-
Medical journals
- Career
A Decade of Therapy Targeting B Lymphocytes in MS
3. 1. 2024
Ocrelizumab is not only a pioneer but also a highly effective and still promising modality for the treatment of multiple sclerosis (MS) with excellent patient adherence and persistence.
Times Are Changing
At the beginning of the 21st century, most neurologists were skeptical about a drug for multiple sclerosis (MS) targeting B lymphocytes. After all, T lymphocytes are to blame in MS! However, the results of the OPERA I and II clinical trials were a breakthrough. In patients with relapsing-remitting MS (RRMS) treated with ocrelizumab, a monoclonal antibody targeting B lymphocytes, at week 96 compared to patients treated with interferon, there was a:
- 47% reduction in the annual relapse rate (ARR),
- 33% slowing of disability progression.
Leading in Primary Progressive MS Treatment
Ocrelizumab also holds another first—it is currently the only biological treatment for primary progressive MS funded by public health insurance in the Czech Republic. The ORATORIO study confirmed a 24%, and then 29.6%, lower relative risk of disability progression after 12 and 24 weeks, respectively, in the ocrelizumab-treated group compared to the placebo group.
How to Treat? Early and Intensively
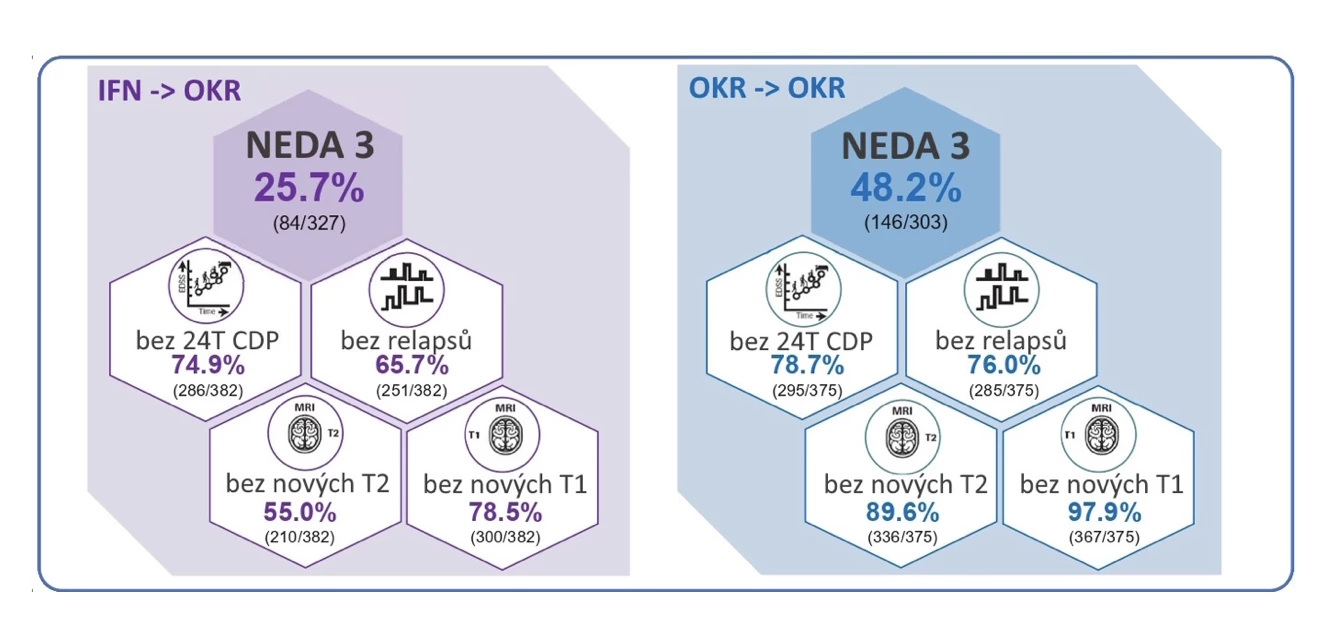
But the successes of ocrelizumab do not end there. In 2022, it became the first drug from the highly effective MS therapy group funded for patients with RRMS from their first attack. The superior effect of its early initiation is also confirmed by long-term data from the extensions of the OPERA I and II studies. After 9 years of therapy, the status of NEDA (no evidence of disease activity) was achieved in 48.2% of patients treated with ocrelizumab from the start compared to 25.7% of those who initially received interferon (see Fig. 1).
Fig. 1 9-year efficacy data from the extensions of the OPERA I and II studies
Safety First
The essential condition for any therapy is safety. Besides upper respiratory tract infections, infusion reactions and flu-like symptoms were most frequently recorded in the registration studies with ocrelizumab. Given the immunosuppressive action mechanism affecting antitumor immunity, there is a significant question regarding the potential incidence of malignancies. Although previous observations suggested a higher incidence of breast cancer, recently published 7-year data did not confirm a higher risk in women treated with ocrelizumab. However, long-term monitoring remains advisable.
Adherence and Persistence on Top
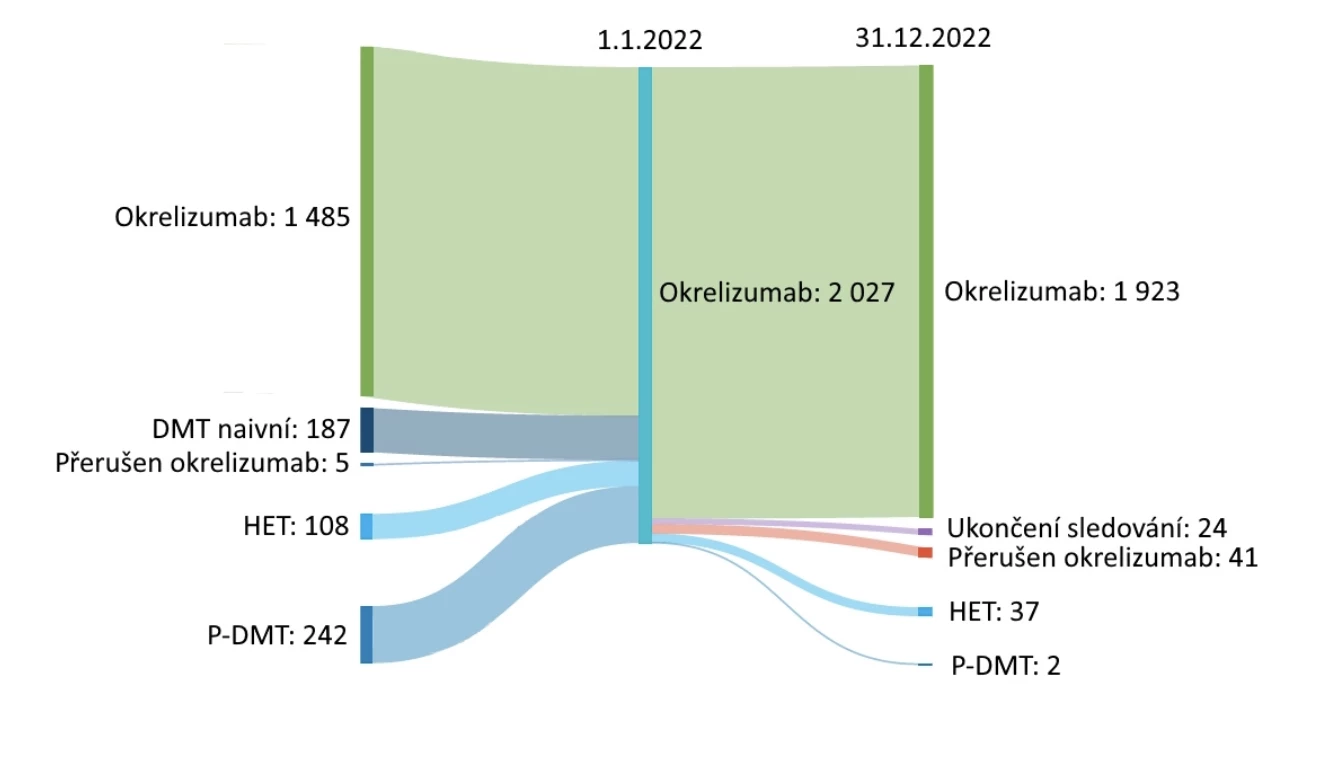
Considering the semi-annual infusion administration of ocrelizumab, the adherence and persistence with this drug are truly excellent compared to many other MS therapies (see Fig. 2). This is also confirmed by data from the Czech ReMuS registry. As of January 1, 2022, 1485 patients in the Czech Republic were treated with ocrelizumab, and by December 31, 2022, 95% of them continued the therapy. An additional 542 patients started ocrelizumab therapy during 2022 (see Fig. 3).
Fig. 2 Persistence after 9 years of ocrelizumab therapy and reasons for discontinuation of follow-up/therapy in the OPERA I, II studies and their extensions
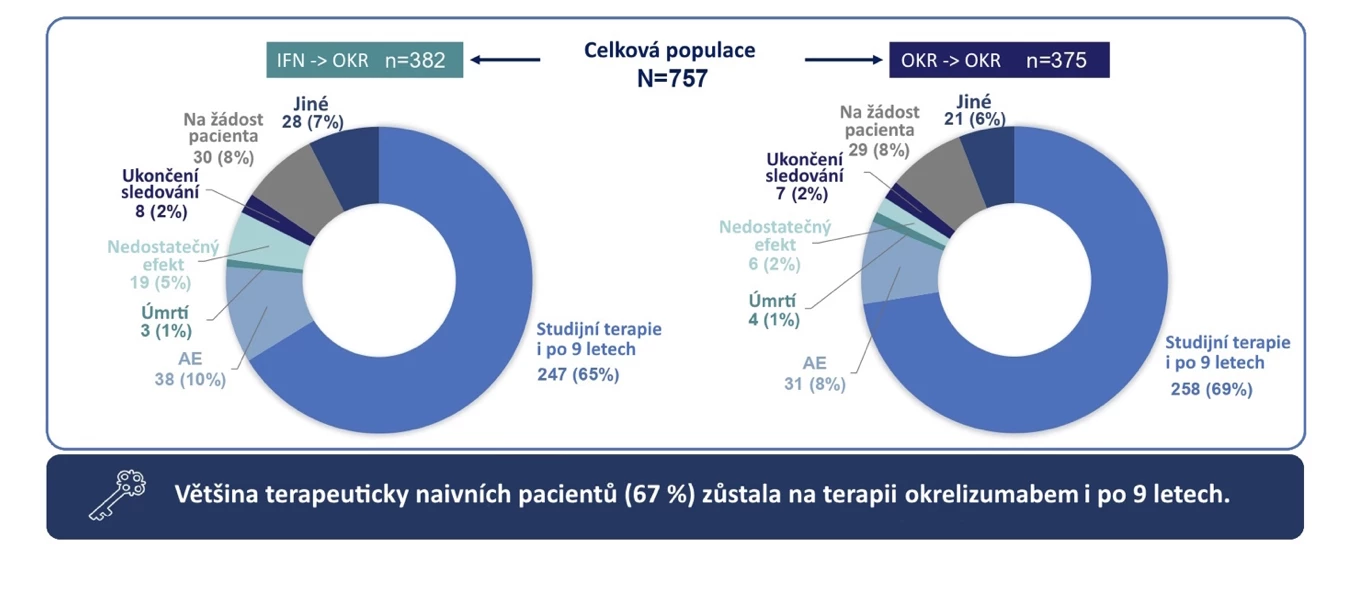
Fig. 3 Current Czech data on adherence and persistence of ocrelizumab therapy – ReMuS registry
(dos)
Sources:
1. Šťastná D., Horáková D. A Decade of Therapy Targeting B Lymphocytes - Adherence and Persistence with Ocrelizumab on Top. Multiple Sclerosis News 2023; 9 (2): 23–28.
2. Hauser S. L., Bar-Or A., Comi G. et al. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med 2017; 376 (3): 221–234, doi: 10.1056/NEJMoa1601277.
3. Montalban X., Hauser S. L., Kappos L. et al. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N Engl J Med 2017; 376 (3): 30–31, doi: 10.1056/NEJMoa1606468.
4. Cerqueira J., Berthele A., Cree B. et al. Long-term treatment with first-line ocrelizumab in patients with early RMS: 9-year follow-up data from the OPERA trial. 5th annual meeting of the American Academy of Neurology (AAN), Boston, April 22–27, 2023.
5. Nicholas J. A., Edwards N. C., Edwards R. A. et al. Real-world adherence to, and persistence with, once - and twice-daily oral disease-modifying drugs in patients with multiple sclerosis: a systematic review and meta-analysis. BMC Neurol 2020; 20 (1): 281, doi: 10.1186/s12883-020-01830-0.
6. Engmann N. J., Sheinson D., Bawa K. et al. Persistence and adherence to ocrelizumab compared with other disease-modifying therapies for multiple sclerosis in U.S. commercial claims data. J Manag Care Spec Pharm 2021; 27 (5): 639–649, doi: 10.18553/jmcp.2021.20413.
7. Moccia M., Affinito G., Berera G. et al. Persistence, adherence, healthcare resource utilization and costs for ocrelizumab in the real-world of the Campania region of Italy. J Neurol 2022; 269 (12): 6504–6511, doi: 10.1007/s00415-022-11320-7.
Did you like this article? Would you like to comment on it? Write to us. We are interested in your opinion. We will not publish it, but we will gladly answer you.
Labels
Neurology
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career


