-
Medical journals
- Career
Excellent effect of sotorasib in a higher line of treatment in a patient with NSCLC with G12C mutation of KRAS – a case study
30. 10. 2024
We present a case study of an elderly patient with non-small cell lung cancer (NSCLC) treated with the selective KRASG12C inhibitor sotorasib in the 5th line of oncological treatment, but with a very exceptional effect.
Introduction
Lung tumors are among the deadliest oncological diagnoses both in the Czech Republic and worldwide (ÚZIS 2021, Globocan 2022). Despite this fact, the area of non-small cell lung cancer (NSCLC) in particular has seen incredible development (refinement of diagnostics, development of treatment strategies, next-generation sequencing). NSCLC thus breaks down into a very heterogeneous group including mutation-driven malignancies and tumors without any predictors. The prognosis of individual patients can therefore vary significantly. In some cases, lung cancer can even be referred to as a chronic disease.1 Each year, along with the growing level of knowledge, the portfolio of preparations with which we can treat patients in accordance with the principle of personalized oncological therapy is expanded even in our conditions.
One of the recently very discussed parameters is the KRAS mutation. In the Caucasian population, it occurs with a frequency of 23–40%, and it is more often detected in smokers.2 Its existence has been known for a long time, but it was perceived only as a negative prognostic factor – the presence of the KRAS mutation in NSCLC is associated with shorter overall survival. Due to the absence of an inhibitor targeting this molecular genetic change, it was not routinely tested either. However, we now have an effective KRAS inhibitor, sotorasib, which targets the KRASG12C subtype. The incidence in the European population is 13–16%, and it is often found as a comutation together with other mutations such as EGFR or ROS1.3
In clinical studies, sotorasib has demonstrated superiority over docetaxel in pretreated patients with NSCLC with KRASG12C positivity. In the Czech Republic, it has been reimbursed for the 2nd line treatment of advanced NSCLC with KRASG12C mutation since spring 2023.
Case Description
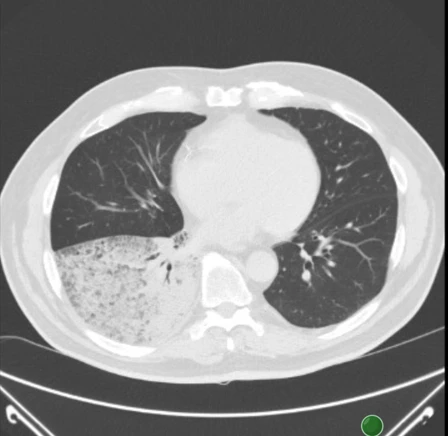
A 73-year-old man, previously treated for hypertension, ischemic heart disease (IHD), and chronic obstructive pulmonary disease (COPD), a retiree and former smoker, was examined in June 2021 for shortness of breath and productive cough. According to X-ray and CT of the chest, a diffuse infiltration of most of the lower lobe of the right lung, T4N1M0 (see Figures 1 and 2), was observed. Lung adenocarcinoma with lepidic spread was diagnosed using bronchoscopy. At that time, the commonly determined predictors EGFR, ALK, and ROS1 were negative, and the expression of the programmed cell death 1 (PD-L1) receptor ligand was <1%. The patient was in very good clinical condition with a performance status (PS) of 0–1.
Figure 1 Chest X-ray at the time of diagnosis (6/2021)
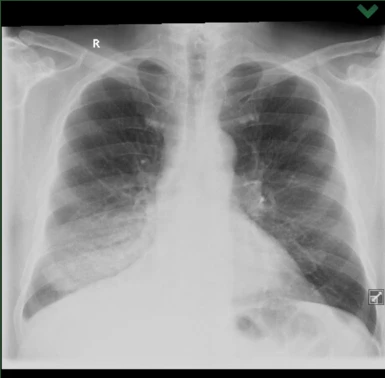
Figure 2 Chest CT at the time of diagnosis (6/2021)
Before starting oncological treatment, an empyema of the right chest developed, necessitating chest drainage and combined antibiotic treatment. The complication was finally successfully managed, and in July 2021, the 1st line treatment was initiated with a combination of carboplatin/vinorelbine/bevacizumab (according to the then standard of care). Four cycles of oncological therapy were administered without complications; however, disease progression was evident on follow-up chest CT. From September 2021 to January 2022, the patient received 5 cycles of atezolizumab in the 2nd line, unfortunately with further disease progression. Docetaxel was chosen for the 3rd line, with a total of 4 cycles of chemotherapy administered from February to April 2022, although its effect was very short-lived. By June 2022, tumor progression was again detected. Erlotinib was initiated in the 4th line, but this treatment was discontinued after one month due to further deterioration.
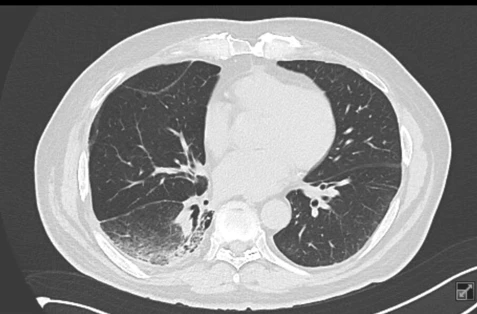
Given the patient's sustained excellent clinical condition, we considered what further options could be offered. We decided on next-generation sequencing (NGS) from the original tumor sample. The result was very unexpected – a KRASG12C mutation was detected. In light of the exhausted possibilities of oncological treatment, we applied for individual approval from the health insurer, which was granted. Thus, we initiated sotorasib treatment for the patient in December 2022 (see Figure 3). Initially, an increase in blood pressure was observed, which was compensated with an increased dose of antihypertensives without major issues. A mild rash on the trunk also appeared but resolved spontaneously. After 6 months of sotorasib treatment, a significant regression of the tumor infiltration in the lower lobe of the right lung was observed on follow-up chest CT (see Figure 4). The patient felt very well physically, and the expectoration of sputum, which is typical for lepidic-type lung carcinoma, significantly decreased.
The patient has been treated with sotorasib for 1.5 years to date, with very good effect and minimal toxicity. The most recent chest CT shows only residual tumor infiltration in the right lung base (see Figure 5).
Figure 3 Chest CT at the time of initiation of sotorasib treatment (12/2022)

Figure 4 Chest CT after 6 months of sotorasib treatment – partial regression

Figure 5 Chest CT after 1.5 years of sotorasib treatment – further partial regression, only residual infiltration in the right lower lobe (3/2024)
Discussion
Sotorasib has become a new addition to the treatment strategy for patients with NSCLC with KRASG12C positivity. According to the phase III registration study, it achieved a time to progression (PFS) in pretreated patients compared to docetaxel of 5.6 vs. 4.5 months (hazard ratio [HR] 0.66; p = 0.0017).3 Its effectiveness has also been noted in pretreated patients with colorectal cancer with KRASG12C mutation.4 The drug is taken daily at a dose of 960 mg/day until progression or the emergence of unacceptable toxicity. The most common adverse effects include anemia, headaches, nausea, and elevated liver tests.
Given these new possibilities, it is crucial to include KRAS mutation testing at the time of diagnosis. In the patient from our case study, KRASG12C positivity was detected based on NGS only after many lines of oncological treatment. Despite the patient's significant pre-treatment, sotorasib subsequently demonstrated an very exceptional effect. The patient has been treated for over 1.5 years without signs of progression. However, without this finding and the resulting targeted therapy, a rapid deterioration of condition and death would have probably followed.
Another rarity of our case is that it involved the lepidic type of lung adenocarcinoma, which is very rare. At the same time, the diagnosis of this type of tumor is very challenging. It is often mistaken for pneumonia or an interstitial lung process, and the time to final diagnosis is therefore quite long. However, if lepidic lung adenocarcinoma is detected at an early stage and surgically removed, its prognosis is significantly better than that of solid tumor types.5 Advanced and disseminated stages of the disease are, on the other hand, harder to influence and their survival shorter. Due to the nature of the involvement, some treatment modalities, such as radiotherapy, cannot be used, and the tumor extent is difficult to measure due to its infiltrative growth. In addition to dyspnea, a typical symptom is also massive expectoration of thin sputum – interestingly, if the treatment is effective, this symptom can be positively affected.
Conclusion
Diseases from the NSCLC group are currently the driver of personalized oncological therapy. The inexorable trend is the early detection of targeted treatment predictors using NGS and the arrival of new effective substances and treatment strategies. Prolonged survival can be caused by highly effective targeted therapy, appropriate chaining of individual treatment lines, or a combination of both (as in our case study). Sotorasib represents a new effective tool for patients with KRASG12C mutation, making the poor prognosis associated with this mutation relative.
MUDr. Monika Bratová
Clinic of Pulmonary Diseases and TBC LF MU and FN BrnoReferences:
1. Bratová M., Karlínová B., Skřičková J. et al. Non-small cell lung cancer as a chronic disease – a prospective study from the Czech TULUNG registry. In Vivo 2020; 34 (1): 369–379, doi: 10.21873/invivo.11783.
2. Wu S. G., Liao W. Y., Su K. Y. et al. Prognostic characteristics and immunotherapy response of patients with nonsquamous NSCLC with Krasmutation in East Asian populations: a single-center cohort study in Taiwan. JTO Clin Res Rep 2020; 2 (2): 100140, doi: 10.1016/j.jtocrr.2020.100140.
3. de Langen A. J., Johnson M. L., Mazieres J. et al. Sotorasib versus docetaxel for previously treated non-small-cell lung cancer with KRASG12C mutation: a randomised, open-label, phase 3 trial. Lancet 2023; 401 (10378): 733–746, doi: 10.1016/S0140-6736(23)00221-0.
4. Hong D. S., Fakih M. G., Strickler J. H. et al. KRASG12C inhibition with sotorasib in advanced solid tumors. N Engl J Med 2020; 383 (13): 1207–1217, doi: 10.1056/NEJMoa1917239.
5. Sato D., Matsubara H., Matsuoka H. et al. Lepidic growth component as a favorable prognostic factor in non-small cell lung cancer of ≤3 cm. Thorac Cancer 2022; 13 (23): 3274–3283, doi: 10.1111/1759-7714.14680.
Did you like this article? Would you like to comment on it? Write to us. We are interested in your opinion. We will not publish it, but we will gladly answer you.
Labels
Clinical oncology Pneumology and ftiseology
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career


