-
Medical journals
- Career
Reduction of Inflammation in Vertebral Bodies and Posterolateral Structures of the Spine in Patients with Ankylosing Spondylitis Treated with Tofacitinib
23. 11. 2023
A post hoc analysis of a phase II study with tofacitinib in patients with active ankylosing spondylitis (AS) evaluated the suppression of spinal inflammation using magnetic resonance imaging (MRI) based on the Canadian-Danish scoring system (CANDEN), which allows for the assessment of changes in posterolateral structures of the spine, characteristic of AS involvement.
Location of Inflammatory Lesions in the Spine
In patients with AS, lesions of the posterolateral structures of the spine are often present, which may be more common in early axial AS than changes in vertebral bodies. Inflammation of the posterolateral structures can disrupt the mobility and function of the spine. Existing AS scoring systems based on MRI images do not include some spinal structures affected by AS.
The CANDEN scoring system was developed for a comprehensive and detailed assessment and quantification of inflammation (bone marrow edema) and structural lesions (fat deposits, bone erosion, and bone formation) in all parts of the spine including the posterolateral structures, i.e., costovertebral joints, transverse and spinous processes, ribs, and soft tissues (see Figure 1).
Figure 1 Anatomical localization of lesions in the spine of patients with AS assessed on MRI images according to the CANDEN scoring system
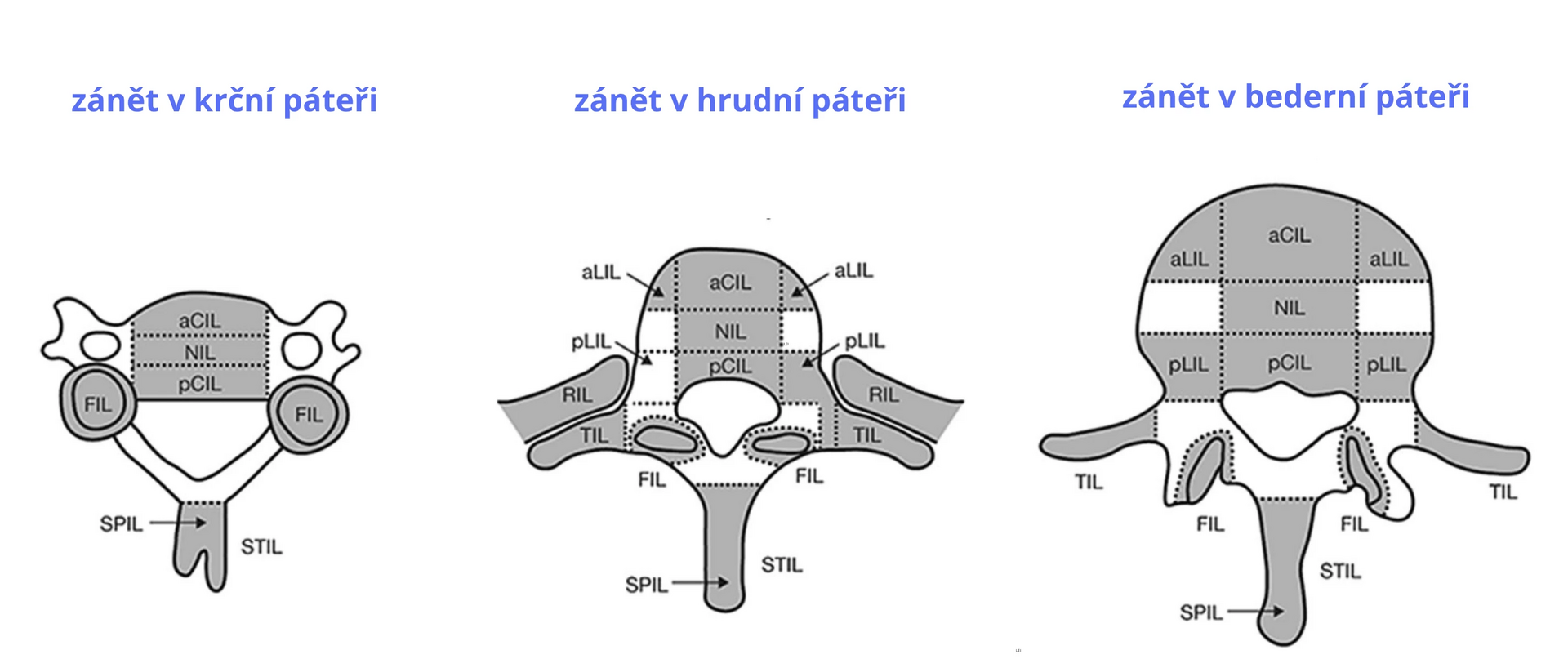
Note: aCIL – inflammatory lesion of the anterior part of the vertebral body; aLIL – inflammatory lesion of the anterior lateral part of the vertebral body; FIL – inflammatory lesion of intervertebral joints; NIL – inflammatory lesion of the vertebra outside the corner; pCIL – inflammatory lesion of the posterior part of the vertebral body; pLIL – inflammatory lesion of the posterior lateral part of the vertebral body; RIL – inflammatory lesion of the rib; SPIL – inflammatory lesion of the spinous process; STIL – inflammatory lesion of soft tissues; TIL – inflammatory lesion of the transverse process.
Anti-inflammatory Effect of Tofacitinib
Tofacitinib is an oral Janus kinase inhibitor (JAKi) indicated for the treatment of AS in adult patients. Its efficacy and safety were evaluated in patients with active AS and inadequate response or intolerance to nonsteroidal anti-inflammatory drugs (NSAIDs) in a 16-week phase II study and a 48-week phase III study.
In the phase II study, tofacitinib at doses of 5 or 10 mg twice daily demonstrated greater clinical efficacy than placebo in relieving AS symptoms and its safety was similar to its use in other indications. MRI assessment in this study showed significant reduction in SPARCC scores in the sacroiliac joint and spine after 12 weeks of tofacitinib treatment compared to baseline, and also compared to placebo. Until the publication of the below-cited post hoc analysis, the efficacy of tofacitinib on inflammation of entheses and joint connections of posterolateral spinal structures had not been evaluated.
Analysis Methodology
The recently published post hoc analysis of the described phase II study evaluated the efficacy of tofacitinib on inflammation and structural lesions of the spine on MRI according to the CANDEN scoring system in patients with active AS. In the 16-week, double-blind study, patients were randomized to receive tofacitinib 2, 5, or 10 mg twice daily or placebo. For the purposes of the post hoc analysis, MRI images of patients receiving tofacitinib at 5 or 10 mg twice daily or placebo were independently assessed by two blinded radiologists evaluating changes according to the CANDEN scoring system between baseline and week 12 of the treatment.
Within the MRI image assessment, the following scores were calculated:
- Total spine inflammation score: sum of aCIL, pCIL, aLIL, pLIL, FIL, NIL, TIL, RIL, SPIL, and STIL.
- Vertebral body inflammation subscore: sum of aCIL, pCIL, aLIL, and pLIL.
- Posterior spinal structures inflammation subscore: sum of FIL, TIL, RIL, SPIL, and STIL.
- Corner inflammation subscore: sum of aCIL and pCIL at 23 levels (C2/C3–L5/S1), aLIL at levels C7/Th1–L5/S1, and pLIL at levels Th12/L1–L5/S1.
- Non-corner vertebral body inflammation subscore: sum of NIL.
- Intervertebral joint inflammation subscore: sum of FIL.
- Posterolateral structures subscore: sum of TIL, SPIL, and STIL at 23 levels, RIL and pLIL at levels C7/Th1–Th11/Th12.
Results
Data from 137 patients were analyzed. After 12 weeks of the evaluated treatment, the spine inflammation scores and individual subscores for vertebral body inflammation, posterior structures, vertebral corner, non-corner vertebral body, intervertebral joints, and posterolateral structures were significantly more reduced with tofacitinib (combined data of both doses) compared to placebo (all p < 0.0001, except for non-corner vertebral body subscore where p < 0.05). Treatment with tofacitinib also resulted in a numerical increase in fat lesion scores, indicating a resolution of the inflammatory response.
Figure 2 Average change in MRI inflammatory lesion scores according to CANDEN with tofacitinib and placebo in AS patients at week 12 compared to baseline

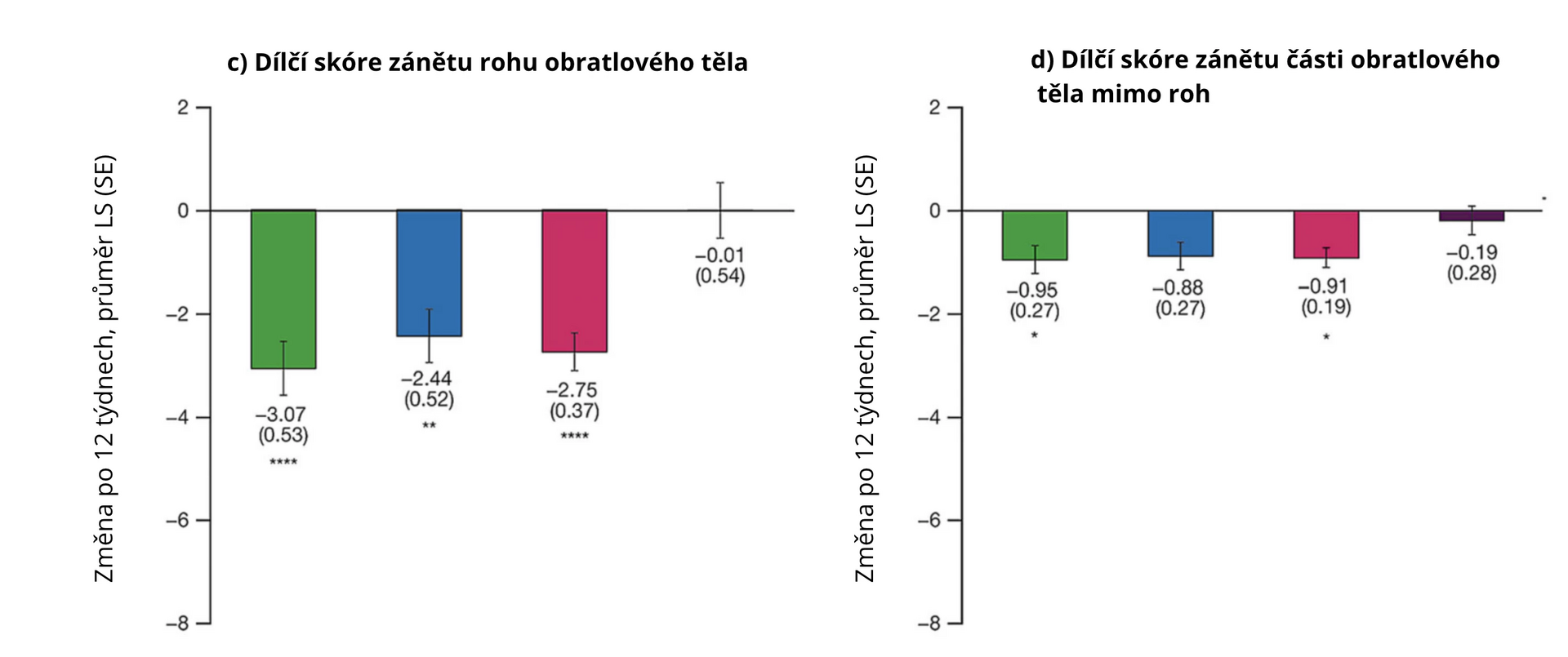
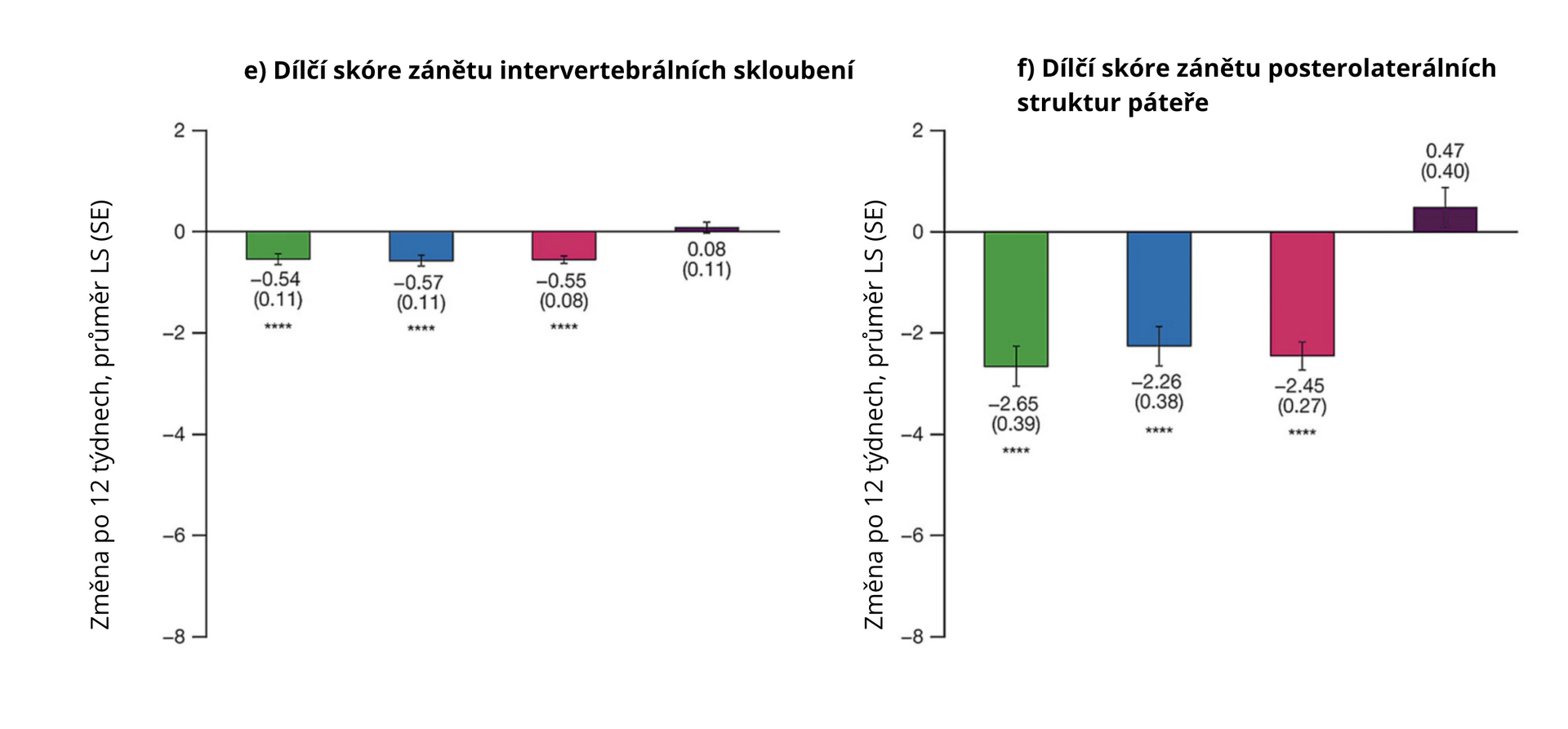
Note: LS mean – least squares mean; SE – standard error of the mean; * p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001.
Conclusion
In patients with AS, treatment with tofacitinib led to a significant reduction in spine inflammation scores on MRI assessed according to the CANDEN scoring system. In this post hoc analysis, tofacitinib also reduced inflammation in posterolateral structures of the spine and intervertebral joints, which had not been previously described.
(zza)
Source: Østergaard M., Wu J., Fallon L. et al. Tofacitinib reduces spinal inflammation in vertebral bodies and posterolateral elements in ankylosing spondylitis: results from a phase 2 trial. Rheumatol Ther 2023 Aug; 10 (4): 1001–1020, doi: 10.1007/s40744-023-00564-y.
Did you like this article? Would you like to comment on it? Write to us. We are interested in your opinion. We will not publish it, but we will gladly answer you.
Labels
Paediatric rheumatology Rheumatology General practitioner for adults
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

