-
Medical journals
- Career
Reduction of Enthesitis in Patients with Psoriatic Arthritis Taking Tofacitinib
24. 11. 2023
Inflammation in the area where a tendon, ligament, or joint capsule attaches to the bone occurs in 35–50% of patients with psoriatic arthritis (PsA). Its presence is associated with higher activity of PsA, and its intensity correlates with the degree of joint damage. Patients with enthesitis are generally in worse functional status and report greater fatigue, pain, and decreased productivity. How can treatment with tofacitinib affect this important domain of PsA?
Focus on Enthesitis
Tofacitinib is an oral Janus kinase inhibitor (JAKi) used in the treatment of PsA. Its efficacy and safety at doses of 5 mg twice daily (recommended dose) and 10 mg twice daily were demonstrated in two phase III studies from the OPAL program in patients with active PsA. In both studies, patients in the tofacitinib groups showed greater reduction in enthesitis after 3 months of treatment.
OPAL Broaden was a 12-month study involving PsA patients without prior treatment with tumor necrosis factor inhibitors (TNFi), who also had insufficient response to conventional synthetic disease-modifying antirheumatic drugs (csDMARDs). Patients received tofacitinib 5 or 10 mg twice daily, adalimumab 40 mg s.c. every 2 weeks, or placebo (switched to tofacitinib after 3 months). OPAL Beyond was a 6-month study that included PsA patients with an insufficient response to TNFi. These patients received tofacitinib 5 or 10 mg twice daily or placebo, also switched to tofacitinib after 3 months. In both studies, all participants used a stable dose of one csDMARD.
Analysis of Pooled Data
A recently published post hoc analysis pooled data from patients in OPAL studies who were treated with tofacitinib until month 6 or who received placebo until month 3 and assessed the effects of this drug on enthesitis in a subset of patients with this condition at study entry, based on the localization and intensity of enthesitis, and its impact on PsA activity and patient-reported outcomes (PROs).
Scoring Systems and Other Evaluated Parameters
Enthesitis in the OPAL studies was assessed using two scoring systems. The LEI score (Leeds Enthesitis Index) is based on the evaluation of tenderness at 3 bilateral sites and ranges from 0–6. The SPARCC score (Spondyloarthritis Research Consortium of Canada Enthesitis Index) evaluates tenderness at 8 bilateral sites and ranges from 0–16 (always presence/absence of tenderness at a specific anatomical site). The evaluated sites are shown in Figure 1.
Figure 1 Evaluated sites of enthesitis according to LEI and SPARCC scores
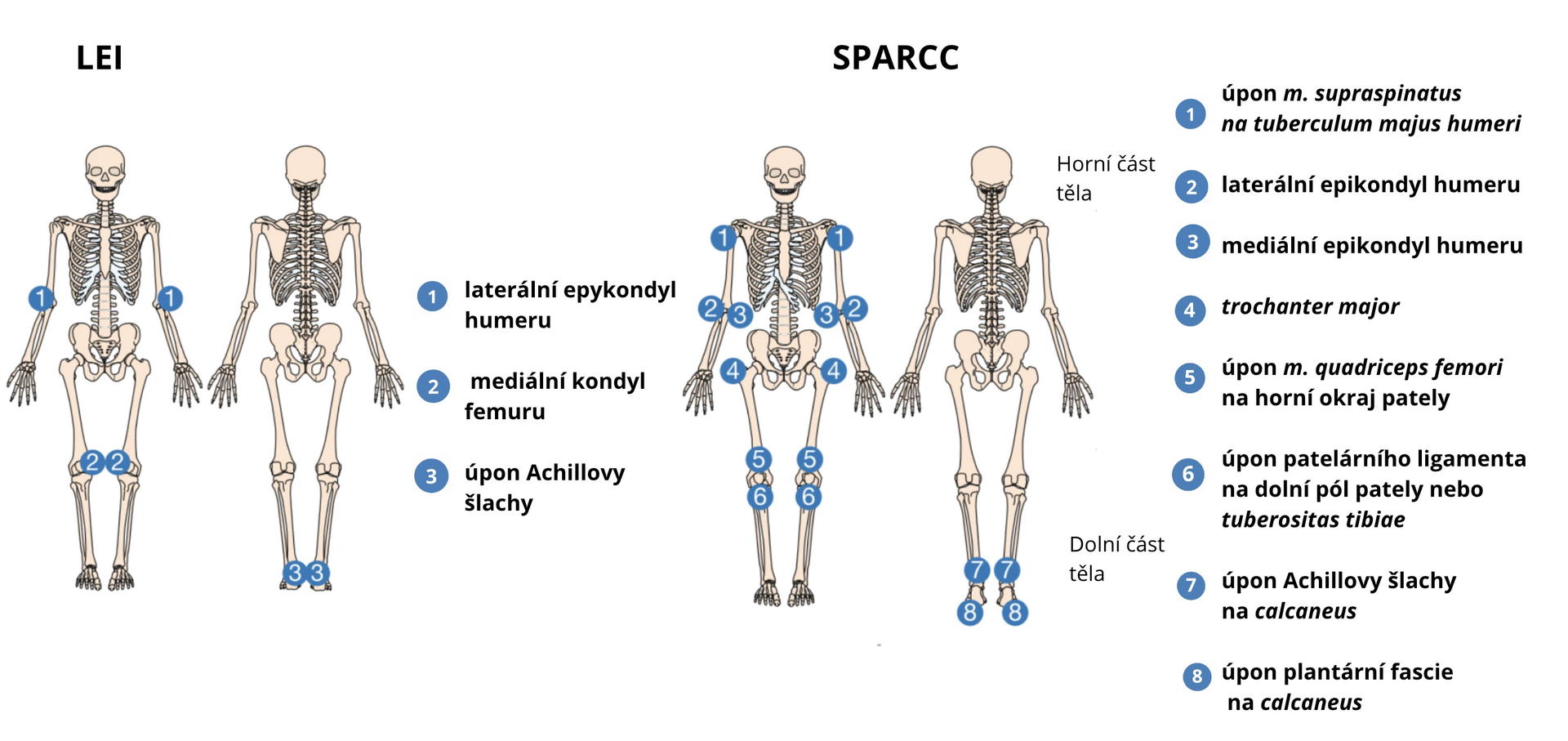
Patients were stratified by the presence (LEI > 0 or SPARCC > 0) or absence (LEI = 0 and SPARCC = 0) of enthesitis at study entry. They were further divided into 3 subgroups according to the location of enthesitis (upper body, lower body, upper and lower body) and into 3 subgroups according to the intensity of enthesitis (1, 2, or 3–6 affected sites based on LEI score and 1–2, > 2 to ≤ 5, or > 5 affected sites based on SPARCC score).
The monitored parameters included changes in LEI and SPARCC scores from study entry, the proportion of patients with enthesitis, relapses of enthesitis after resolution, occurrence of de novo enthesitis, achievement of low PsA activity or remission, scores of several PsA activity indices, and patient-reported outcomes (PROs): fatigue according to FACIT-F (Functional Assessment of Chronic Illness Therapy-Fatigue) and arthritis pain score on the visual analog scale (VAS).
Results of the Analysis
Of the 710 patients evaluated, 67.5% had enthesitis at study entry according to LEI score and 76.8% according to SPARCC score. Conversely, 19.2% did not have this complication at study entry. The demographic and baseline characteristics of patients in subgroups by location and intensity of enthesitis were mostly comparable. Higher levels of C-reactive protein (CRP) and pain scores were measured in patients with enthesitis at study entry compared to participants without enthesitis.
The average reduction in LEI and SPARCC scores over 3 months was greater across all subgroups in terms of enthesitis location and severity with tofacitinib treatment (–2.0 to 0.4 and –3.5 to 0.2, respectively) compared to placebo (–0.9 to 0.4 and –1.5 to 1.1, respectively), as shown in Figure 2. The reduction in both scores persisted for the entire 6 months regardless of baseline enthesitis location and intensity, except for patients with 1 affected site according to LEI score and 1–2 affected sites according to SPARCC score at study entry.
Figure 2 Change in LEI and SPARCC scores in patients with enthesitis at study entry with tofacitinib or placebo treatment depending on enthesitis location and severity
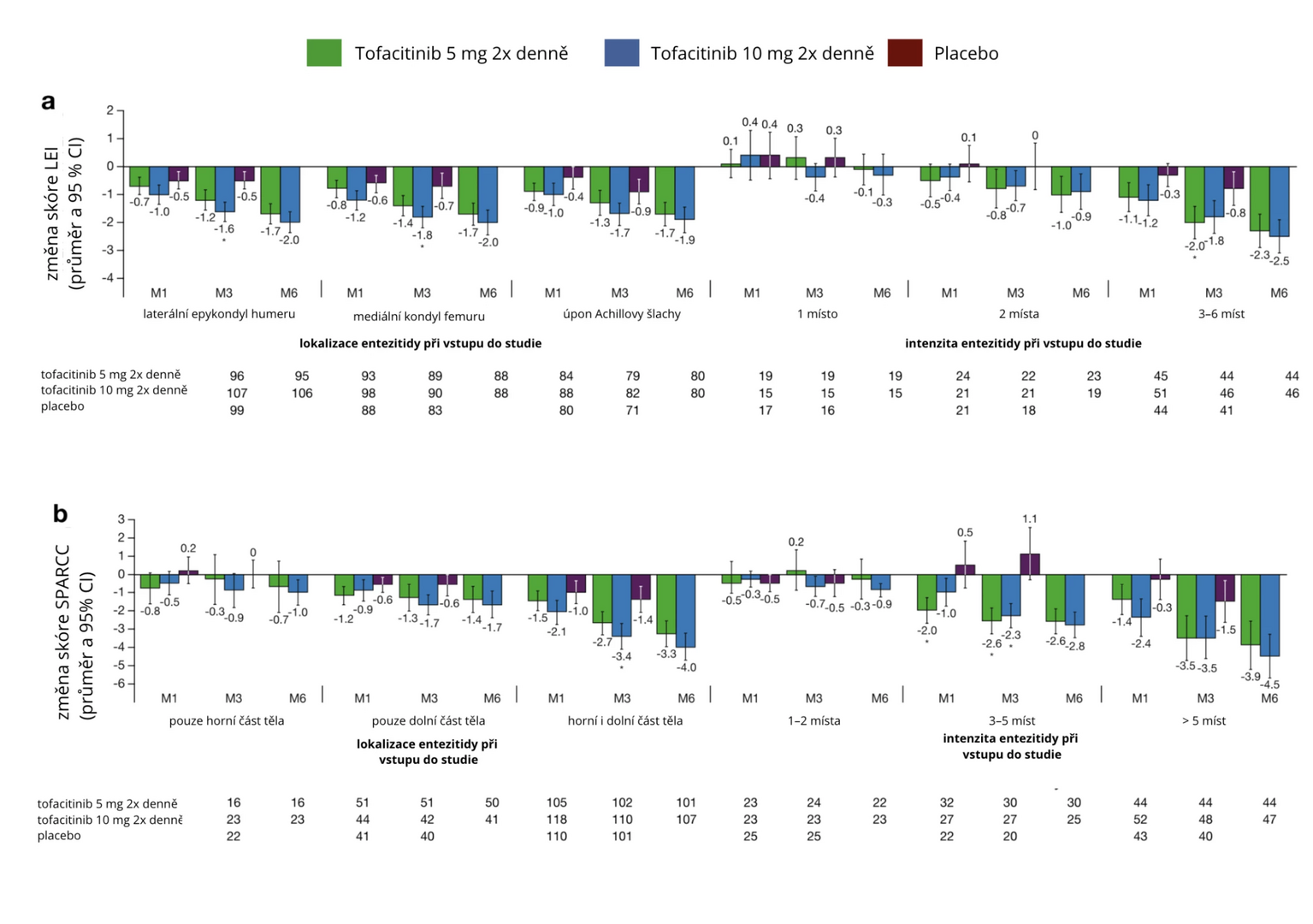
Note: M – month; CI – confidence interval.
After 6 months, the occurrence of enthesitis was higher in patients with higher baseline LEI or SPARCC scores. Among patients with enthesitis at study entry who experienced resolution of this complication within 3 months of tofacitinib treatment, the incidence of enthesitis relapses after 6 months was ≤ 40%. In patients without enthesitis at study entry, de novo enthesitis occurred in 14% of cases. The proportion of participants achieving low PsA activity/remission generally increased with tofacitinib treatment.
There was a correlation between enthesitis localized in the area of the lateral epicondyle of the humerus and the Achilles tendon attachment with reduction in arthritis pain, and between greater severity of enthesitis according to SPARCC score (> 5) and reduction in fatigue and arthritis pain with tofacitinib treatment.
Conclusion
Tofacitinib leads to a reduction in enthesitis in PsA patients regardless of its location and intensity.
(zza)
Source: Mease P. J., Orbai A. M., FitzGerald O. et al. Efficacy of tofacitinib on enthesitis in patients with active psoriatic arthritis: analysis of pooled data from two phase 3 studies. Arthritis Res Ther 2023 Aug 22; 25 (1): 153, doi: 10.1186/s13075-023-03108-5.
Did you like this article? Would you like to comment on it? Write to us. We are interested in your opinion. We will not publish it, but we will gladly answer you.
Labels
Paediatric rheumatology Rheumatology
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

