-
Medical journals
- Career
When You Say Amyloid…
12. 12. 2022
The term 'amyloid' was first used by the German doctor Rudolf Virchow in the mid-19th century. He described it as a pathological substance which he originally thought was starch. Although it was quickly discovered that it was a substance of protein nature, the reference to starch (from the Greek amylon) remained in the name.
Structure of Amyloid
For the next 100 years, the term amyloid was associated with various extracellular substances. A more detailed understanding of their structure and properties, however, came with the X-ray analysis of protein structures in the 1970s.
Amyloids are fibrous proteins. Their basic structure consists of fibers (fibrils) typically composed of 2 or more protofilaments. These form polypeptide chains arranged in the form of what is known as a beta-pleated sheet (see Fig. 1), which is one of the natural forms of the secondary structure of proteins.
Image 1. Structure of amyloid fibril (adapted from: 2) 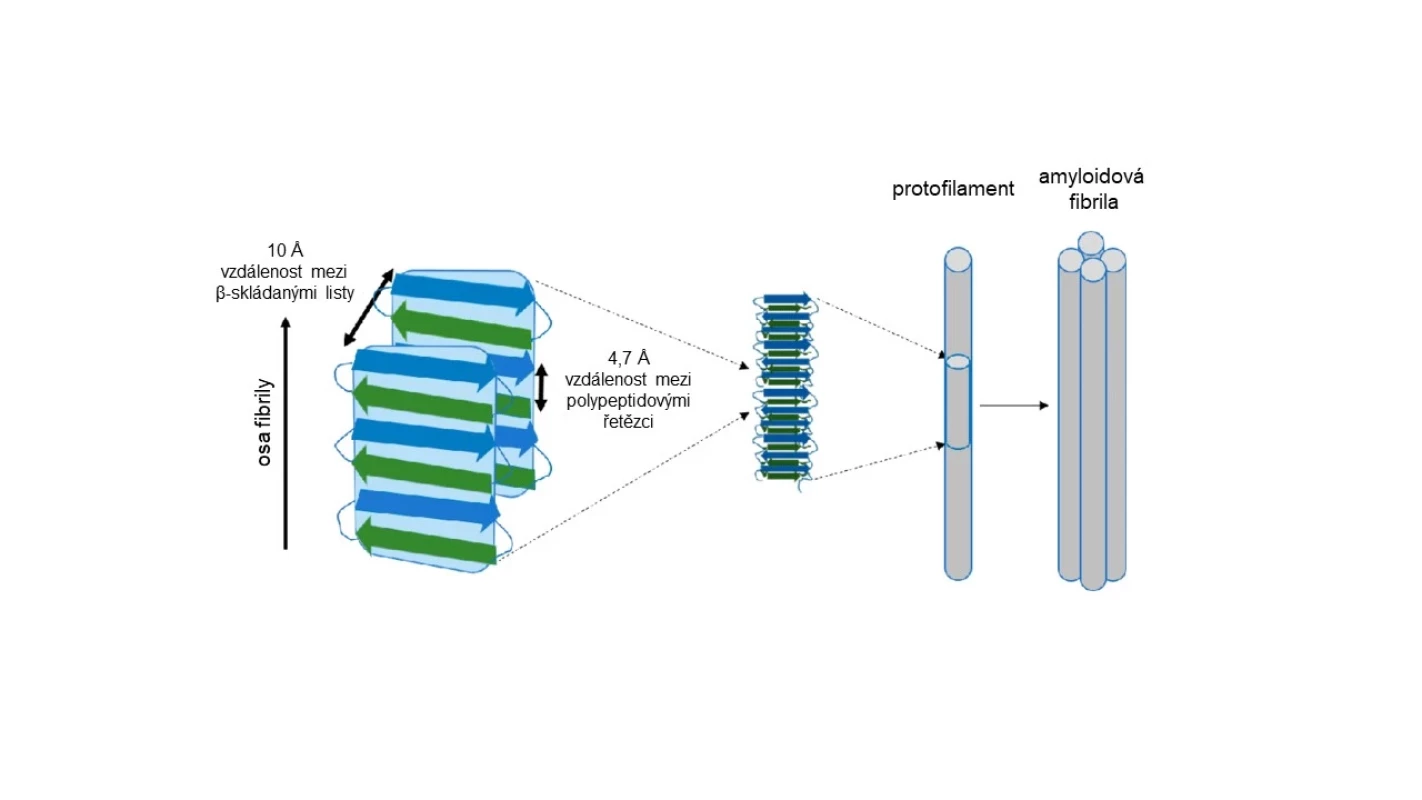
Since 2018, the official nomenclature has considered any fiber consisting of beta-pleated sheets to be an amyloid fibril. The term thus lost its original pathological connotation, which we were accustomed to in medicine, as the term amyloid was mainly used for pathological deposits of specific protein aggregates found during histopathological examination of tissues. The reason for this change was the recognition that fibrous proteins with beta-pleated sheet structures also fulfill physiological functions in nature:
- In humans, amyloid fibrils are involved in the formation of polypeptide hormone reserves or in the structure of the melanin transporter in melanosomes.
- The silkworm produces the protein fibroin with the structure of an amyloid fiber (i.e., natural silk).
- Bacteria secrete amyloid fibrils as part of the biofilm.
Amyloid in Histological Preparations
When observed by optical microscopy, amyloid appears as an amorphous substance that stains with Congo red and then exhibits specific optical properties. When polarized light passes through amyloid with intercalated dye, birefringence and color changes of the structure occur. It is most often stated that a green coloration can be observed, but recent practice clarifies that yellow and red colors can also be seen, depending on the tissue section and the orientation of the fibrils in situ.
Amyloid Deposits and Amyloidoses
In addition to functional amyloid fibers, pathological deposits can also form in the organism. These include not only the protein itself but also other proteins and proteoglycans, whose functions are not yet fully understood. Among the most well-known deposits are the so-called beta-amyloid plaques found in the cerebral cortex of patients with Alzheimer's disease (AD). The term 'plaque' is incorrect, as it suggests flat formations. In reality, amyloid fiber deposits are more spherical. While it may not be possible to eliminate the term 'plaques' from AD terminology, it should not be used for other amyloid aggregates.
Amyloidosis is therefore referred to as a disease caused by the accumulation and pathological clumping of amyloid fibrils or their improper formation. Currently, 18 amyloid proteins have been identified that cause systemic diseases, and 22 amyloids whose pathological deposits have been found locally. An example of a systemic disease is AL amyloidosis, where deposits of structurally abnormal immunoglobulin light chains accumulate in various organs, or transthyretin amyloidosis affecting, for example, peripheral nerves and the myocardium. A serious disease that occurs most frequently in acquired form in the Czech Republic is cardiomyopathy caused by transthyretin amyloidosis (ATTR-CM). Early diagnosis and subsequent therapy are crucial for improving the prognosis of these patients. A useful tool for assessing the probability of ATTR-CM in patients with heart failure is the web application wtATTR-CM estimATTR, based on a machine learning method.
Some diseases meet the characteristics of amyloidoses, but are not yet officially called this. This group includes, for example, Alzheimer's or Parkinson's disease with the occurrence of amyloid aggregates locally in the CNS.
(jam)
Sources:
1. Benson M. D., Buxbaum J. N., Eisenberg D. S. et al. Amyloid nomenclature 2020: update and recommendations by the International Society of Amyloidosis (ISA) nomenclature committee. Amyloid 2020; 27 (4): 217−222, doi: 10.1080/13506129.2020.1835263.
2. Vahdat Shariat Panahi A. The importance of macrophages, lipid membranes and seeding in experimental AA amyloidosis. Linköping University Electronic Press, 2019, doi: 10.3384/diss.diva-159658.
Did you like this article? Would you like to comment on it? Write to us. We are interested in your opinion. We will not publish it, but we will gladly answer you.
Labels
Neurology
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

