physical dependence manifest themselves within a few weeks, many patients continue to use these drugs to this day, and often for years due to improper prescribing15).
Apart from the dependence itself, long-term therapy is also heavily questioned because of the possibility of grave adverse effects16). This discussion has led to serious controversy in some countries17).
Benzodiazepines mechanism of action and their distribution
All BZDs affect the GABAA receptor complex in the central nervous system. A major part of the GABAA receptor is composed of α, β, and γ subunits, which are further divided into different subgroups labelled by numerical indexes. BZDs are normally bound at the interface between γ2 and α1, α2, α3 or α5 subunits and are inactive towards α4 and α6 ones. More than 90% of GABAA receptors containing the α1, α2 and α3 subunits are located in different parts of the brain, especially in the cerebral cortex and limbic system18). Here BZDs act as agonists, allosterically modulating the activation of GABAergic neurotransmitter receptors by GABA. This increases the transmembrane entry of Cl– ions via chloride channels to intracellular space, resulting in a CNS depression. The BZDs act via the same receptors as natural endogenous ligands endozepins, which are produced by the human body19). Activation of receptors containing a α1 subunit gives BZDs their sedative and hypnotic effect. Activation of receptors containing α2 and α3 provides the anxiolytic effect. Receptors containing the α5 subunit may play a role in memory processes. However, these receptors form only a small population of GABAA receptors. In connection with these findings, it is assumed that the formation of dependence is determined by activation of specific subunits of these receptors18).
Onset of BZD effects is very fast and treated symptoms are alleviated within a few minutes. Therapeutic benefit has a tendency to increase in a few weeks and the therapeutic effects are maintained even for months19). Administration of BZDs results in tolerance and therefore, due to addiction prevention, the duration of BZD therapy should be roughly 2–4 weeks long6).
Individual BZDs have different pharmacokinetics or biological half-life. They can be divided into BZDs with short (less than 6 hours), mid (6–24 hours), or long half-life (more than 24 hours)20), and according to potency, labelled as either high or less effective (see Table 1)21).
Tab. 1. Benzodiazepines division according to time of an action21) 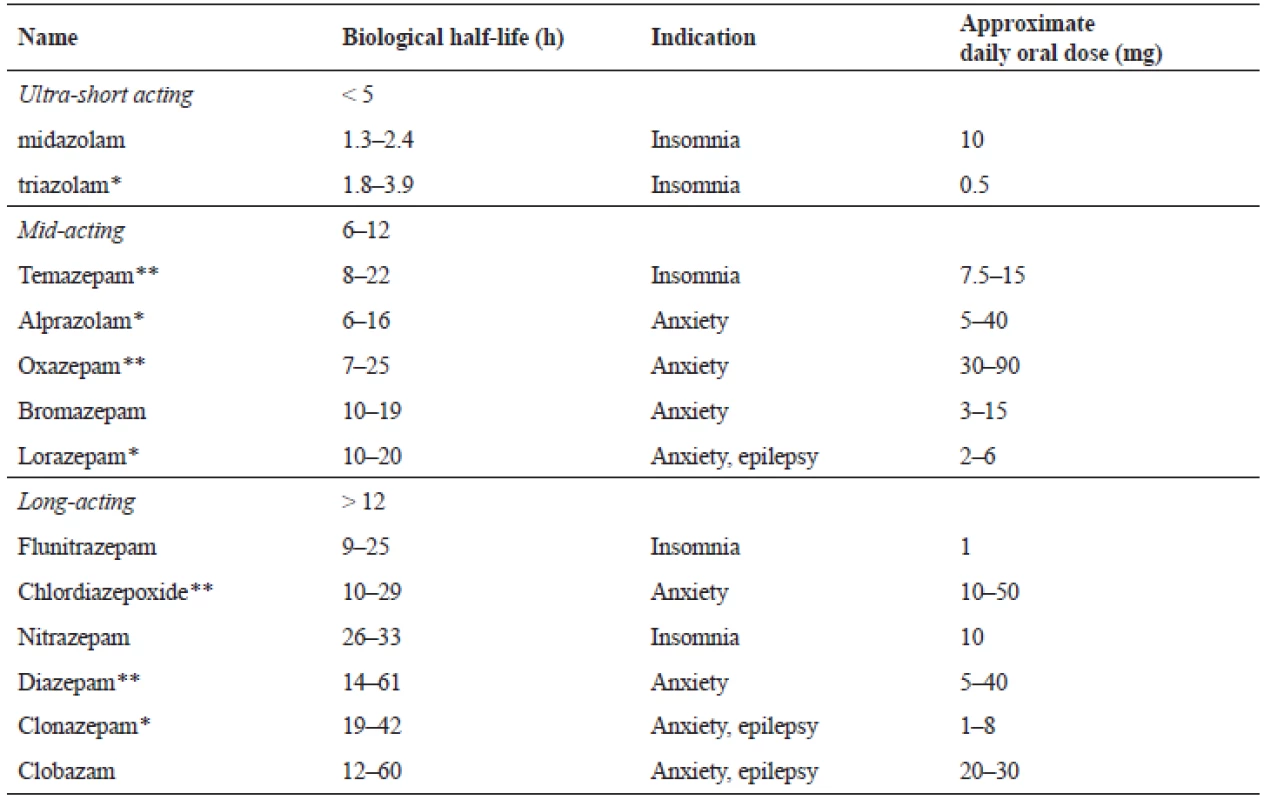
* highly efficient, **lower efficient Benzodiazepines prescription
The INCB (International Narcotics Control Board) statistics for 2009 show that Europe has the highest average consumption of both sedative-hypnotics and anxiolytics, expressed as defined daily doses for statistical purposes (S-DDD) per 1,000 inhabitants per day. In 2007, the global consumption of anxiolytics was around 21 billion S-DDD22).
There is a lack of self-reported prevalence data on the use of BZDs in the general population. Monitoring is limited by the very broad range of BZD products available in Europe and the lack of clear definitions for the general population to report their levels of use. Among 15 - to 16-year-old school students, lifetime prevalence of the use of tranquillizers or sedatives without a doctor’s prescription ranged from 2% to 15% in the 24 EU Member States and Norway with ESPAD (the European School Survey Project on Alcohol and Other Drugs) surveys in 201122).
One especially endangered group are geriatric patients. According to Beers criteria published by the American Geriatrics Society (AGS), short - and mid-acting BZDs (alprazolam, estazolam, lorazepam, oxazepam, temazepam, triazolam) and long-acting BZDs (clorazepate, chlordiazepoxide – alone or in combination with amitriptyline or clidinium, clonazepam, diazepam, flurazepam and quazepam) are highly inappropriate in geriatric indications. AGS recommends strictly avoiding them. The reason lies in polypharmacy of the elderly patients, changes in pharmacokinetics and pharmacodynamics and therefore associated development of serious adverse effects23). In the pharmacokinetic area, it is mainly an increase in the distribution volume for lipophilic drugs, which is caused by the increase in fat and a decrease in the muscle tissue, as well as decreased renal clearance and biotransformation, leading to a prolongation of the biological half-life and subsequent BZD accumulation.
Table 2 offers a comparison of the biological half-life in middle-aged and elderly patients24). From a pharmacodynamics perspective, seniors have a higher tendency to orthostatic hypotension, falls, impairment of cognitive function, and behavioral disorders. Delirium states occur more frequently. This could be erroneously perceived as the beginning of dementia, which is then completely unnecessarily treated, ultimately proving harmful for the patients. BZDs can also impair thermoregulatory mechanisms with the subsequent risk of hypothermia25). These similar complications are also typically associated with Z-drugs, although senior patients take these drugs routinely with few negative consequences26). For the treatment of the elderly it is therefore preferable to employ a mid - or short-acting BZDs and therapy should not exceed two weeks for a hypnotic indication, 4 weeks for anxiolytic indication and 12 weeks in the inaugural treatment of panic disorder27). It is also not suitable to combine multiple BZDs at once28).
Tab. 2. Comparison of the biological half-life24) 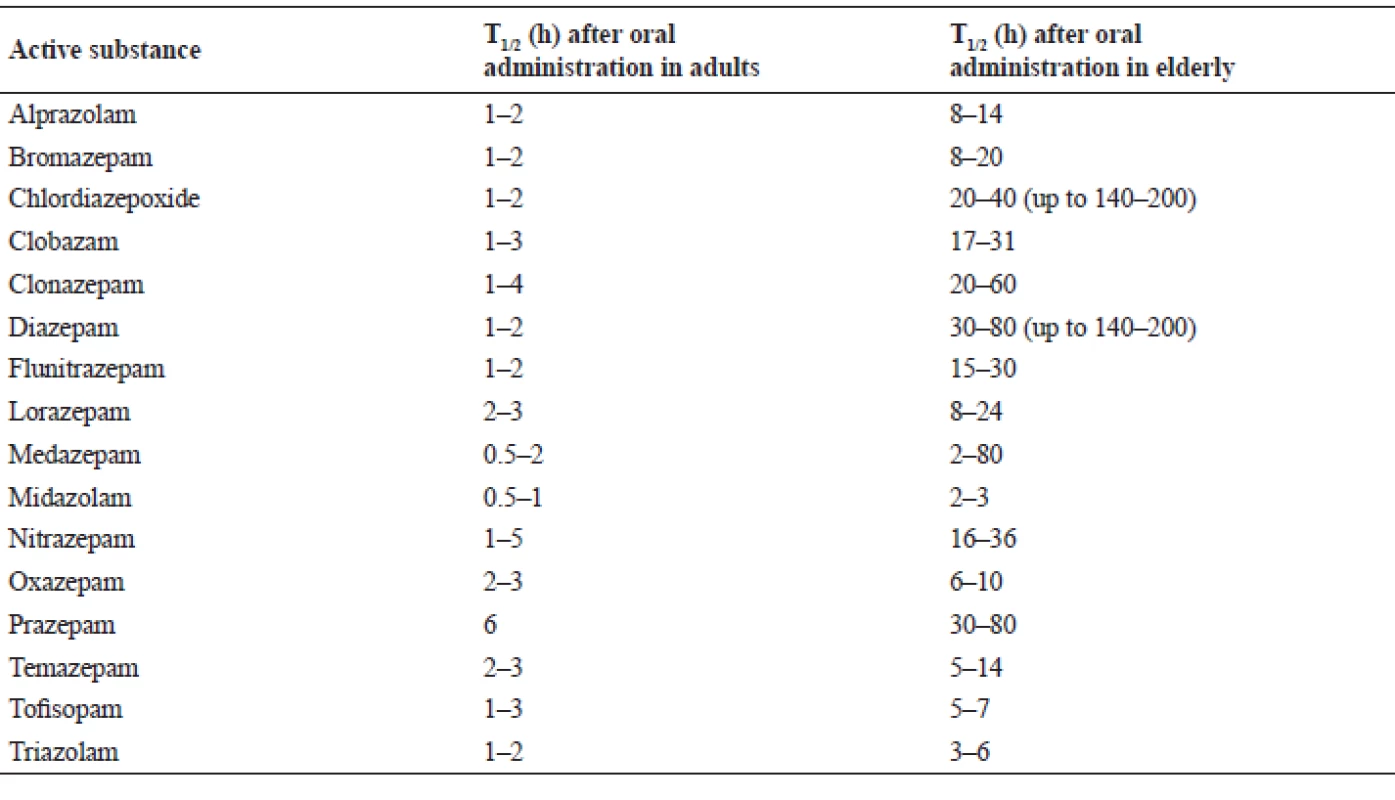
Benzodiazepines addiction
When BZDs and Z-drugs first came into use, experts erroneously believed that these substances cannot develop dependence29). However, nowadays it is the dependence on BZDs and the Z-drugs which seems to be, with the exception of nicotinism and alcoholism, the most pressing problem worldwide, mainly in developed countries30). In addition to the therapeutic indications, they are misused by patients who even demand them from more than one doctor (Ashton 2005) or by drug users to suppress the “roll out” effect of psychoactive substances or for sedation after psychostimulants31). Dependence on BZDs develops in 5% of patients after half a year of usage and in approximately 40% after one year. Problematic groups are benzodiazepine anxiolytics, which are commonly prescribed agents and administered in amounts exceeding recommended dosing (alprazolam, chlordiazepoxide and diazepam)32).
Chronic use of BZDs leads not only to mental, but often to heavy somatic addiction. As a consequence, dependence on them significantly reduces the quality of life, manifesting as reduced vitality, impaired social functioning and mental health, and reduced labour intensity33). BZD use has been associated with increased risk of severe anxiety, depression, and sleep disorders. Consumption of alcohol can potentiate their sedative and anxiolytic effects, reduce withdrawal symptoms, etc.34)
BZD withdrawal syndrome is often compared with withdrawal symptoms of heroin or cocaine but are also more serious because BZD cannot be discontinued abruptly (cold turkey) (Busto et al. 1986). Withdrawal symptoms manifest as restlessness, insomnia, tremor, dysphoria, anger or even aggression, paranoid ideas, agoraphobia, and panic attacks35). Deterioration of spatial vision, memory and attention disorders, including return of difficulties occurring before the medication (rebound phenomenon) are often observed36). Sometimes withdrawal syndrome may develop into seizures, which can lead to death15). Based on physical indicators, difficultly localized abdominal pain, neuralgia and paraesthesia can be observed37). In some cases, it can even lead to malignant tachycardia or fatal hyperthermia. The somatic state of withdrawal from BZD is therefore more dangerous than that of opioids38, 39). The psychological component of dependency subsequently creates an increased need for medicine40).
Benzodiazepines addiction treatment
Primarily, emphasis should be placed on addiction prevention and patient education during the introduction of BZD treatment.
Treatment of already developed dependence is considered to be fully justified because of the possibility of fatal withdrawal symptoms and improvement of life quality in patients dependent on BZDs41). An important task is also to provide individual, flexible, and supportive counselling for the BZD-addicted, ideally in specialized departments/clinics, although only a minimum of patients selects such intensive intervention. Facilities for people addicted to alcohol and illicit drugs are not suitable for users of BZDs, because the patient usually becomes addicted unknowingly with no fault of their own42). Doctors (practical or specialist) should monitor their patients to observe any addiction development. With early intervention, patients can achieve very good results in a relatively short period of time. If the doctor has enough experience and capabilities, he or she can treat the patient himself/herself. If an addiction treatment would already exceed the capabilities of the doctor, then it is possible to use psychiatrists – addictology specialists43). However, most treatments take place on an ambulatory basis and the current, generally valid opinion about the appropriateness of supportive psychotherapy (but not addictological intervention) during withdrawal is under dispute. From the results of long-term abstinence studies, psychotherapy itself has no significant effect on long-term abstinence in patients who go through a psychotherapy abstinence program44).
The very principle of withdrawal is to set a stable, slow decline of BZD concentrations in the blood and tissues so that the natural sedative system of the body can be restored, through mediation by the GABA neurotransmitter. During prolonged use of short - and mid-acting BZDs, plasma levels fluctuate during the day. This is a heavy burden for the nerve receptors, which may lead to an arbitrary increase of the dose by the patient in order to compensate for the resulting unpleasant state. During gradual reduction of the drug dose during detoxification, these plasmatic differences are further exacerbated, making gradual withdrawal of BZD extremely difficult for many users. Performed too quickly, the withdrawal may additionally lead to complications in the form of agitation like tinnitus and involuntary movement45) and even catatonic state46). For this reason, the replacement of long-term-use BZD by diazepam in the dose equivalent to the original treatment is used32). The organism is thus stabilized at the same plasma level, thereby minimizing the withdrawal symptoms associated with plasma level fluctuations during the day, which itself is already perceived as a relief46). It is still a classic mechanism of detoxification, though attempts are being made to replace diazepam with acetylcholinesterase inhibitors (donepezil, galantamine and rivastigmine) that also possess the effect of inhibiting GABA receptors, and act as an indirect antagonist, to be applied in the rapid detoxification treatment of BZD and Z-drug dependence47). Diazepam is then eliminated slowly and to achieve stable blood concentration it requires administration only once or twice a day41). Scientists generally agree that the withdrawal should be gradual and long-term. A precondition for successful treatment is the creation of a withdrawal schedule for each individual. A withdrawal timetable is compiled on the basis of what BZD the patient is taking and for how long41). Of course, there are other approaches where diazepam is not found to be sufficiently effective48). Despite the fact that BZDs were to replace barbiturates because of their high addictiveness and narrow therapeutic index, there are attempts to switch the BZD dependent patient to phenobarbital. It has a similarly long biological half-life, and operates on similar receptors as BZDs49). Nevertheless, substitution by diazepam is still used as a first course of action.
In order to perform a suitable substitution (switching) of BZD/Z-drugs for diazepam, it is necessary to know the equivalent ratios. A dose of 10 mg of diazepam empirically corresponds to 1 mg of alprazolam, 6 mg of bromazepam, 1 mg of clonazepam, 1 mg of lorazepam, 30 mg of oxazepam, or 15 mg of zopiclone36).
The optimal dose reduction of diazepam has been insufficiently explored and withdrawal plans are highly non-uniform. There is a general consensus that at the outset it is possible to discontinue up to 25% of the dose (or an even greater amount in larger doses) with a subsequent reduction ranging from 10% to 25% per week, while the last 25% of the original dose should be withdrawn more slowly41). In most cases, the discontinuation takes approximately 8–12 weeks. For repeatedly treated patients, the withdrawal should take up to 6 months. The minimum period from the original dose to complete cessation is 4 weeks. Many authors speak about an optimal time of 6–8 weeks, but very often it is a much longer period (3–6 months) and in some cases, discontinuation may even take more than a year6). Everything depends on the original dose used by the patient. In a life-threatening addiction, the withdrawal rate is quicker – the diazepam equivalent of the original BZD dose is taken only on the first day. On the second day it is reduced by 30%, followed by a daily dose reduction of 5%50). In patients receiving therapeutic doses, the daily BZD dose should be lowered by between approximately one eighth and one tenth every 1–2 weeks. Shorter times are recommended for highly potent BZDs or BZDs with short half-lives51).
Substitution can be adapted to the needs of the patient and his/her individual tolerance. For example, if a patient suffers from insomnia, most of the dose is administered before sleep. It therefore follows that an individual withdrawal plan should be conceived52). For high doses of alprazolam, exemplary schedule suggested by Ashton on the basis of “clinical impression” and successfully applied to dependent patients is listed in Table 336). This can serve rather as an indicative guideline.
Tab. 3. Withdrawal from high doses of alprazolam (2 mg, 3 times per day) by diazepam substitution36) 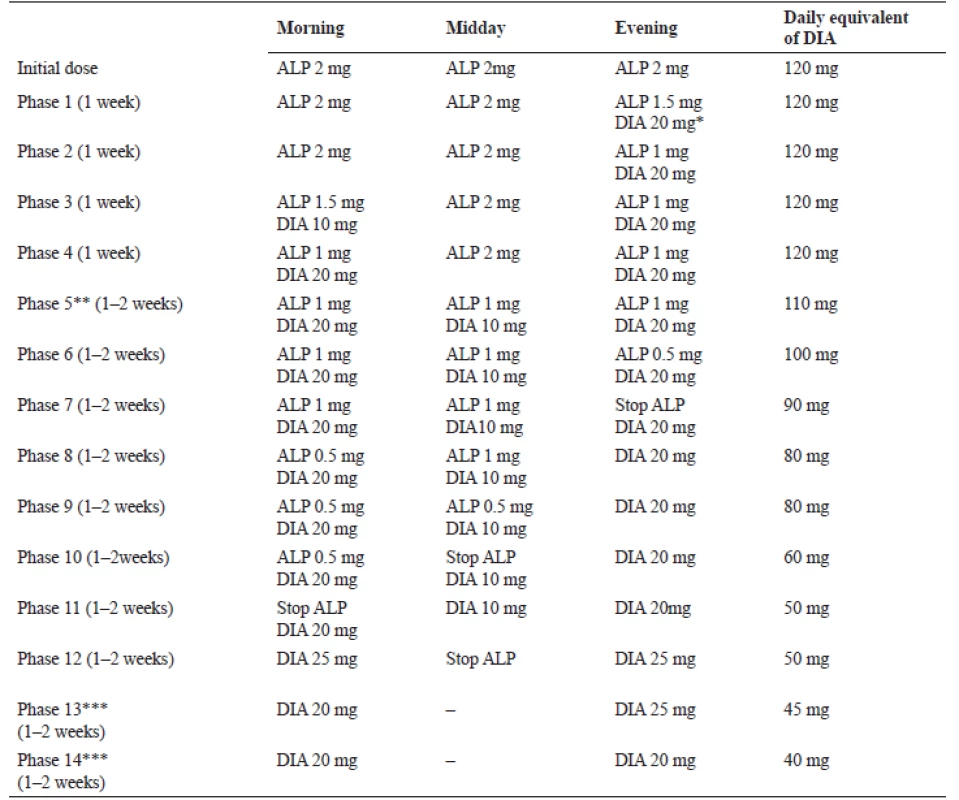
DIA – diazepam, ALP – alprazolam
*Evening dose of DIA can also be taken before bedtime, as it is better than administration at the same time with ALP.
**Total switch to DIA in phases 5–11.
***DIA is a long-acting BZD, therefore it is not needed to be taken more than twice a day.Due to its interaction with GABA receptors, patients should avoid alcohol at least during detoxification and ideally on a more long-term basis. During cessation it is also recommended to use a wide range of adjuvant drugs6, 52). In order to prevent epileptic seizures, carbamazepine53) or valproate54) may be used. If the dependent patient is suffering from major depressive disorders, a viable option is the administration of a suitable antidepressant55). For anxiety relief, despite earlier scepticism56) it is possible to take non-BZD anxiolytics (e.g. buspirone)57) or use the anxiolytic effect of some antidepressants (e.g. paroxetin)58). Sleep disorders can be treated with sedative antidepressants, like mirtazapine59), trazodone60) or agomelatine61). Sometimes, if needed, small doses of anxiolytic antipsychotics, for example cyamemazin62), tiapride63) or quetiapine64), can be administered. For tachycardia adjustment, beta-blockers are recommended, most commonly propranolol38, 65).
Psychogenic problems with withdrawal
However, even if we find an adequate dose of diazepam, select the appropriate individualized withdrawal schedule and choose the necessary well-tolerated and effective complementary medicines, a predisposed patient is usually subject to several moments of high stress. These moments can make successful withdrawal difficult or even impossible.
The first stressful experience (stage) is the awareness of the dependence itself (Realize), usually accompanied by side-effects and unsuccessful efforts to discontinue medication62). Second, the need for a psychiatrist, specifically addictology specialist (Counselling), which is often perceived by the patient as a negative fact66). From the psychological point of view, a transition from the used drug to diazepam is the third stressful stage (Switching), which can cause a fear of the unknown. It manifests itself even in the substitution of generic drugs67). Fourth, reduction of the diazepam dose is responsible for both psychogenic and physiological problems during the withdrawal period (Tapering) and people often need medical support68). Fifth, and according to many patients the hardest step, is the actual withdrawal (Withdrawal), even when dealing with the smallest doses69). This last step, however, as it will be discussed below, presenting an opportunity for pharmacists and pharmaceutical service, opens itself: the preparation of a safe dosage form which can practically decompose accompanying phobias connected particularly with third, fourth, and fifth stages70) (see Table 4).
Tab. 4. Stages and detoxification complication(s)70) 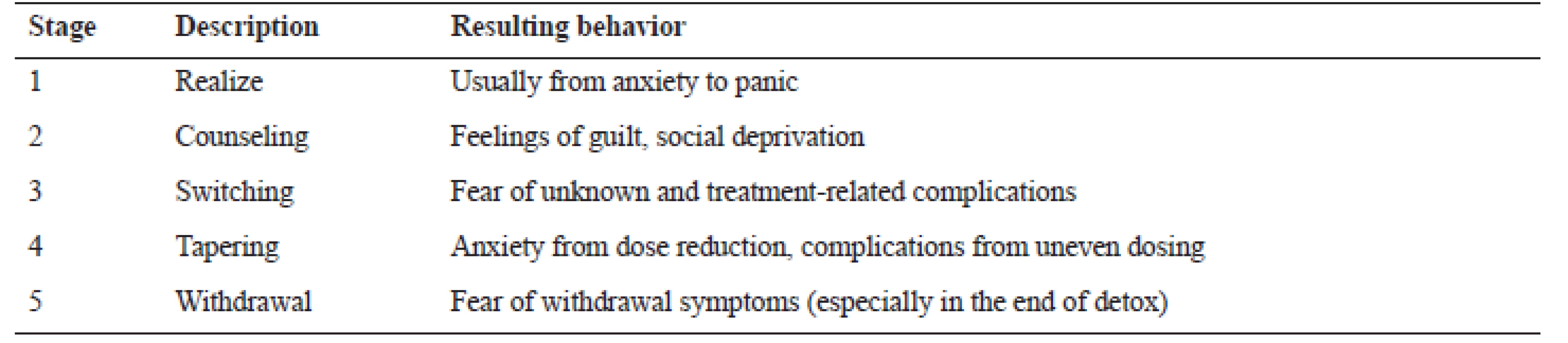
The issue of individualized detoxification
It is apparent that withdrawal from BDZ may be technically and organizationally difficult. In the EU, diazepam is administered orally in tablet and liquid forms, rectally as a gel, and parenterally as intramuscular injections. It is commonly prescribed in tablet form, which comes in three strengths – 2, 5, and 10 mg71). The relatively exact dose of diazepam could be achieved from a commercial or individually prepared solution.
However, even here precise dosing is difficult and in the cases of ambulant treatment it depends on the accuracy and self-control of the patient. The transition from a solid to a liquid dosage form (solution, suspension) may be another stress factor, as well as an ever-decreasing volume of fluid. This solution enhances the psychological stress of the third, fourth, and fifth stages. Moreover, in the cases of suspensions prepared from BZD tablets, which are also sometimes recommended, inadequate dose preparation can occur72). Kinetic instability of two separated phases can occur due to sedimentation, which prevents accurate dosing. The usage of registered oral tablets is also very problematic; tablet breaking does not represent a precise method for obtaining an exact dose of diazepam73). Moreover, in this way the patient is fully aware of the reduction in substance intake, which may have a significant negative psychogenic effect.
All of these disadvantages could be overcome through individualized treatment, which would be based on hard gelatine capsules containing the exact required diazepam dose with an individual gradual dose decrease over time. The essential step is to find such a technique that would actually provide the exact BZD dose, because the responsible receptors are sensitive to a fluctuating BZD plasmatic level. These capsules could be filled by a mass prepared by special technological procedures in a pharmacy-like, optimized mixing process74), granulation75) or impregnation76) or others. The capsules could also include adjuvant drugs, e.g. an antiepileptic, antidepressants, or beta-blockers.
In this case a patient would get professionally prepared medicine with good content uniformity, but moreover the patient would not be additionally stressed by the switching to diazepam and knowledge of dose reduction because he would still receive the same organoleptically indistinguishable formulation. Assuming good management and compliance, a patient could reach the placebo receiving stage without even realizing it.
Therefore, this administration form significantly eases and eventually eliminates stress factors of the third, fourth, and fifth stages of the detoxification process. After complete diazepam withdrawal, the dose of adjuvant psychotropic treatment would be tapered as well without changing of the dosage form design. Considering the withdrawal schemes and pharmacopoeia requirements for content uniformity of dosage forms, it would be pharmaceutically possible to reduce the dose by 15%, since this is also the Pharmacopoeia limit for content uniformity (European Pharmacopoeia). A reduction of less than 15% to the diazepam dose is difficult to ensure from the viewpoint of pharmaceutical technology.
Another possibility for detoxification could be seen in incorporating BZDs and Z-drugs into dosage forms of higher generations with prolonged drug release. In this respect, they seem to be suitable for a stable plasma level, and the possibility of accurate dose reduction, resulting in higher patient compliance. Tablets77) or microparticles78, 79) are considered suitable dosage forms for 12 - or 24-hour oral drug delivery of short - or mid-acting BZDs or Z-drugs. In the cases of prolonged release, there is no need to switch patients to diazepam at all. This seems to be particularly an advantage for Z-drugs, which act selectively only on some of the GABA receptors and a switch to diazepam would bring only a needless increase in receptor stress.
Conclusion
The success of BZD addiction treatment closely relates to the patient’s comfort during treatment. This criterion can be fulfilled by individualized detoxification through the preparation of oral capsules with an accurate dose of diazepam. This could be ensured by solid filling of capsules prepared by special technological procedures to provide content uniformity of the filled mass. This formulation also reduces or even eliminates stress during switching, tapering and withdrawal.
Conflict of interest: none.
Department of Pharmaceutics, Faculty of Pharmacy
University of Veterinary and Pharmaceutical Sciences
Palackého 1946/1, 612 42 Brno, Czech Republic
e-mail: franca@vfu.cz
J. Šaloun
Department of Applied Pharmacy, Faculty of Pharmacy
University of Veterinary and Pharmaceutical Sciences Brno, Czech Republic
Zdroje
1. Lavania S., Ram D., Praharaj S. K., Khan A. H. Pattojoshi A. Deliberate self-harm in nondepressed substance-dependent patients. J. Addict. Med. 2012; 6(4), 247–252.
2. Soumerai S. B., Simoni-Wastila L., Singer C., Mah C., Gao X. M., Salzman C., Ross-Degnan D. Lack of relationship between long-term use of benzodiazepines and escalation to high dosages. Psychiatr. Serv. 2003; 54(7), 1006–1011.
3. Janhsen K., Patrik R., Hoffmann K. The problems of long-term treatment with benzodiazepines and related substances. Dtsch. Arztebl. Int. 2015; 112(1–2), 1–7.
4. Linnet K., Gudmundsson L. S., Birgisdottir F. G., Sigurdsson E. L., Johannsson M., Tomasdottir M. O., Sigurdsson J. A. Multimorbidity and use of hypnotic and anxiolytic drugs: cross-sectional and follow-up study in primary healthcare in Iceland. BMC Fam. Pract. 2016; 17(1), 69.
5. Olfson M., King M., Schoenbaum M. Benzodiazepine use in the United States. JAMA Psychiatry 2015; 72(22), 136–142.
6. Ashton H. The diagnosis and management of benzodiazepine dependence. Curr. Opin. Psych. 2005; 18(3), 249–255.
7. Lader M. History of benzodiazepine dependence. J. Subst. Abuse Treat. 1991; 8(1–2), 53–59.
8. Argyropulous S. V., Nutt D. J. The use of benzodiazepines in anxiety and other disorders. Eur. Neuropsychopharmacol. 1999; (9), S407–S412.
9. Atack J. R. The benzodiazepine binding site of GABAA receptors as a target for the development of novel anxiolytics. Expert. Opin. Investig. Drugs 2005; 14(5), 601–618.
10. Gould R. A., Ott M. W., Pollack M. H. A meta-analysis of treatment outcome for panic disorder. Clin. Psychol. Rev. 1995; 15(8), 819–844.
11. Silber M. H. Chronic insomnia. N. Engl. J. Med. 2005; 353(8), 803–810.
12. Riss J., Cloyd J., Gates J., Collins S. Benzodiazepines in epilepsy: pharmacology and pharmacokinetics. Acta Neurol. Scand. 2008; 118(2), 69–86.
13. Mayo-Smith M. F., Beecher L. H., Fischer T. L., Gorelick D. A., Guillaume J. L., Hill A., Melbourne J. Management of alcohol withdrawal delirium: an evidence-based practice guideline. Arch. Intern. Med. 2004; 164(13), 1405–1412.
14. Dell’osso B., Lader M. Do benzodiazepines still deserve a major role in the treatment of psychiatric disorders? A critical reappraisal. Eur. Psychiatry 2013; 28(1), 7–20.
15. Salzman C. 1990. Benzodiazepine dependence, toxicity, and abuse: a task force report of the American psychiatric association. Washington D.C.: American Psychiatric Pub. 1990.
16. Lader M., Tylee A., Donoghue J. Withdrawing benzodiazepines in primary care. CNS Drugs 2009; 23(1), 19–34.
17. Lader M. Benzodiazepines revisited – will we ever learn? Addiction 2011; 106(12), 2086–2109.
18. Licata S. C., Rowlett J. K. Abuse and dependence liability of benzodiazepine-type drugs: GABA a receptor modulation and beyond. Pharmacol. Biochem. Behav. 2008; 90(1), 74–89.
19. Tanimoto Y., Onishi Y., Sato Y., Kizaki H. Benzodiazepine receptor agonists modulate thymocyte apoptosis through reduction of the mitochondrial transmembrane potential. Jpn. J. Pharmacol. 1999; 79(2), 177–183.
20. Šonka K. Hypnotika. Remedia 2003; 13(6), 434–441.
21. Greenblatt D. J., Shader R. I., Divoll M., Harmatz J. S. Benzodiazepines: a summary of pharmacokinetic properties. Br. J. Clin. Pharmacol. 1981; 11(S1), 11–16.
22. Hibell B., Guttormsson U., Ahlström S., Balakireva O., Bjarnason T., Kokkevi A., Kraus L. The 2011 ESPAD report: substance use among students in 36 European countries. Stockholm: Swedish Council for Information on Alcohol and other Drugs 2012; 123–124.
23. Radcliff S., Yue J., Rocco G., Aiello S. E., Ickowicz E., Hurd Z., Beers M. H. Updated Beers criteria for potentially inappropriate medication use in older adults. J. Am. Geriatr. Soc. 2015; 63(22), 2227–2246.
24. Ballóková A. Rational use of benzodiazepines in elderly. Farmi. News 2012; 9, 12–13.
25. Echizenya, M. Mishima, K. Satoh, K. Enhanced heat loss and age-related hypersensitivity to diazepam. J. Clin. Psychopharmacol. 2004; 24(6), 639–646.
26. Gérardin M., Victorri-Vigneau C., Guerlais M., Guillou-Landreat M., Grall-Bronnec M., Jolliet P. Benzodiazepines consumption: does dependence vary with age? Subst. use Misuse 2014; 49(11), 1417–1425.
27. Preskorn S. H. A way of conceptualizing benzodiazepines to guide clinical use. J. Psychiatr. Pract. 2015; 21(6), 436–441.
28. Fialová D., Topinková E. Appropriate choce of drugs and drug dosing in geriatric patients. Klin. Farmakol. Farm. 2013; 27, 18–22.
29. Hajak G., Müller W. E,. Wittchen H. U., Pittrow D., Kirch W. Abuse and dependence potential for the non‐benzodiazepine hypnotics zolpidem and zopiclone: a review of case reports and epidemiological data. Addiction 2003; 98(10), 1371–1378.
30. Casati A., Sedefov R., Pfeiffer-Gerschel T. Misuse of medicines in the European Union: a systematic review of the literature. Eur. Addict. Res. 2012; 18(5), 228–245.
31. Lessenger J. E., Feinberg S. D. Misuse of prescription and over-the-counter medicines in South London nightclubs. J. Subst. Use 2016; 21(5), 495–500.
32. Lugoboni F., Mirijello A., Faccini M., Casari R., Cossari A., Musi G., Addolorato G. Quality of life in a cohort of high-dose benzodiazepine dependent patients. Drug Alcohol Depend. 2014; 142, 105–109.
33. Lozano Ó. M., Rojas A. J., Fernandez C. F. Psychiatric comorbidity and severity of dependence on substance users: how it impacts on their health-related quality of life? J. Ment. Health 2017; 26(2), 119–126.
34. Mant A., Walsh R. A. Reducing benzodiazepine use. Drug Alcohol Rev. 1997; 16(1), 77–84.
35. Smith D. E., Wesson D. R. The benzodiazepines: current standards for medical practice. Springer Science & Business Media 2012.
36. Ashton H. Benzodiazepines: how they work & how to withdraw: medical research information from a benzodiazepine withdrawal clinic. University of Newcastle 2001.
37. Sellers E. M. Alcohol, barbiturate and benzodiazepine withdrawal syndromes: clinical management. Can. Med. Assoc. J. 1988; 139(2), 113.
38. Abernethy D. R., Greenblatt D. J., Shader R. I. Treatment of diazepam withdrawal syndrome with propranolol. Ann. Intern. Med. 1981; 94(3), 354–345.
39. da Silva P. S. L., Reis M. E., Fonseca T. S. M., Fonseca M. C. M. Opioid and benzodiazepine withdrawal syndrome in PICU patients: which risk factors matter? J. Addict. 2016; 10(2), 110–116.
40. Vorma H., Naukkarineh H. Symptom severity and quality of life after benzodiazepine withdrawal treatment in participants with complicated dependence. Addict. Behav. 2004; 29(6), 1059–1065.
41. Frare F., Perugi G. Managing benzodiazepine withdrawal. Heroin Add. & Rel. Clin. Probl. 2000; 2, 1–18.
42. Ashton H. Benzodiazepine withdrawal: an unfinished story. BMJ 1984; 288(6424), 1135.
43. Voshaar R., Gorgels W., Mol A., van Balkom A., Mulder J., van de Lisdonk E. Long-term outcome of two forms of randomised benzodiazepine discontinuation. Br. J. Psychiatry 2006; 188, 188–189.
44. Ashton H. How to wean patients off benzodiazepines. Pulse 2004; 64, 50–53.
45. Busto U., Sellers E. M., Naranjo C. A., Cappell H., Sanchez-Craig M., Sykora K. Withdrawal reaction after long-term therapeutic use of benzodiazepines. N. Engl. J. Med. 1986; 315(14), 854–859.
46. Rosebush P. I., Mazurek M. F. Catatonia after benzodiazepine withdrawal. J. Clin. Psychopharmacol. 1996; 16(4), 315–319.
47. Lin S. K. Rapid detoxification of benzodiazepine or Z-drugs dependence using acetylcholinesterase inhibitors. Med. Hypotheses 2014; 83(1), 108–110.
48. Lader M. Benzodiazepine harm: how can it be reduced? Br. J. Clin. Pharmacol. 2014; 77(2), 295–301.
49. Kawasaki S. S., Jacapraro J. S., Rastegar D. A. Safety and effectiveness of a fixed-dose phenobarbital protocol for inpatient benzodiazepine detoxification. J. Subst. Abuse Treat. 2012; 43(3), 331–334.
50. Nešpor K. Substance abuse preventinon and approaches to managing of cure dependence. Prakt. Lék. 2010; 90, 93–96.
51. Authier N., Balayssac D., Sautereau M., Zangarelli A., Courty P., Somogyi A. A. Eschalier A. Benzodiazepine dependence: focus on withdrawal syndrome. Ann. Pharm. Fr. 2009; 67(6), 408–413.
52. Lader M., Kyriacou A. Withdrawing benzodiazepines in patients with anxiety disorders. Curr. Psychiatry Rep. 2016; 18(1), 8.
53. Ries R. K. Use of anticonvulsants in benzodiazepine withdrawal. Am. J. Addic. 1998; 7(3), 198–204.
54. McElroy S. L., Keck P. E., Mawrence J. M. Treatment of panic disorder and benzodiazepine withdrawal with valproate. J. Neuropsychiatry Clin. Neurosci. 1991; 3, 232–233.
55. Zitman F. G., Couvee J. E. Chronic benzodiazepine use in general practice patients with depression: an evaluation of controlled treatment and taper off. Br. J. Psychiatry 2001; 178(4), 317–324.
56. Lader M., Olajide D. A. Comparison of buspirone and placebo in relieving benzodiazepine withdrawal symptoms. J. Clin. Psych. 1987; 7(1), 11–15.
57. Chiae R. P., Pancheri P., Casacchia M., Stratta P., Kotzalidis G. D., Zibellini M. Assessment of the efficacy of buspirone in patients affected by generalized anxiety disorder, shifting to buspirone from prior treatment with lorazepam: a placebo-controlled double-blind study. J. Clin. Psych. 1995; 15(1), 12–19.
58. Nakao M., Takeuchi T., Nomura K., Teramoto T., Yano E. Clinical application of paroxetine for tapering benzodiazepine use in non‐major‐depressive outpatients visiting an internal medicine clinic. Psychiatry Clin. Neurosci. 2006; 60(5), 605–610.
59. Chandrasekaran P. K. Employing mirtazapine to aid benzodiazepine withdrawal. Singapore Med. J. 2008; 49(6), 166–167.
60. Ansseau M., de Roeck J. Trazodone in benzodiazepine dependence. J. Clin. Psych. 1993; 54(5), 189–191.
61. Müller H., Seifert F., Maler J. M., Kornhuber J., Sperling W. Agomelatine reduces craving in benzodiazepine addicts: a follow-up examination of three patients. Singapore Med. J. 2012; 53(11), e228–e230.
62. Benyamina A., Naassila M., Bourin M. Potential role of cortical 5-HT 2A receptors in the anxiolytic action of cyamemazine in benzodiazepine withdrawal. Psychiatry Res. 2012; 198(2), 307–312.
63. Pecinovská O. Delirium u závislostí na návykových látkách. Neurológia pre prax 2011; 12(5), 210–214.
64. Sattar S. P., Bhatia S. C., Petty F. Potential benefits of quetiapine in the treatment of substance dependence disorders. J. Psychiatry Neurosci. 2004; 29(6), 452–457.
65. Cantopher T., Olivieri S., Cleave N., Edwards J. G. Chronic benzodiazepine dependence: a comparative study of abrupt withdrawal under propranolol cover versus gradual withdrawal. Br. J. Psychiatry 1990; 156(3), 406–411.
66. Link B. G., Struening E. L., Rahav M., Phelan J. C., Nuttbrock L. On stigma and its consequences: evidence from a longitudinal study of men with dual diagnoses of mental illness and substance abuse. J. Health. Soc. Behav. 1997; 38, 177–190.
67. Håkonsen H., Eilertsen M., Borge H., Toverud E. L. Generic substitution: additional challenge for adherence in hypertensive patients? Curr. Med. Res. Opin. 2009; 25(10), 2515–2521.
68. Nuzzarello A. Does cognitive behavior therapy assist slow-taper alprazolam discontinuation in panic disorder? Am. J. Psychiatry 1994; 151(6), 876–881.
69. Otto M. W., Pollack M. H., Sachs G. S., Reiter S. R., Meltzer-Brody S., Rosenbaum J. F. Discontinuation of benzodiazepine treatment: efficacy of cognitive-behavioral therapy for patients with panic disorder. Am. J. Psychiatry 1993; 150(10), 1485–1490.
70. Franc A., Kubová K., Elbl J., Muselík, J., Vetchý D., Šaloun J., Opatřilová R. Diazepam filled hard capsules intended for detoxification of patients addicted to benzodiazepines and Z-drugs. Eur. J. Hosp. Pharm. 2019; 26(1), 10–15.
71. List of medicinal products: authorised under Article 126a of Directive 2001/83/EC of the of 6 November 2001 on the Community Code relating to medicinal products for human use. Pharmaceuticals – community register, European Parliament and of the Council European Comission – http://ec.europa.eu/health/documents/community-register/html/except_index.htm
72. Newton D. W., Schulman S. G., Becker C. H. Limitations of compounding diazepam suspensions from tablets. AJHP 1976; 33(5), 450–452.
73. van Santen E. D., Barends M., Frijlink H. W. Breaking of scored tablets: a review. Eur. J. Pharm. Biopharm. 2002; 53(2), 139–145.
74. Venables H. J., Wells J. I. Powder mixing. Drug Dev. Ind. Pharm. 2001; 27(7), 599–612.
75. Mahapatra A. K., Sameeraja N. H., Murthy P. N. Development of modified-release tablets of zolpidem tartrate by biphasic quick/slow delivery system. AAPS PharmsciTech. 2015; 16(3), 579–588.
76. Franc A., Rabišková M., Goněc R. Impregnation: a progressive method in the production of solid dosage forms with low content of poorly soluble drugs. EJPPS 2011; 16(3); 85–91.
77. Shanmugam S. Granulation techniques and technologies: recent progresses. Bioimpacts 2015; 5(1); 55–63.
78. Trapani G., Lopedota A., Boghetich G., Latrofa A., Franco M., Sanna E., Liso G. Encapsulation and release of the hypnotic agent zolpidem from biodegradable polymer microparticles containing hydroxypropyl-β-cyclodextrin. Int. J. Pharm. 2003; 268(1–2), 47–57.
79. Lopedota A., Cutrignelli A., Trapani A., Boghetich G., Denora N., La Quintana V., Liso G. Effects of different cyclodextrins on the morphology, loading and release properties of poly (DL-lactide-co-glycolide)-microparticles containing the hypnotic agent etizolam. J. Microencapsul. 2007; 24(3), 214–224.
Štítky
Farmacie Farmakologie
Článek vyšel v časopiseČeská a slovenská farmacie
Nejčtenější tento týden
2019 Číslo 4- Psilocybin je v Česku od 1. ledna 2026 schválený. Co to znamená v praxi?
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
- Ukažte mi, jak kašlete, a já vám řeknu, co vám je
-
Všechny články tohoto čísla
- Nový přístup k detoxikaci pacientů závislých na benzodiazepinech a Z-lécích pro snížení psychogenních komplikací
- Rastlinné inhibítory α-amylázy a ich vplyv na využiteľnosť polysacharidov obsiahnutých v strave
- Teorie a praxe lékopisné kontroly jakosti léčiv a pomocných látek X. Počet paralelních stanovení, zpracování výsledků a jejich použití při hodnocení obsahu léčivých a pomocných látek v Evropském lékopisu (Ph. Eur.)*
- Studie biokompatibility roztoků pro peritoneální dialýzu měřená jako životaschopnost buněk v podmínkách in vitro
- Nefroprotektivní účinek N-acetylglukosaminu u potkanů s akutním poškozením ledvin
- Česká a slovenská farmacie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Nový přístup k detoxikaci pacientů závislých na benzodiazepinech a Z-lécích pro snížení psychogenních komplikací
- Rastlinné inhibítory α-amylázy a ich vplyv na využiteľnosť polysacharidov obsiahnutých v strave
- Nefroprotektivní účinek N-acetylglukosaminu u potkanů s akutním poškozením ledvin
- Teorie a praxe lékopisné kontroly jakosti léčiv a pomocných látek X. Počet paralelních stanovení, zpracování výsledků a jejich použití při hodnocení obsahu léčivých a pomocných látek v Evropském lékopisu (Ph. Eur.)*
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



