-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaStudy of local anaestetics – Part 192*
Formulation of lidocaine into gels with anti-inflammatory effect
Štúdium lokálnych anestetík – časť 192
Formulácia lidokaínu do gélov s protizápalovým účinkomPríspevok pojednáva o formulácii hydrogélov s protizápalovým účinkom pre orálne použitie. Na základe liberácie lidokaínu z hydrogélov s prísadou chloridu zinočnatého a roztoku Bukofitu a tokových vlastností pripravených dermálnych polotuhých liekov sa ukázalo, že prísada chloridu zinočnatého a Bukofitu pozitívne ovplyvňuje liberáciu lidokaínu a vlastnosti samotného gélu.
Kľúčové slová:
gély – lidokaín – Carbopol – antiflogistiká
Authors: J. Čižmárik 1; D. Matušová 2; Z. Vitková 3; B. Brázdovičová 3; P. Herdová 3
Authors place of work: Comenius University Bratislava, Faculty of Pharmacy, Department of Pharmaceutical Chemistry 1; Slovak Medical University, Department of Pharmaceutical Technology 2; Comenius University Bratislava, Faculty of Pharmacy, Department of Galenic Pharmacy 3
Published in the journal: Čes. slov. Farm., 2010; 59, 277-279
Category: Krátká sdělení
Summary
The paper deals with the formulation of hydrogels with anti-inflammatory effect for oral application. Liberation of lidocaine from hydrogels with zinc chloride and Bukofit solution as well as the rheological properties of the prepared dermal semisolid dosage forms revealed that addition of zinc chloride and Bukofit positively influences liberation of lidocaine and the properties of the gel itself.
Key words:
gels – lidocaine – Carbopol – anti-inflammatory drugsIntroduction
The aim of this study was the formulation of an anti-inflammatory gel for application on the oral mucosa with lidocaine, Bukofit solution (an alcoholic plant extract with anti-inflammatory effect) and zinc chloride, the gel-forming agent being Carbomer. Liberation of lidocaine through a hydrophilic membrane into artificial saliva and the influence of particular components on structural viscosity of these gels was evaluated.
Diseases of the oral mucosa occur very frequently and patients ask for an efficient and fast applicable dosage form, which cures inflammation, lowers pain and eliminates malodour discomfort in mouth. Lidocaine acts as a local anaesthetic.
Bukofit solution, which is also used in this cases, is an alcoholic extract of the radix of Rheum palmatum L. Polygonaceae (flavonoids with anti-inflammatory effect and adstringent tanstuffs) and of the herb of Salvia officinalis L. Lamiaceae (ethereal oil and diterpenes with antibacterial effect), the content of ethanol in extract is min. 50% w/w 1). Bukofit solution contains salicylic acid (a nonsteroidal anti-inflammatory drug).
Zinc chloride has anti-inflammatory effect; zinc compounds can lower the sensibility of the dental neck, the amount of anaerobic bacteria and malodour from mouth. They also help to reduce building of tartar.
Gels with the gel-creating agent Carbomer can be well used as mucoadhesive dosage forms, e.g. dental gels 2). The concentration of the gel-forming agent and the concentration of Bukofit (6% w/w) and ZnCl2 (0.3% w/w) were taken from previous studies 3). The content of lidocaine was 0.5, 1 and 2% w/w, respectively, in evaluated gels. In this study we measured the drug release (release of lidocaine) through a hydrophilic membrane into artificial saliva. The acidity of prepared gels and the influence of particular components on structural viscosity of these gels were evaluated.
EXPERIMENTAL PART
Materials
Lidocaine hydrochloride (LID) was obtained from Astra (Sweden), Bukofit solution (BUK) from Calendula a.s. Nová Ľubovňa (Slovak Republic), zinc chloride (ZnCl2) from Lachema o.p. Brno (Czech Republic), Carbopol® 940 from Noveon, Inc. (USA), Tween 80 from Sklochem-Agroekolab, Zvolen (Slovak Republic), Trolaminum, artificial saliva, and hydrophilic membrane from Chemosvit Svit (Slovak Republic).
Instruments
Spectrophotometer – Philips Pyll Unicam Ltd., Cambridge (United Kingdom), permeation apparatus – R & D Laboratory of the Department of Galenic Pharmacy, Faculty of Pharmacy, Comenius University in Bratislava (Slovak Republic), pH meter – Metrohm AG (Switzerland), Viscotester VT 500 – Haake Mess-Technic GmbH (Germany).
Preparation of hydrogel
1.5% Carbopol® 940 hydrogels were prepared without and with lidocaine chloride of 0.5; 1.0 and 2.0% (w/w), other additives being Tween 80, 0.1% (w/w), Bukofit solution (BUK), 6% (w/w), and zinc chloride (ZnCl2), 0.3% (w/w). The mixture was neutralised by trolaminum, then homogenised and left to stand for 7 days.
Evaluation of LID release
A series of six permeation chambers was used. The amount of 3.0 g of the hydrogel under study was placed in the donor chamber and 20 ml of artificial saliva was placed into each acceptor part. The acceptor phase was mixed with a magnetic stirrer. LID was left to permeate at 37 °C through a hydrophilic membrane into the artificial saliva. The amounts of the released drug were determined by spectrophotometry at λ = 261.5 nm after 30, 60 and 90 min. The results were evaluated from released cumulative amounts of LID and represent the averages of 6 measurements.
Determination of rheological properties
Rheological properties were measured by a Viscotester VT 500 at 20 °C (three parallel measurements) 7 days after preparation.
Evaluation of pH of hydrogels
Acidity – pH of hydrogels was measured 7, 21 and 28 days after preparation at 20 °C.
RESULTS
By assessing lidocaine release from hydrogels, the amount of the released lidocaine was found to increase in time (30, 60, 90 min). The best results (statistically significant) were obtained from the hydrogel with 2%(w/w) of lidocaine. Additions of zinc chloride and Bukofit separately and also together increased the amount of the released lidocaine trough the hydrophilic membrane by 0.5%, 1% and 2% of lidocaine in Carbopol gels with one exception of 2% lidocaine gel with ZnCl2 as follows from Table 1.
Tab. 1. Release of lidocaine (LID) from Carbopol gels alternatively containing Bukofit solution (BUK) and/or zinc chloride (ZnCl<sub>2</sub>) after 30, 60 and 90 min 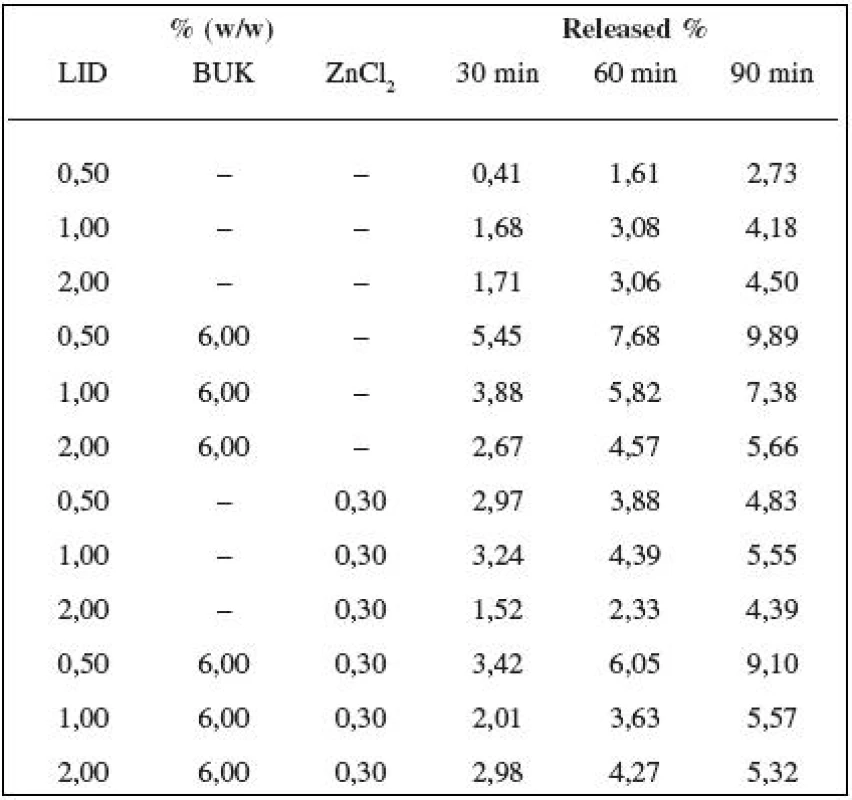
The prepared gels showed week acidity (pH 5.8–6.7).
The evaluation of rheological properties has revealed that with increasing concentration of lidocaine the structural viscosity of hydrogels is decreased. This can be caused by the acidic character of lidocaine hydrochloride, which could destroy some of the net structures of Carbopol gel. It could be useful to prepare an alcoholic solution of lidocaine base and then prepare a gel with it. In that way it would be possible to theoretically increase the penetration of lidocaine without an unwanted effect on viscosity. Addition of ZnCl2 (see Fig. 1) changed the thixotropic character of Carbopol gels to plastic flow. This could be a benefit – a fast regeneration of viscosity which was decreased by mechanic stress. Applied plastic gels stay longer in place, without turning to sols. It is expected that these results will be affirmed in gels from other Carbopols (Carbopol 974P).
Fig. 1. Rheogram of 1.5% Carbopol 940 hydrogel neutralised with trolaminum (TEA) containing Tween 80 and 1% lidocaine hydrochloride (LID) and the same gel with addition of 0.3% ZnCl<sub>2</sub> 
Received 20. October 2010 / Accepted 16. November 2010
Adresa pre korešpondenciu:
PharmDr. Petra Herdová
Katedra galenickej farmácie FaF UK
Odbojárov 10, 832 32 Bratislava, Slovenská republika
e-mail: herdova@fpharm.uniba.sk
Zdroje
1. Enterprise norm Calendula, a.s. Nová Ľubovňa, PN-08-002-02 (2002) Bukofit solution.
2. Kolodziejska, J.: Carbopol 974 P in the prescription of dental anti-inflammatory hydrogels. Polim. Med., 2008; 38, 27–38.
3. Brázdovičová, B., Vitková, Z., Grančaiová, K., Liščáková, A.: Influence of exogenous factors on rheological properties of Carbopol 940 hydrogels. Farm. Obzor., 2008; 77, 211–214.
Štítky
Farmacie Farmakologie
Článek vyšel v časopiseČeská a slovenská farmacie
Nejčtenější tento týden
2010 Číslo 6- Psilocybin je v Česku od 1. ledna 2026 schválený. Co to znamená v praxi?
- Ukažte mi, jak kašlete, a já vám řeknu, co vám je
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
-
Všechny články tohoto čísla
- Orodispersable dosage forms and technologies used in their production
- The use of authorized medicinal products for human use in veterinary practice in the Czech Republic
- A study of the process of homogenization of powder mixtures using NIR spectroscopy
-
Study of local anesthetics. Part 193.
Study of temperature and salt-induced micellization of hepatacainium perchlorate in aqueous solution -
Study of local anaestetics – Part 192*
Formulation of lidocaine into gels with anti-inflammatory effect - On the history of the Pharmacy of the Brothers of Mercy in Skalica till the year 1919
- Z činnosti farmaceutických společností
- Doc. RNDr. Mária Stankovičová, CSc., ocenená Weberovou cenou SFS
- Autorský a věcný rejstřík
- Česká a slovenská farmacie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Orodispersable dosage forms and technologies used in their production
- A study of the process of homogenization of powder mixtures using NIR spectroscopy
- On the history of the Pharmacy of the Brothers of Mercy in Skalica till the year 1919
- The use of authorized medicinal products for human use in veterinary practice in the Czech Republic
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



