-
Medical journals
- Career
A performance tester of defibrillator accumulators for clinical purposes
Authors: Jan Suchomel; Ondřej Čadek
Authors‘ workplace: Czech Technical University in Prague, Faculty of Biomedical Engineering, Kladno, CZ
Published in: Lékař a technika - Clinician and Technology No. 4, 2012, 42, 28-31
Overview
Defibrillator battery life length depends on the way of the utilization of the defibrillator, the battery type and effectiveness of the defibrillator’s internal charging mechanism, as well as access of the personal. This paper describes the basics of energy discharging of the accumulator during the defibrillator clinical utilization. The main parameters and its monitoring are described as well. The methods of testing have been designed. The main part of this thesis describes the construction of the device for the testing of defibrillator’s accumulators and its use in the Department of medical devices in General faculty hospital in Prague. Several batteries were tested, the old an often used as well as the new batteries bought from the producer of the defibrillators. Based on the principles of lead-acid battery there is a predicted improvement in the acu-pack capacity while periodically being tested using our testing routine.
Keywords:
defibrillator, lead-acid battery, measurement of capacity, performance testerIntroduction
An integral part of the defibrillator is, regarding to its structural arrangement and principle of operation, its own battery. Battery status is checked every safety-related control, as well as the operational test. There are different kinds of intelligent chargers and testers, but they mostly focus on the basic diagnostics of battery life, and identification of defective battery pack, despite their high cost. None of these tests give us accurate information about the number of defibrillation shocks that can be made, considering the condition of acu-pack.
The motivation for this work stems from design criteria requested by the General faculty hospital in Prague (VFN v Praze) to produce a simple tester for the ZOLL M-series defibrillator acu-pack. Criteria required by the VFN v Praze is to be a tester for clinical purposes, which is portable, easy to use and tests the battery on the number of discharges which it is able to supply the defibrillator. Therefore, this work focuses solely on one specific type of defibrillator. Another reason to focus on one type of battery is that it has its own mechanical battery pack design, which is different for each device.
VFN v Praze currently uses a manufacturer recommended battery tester, with the output information indicating only three states that say if the battery is in order, or has a reduced capacity or is defective. This information does not define a specific battery status, under which it would be possible to detect the approaching end of battery life. Our design is therefore based on actual energy balances of defibrillators and simulates the cyclic operation. Therefore, the result of the routine test is information about the number of defibrillation shocks realistically achievable given by the tested battery in the defibrillator. This cyclic testing according to assumptions about the characteristics of lead-acid batteries, leads to the revival of electrochemical processes and partial restoration of the original cell capacity along with the extension of the life cycle. A pilot test in VFN Prague shows that this theory seems to be true. To prove that this theory is true further testing is necessary, especially using more batteries at different stages of life.
Design of testing routine
A ZOLL tester
The ZOLL tester works with a routine based on the measurement of capacity during charging, but not during discharging as its real use of the battery is in the defibrillator. During charging the battery tester works with a much lower current, which would in practice correspond to the regime for monitoring, but not in the case of defibrillation shock. The tester also ignores the multiple loading during repeated defibrillation of the patient. The following chart indicates the charging mode, including a fragment of a testing routine. First, the battery is discharged to the voltage of 9 V (point 3 to 4 in Fig. 1) and then charged at a constant current of 1 A until the terminal voltage is 11 V. The points 4 and 5 in Fig. 1 shows capacity testing charges the current by about 1 A and measuring the voltage gradient.
Fig. 1: The description of the voltage dependence on time for charging the battery in the charger ZOLL. 1 – acu-pack placement into the charger; 2 – power charger switched on; 3 – discharging battery started; 4 – battery empty – switching to charging mode, current approx. 1 A; Reducing charging current to approx. 0.45 A; 7 – maximum battery voltage; 8 – end of the charging cycle. 
A ZOLL tester – main limitations
Acu-pack testing is made during the charging not by the discharging. Testing current is approx. 1 A, but during the defibrillation the current is up to 8.5 A [3].
Our testing routine
It should be based on worst case scenario of operation, i.e., the patient is being defibrillated again and again with energy of 200 J biphasic shock, until the battery is empty. When charging the defibrillator at 200 joules, battery is loaded by the current 8.5 A at 12 V for 5 seconds then drops to about 0.5 A at 12 V in a waiting mode and monitoring of vital functions. The results of this collected energy from the battery 510 J per discharge and approximately 39 % efficiency, according to the following calculation (Equation 1):
Edefib = U·I·t (V, A, s)
Edefib = 12·8.5·5 (1)
Edefib = 510 J
Defibrillator ZOLL M-series works in testing routine until the battery voltage drop to 9 V. At this moment automatically switch off. For the test routine, the battery is periodically loaded in cycles of 15 s. The first 5 s is the loading current set to 8.5 A. The tester simulates charging of the defibrillator for the shock. Another 10 s is loading current set to 0.5 A. This second part is the simulation of the patient vital signs monitoring. The number of cycles is calculated. The test is stopped when battery voltage decreased to 9 V and tester displays the number of cycles. After this period the whole test is terminated. Number of cycles represents the available battery power for clinical operation i.e. the amount of discharges, which can be handled by the defibrillator battery. The internal structure regarding to a text above is shown on Fig. 2.
Fig. 2: A block diagram of a testing device conception. 
During the main testing, the battery is cyclically loaded with the current up to 8.5 A, until final discharge at 9 V. For testing it is necessary that the battery is fully charged at room temperature and several hours after the disconnection of the charger. At the end of the test the battery is fully discharged and must be recharged immediately. Equipment cannot be used for the precise measurement of capacity, but the indicative testing resistance of the battery under the load. Because the battery is loaded much more intensively than during the real utilization in the defibrillator for clinical use, the information about the number of discharges is lower than the actual lifetime of the battery in a real defibrillator. This is not a problem because this is a guaranteed minimal number of discharges.
Design of the testing device
Unfortunately there is no standard design of the battery case for the medical devices. In the tester must be used original battery bed of ZOLL defibrillator. The device is based on commercially available components. Internal PCB’s arranged in a modular concept with the possibility of future expansion.
The construction was made considering the large power losses that arise during the testing. It is necessary to assume the range of power lost is about 100 W.
According to the principle of function when it is necessary to load the battery using defined current and measuring the voltage, the commonly used PCBs were used. A current source printed circuit board is realized using a feedback controller with the reference voltage and timing control block. Its accuracy is ensured by a crystal. Measuring circuits are built on precision instrumentation amplifiers produced by Texas Instruments [4].
Overall, the device is designed as a compact and durable box that corresponds to the endurance of daily workload. In order to achieve long life battery pack connecting terminals were used in the construction of the original bed of the battery pack ZOLL.
Fig. 3: Mechanical conception of the testing device. A - an original ZOLL lead-acid battery pack, B – a battery pack berth (original ZOLL), C – metallic box with the printed circuit boards. 
Results
Several lead-acid ZOLL M-series defibrillator compatible acu-packs were tested. The testing was realized in the Department of medical devices in General faculty hospital in Prague including batteries in different state of battery life cycle. This gives us an opportunity to see the differences between new fresh batteries and the old items with reduced capacity. The comparison between different types (pieces) shows Tab. 1.
1. The comparison between different types of batteries tested with our device 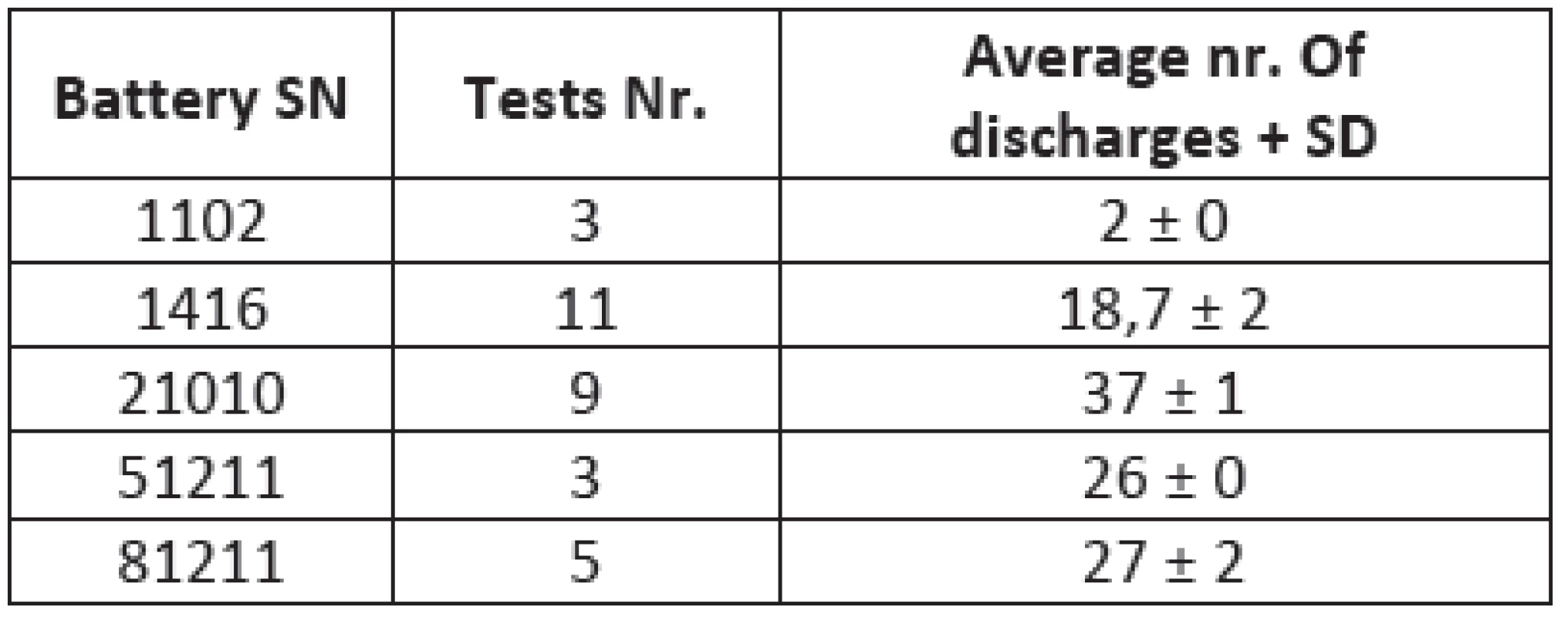
The result from lead-acid batteries can be seen in Fig. 4. The comparison between the old batteries and the newer ones is made.
Fig. 4: A comparison between different acu-packs. Amount of discharges depends on the age of acid-lead batteries. Figure shows differences between the extremely old (Nr. 1102), mid age batteries (Nr. 1416, 51211 and 81211) and a new one (Nr. 21010) represented by its serial numbers for the database. 
The device is considered to be very useful for the purposes of General faculty hospital in Prague. The main goal of this paper was to describe a testing routine and our device. Some results from the testing were published. Testing of the device still continues and detailed analysis of the results will be achieved in a few months.
Conclusion
Main goal of this paper was to fulfill a very specific requirement of General faculty hospital in Prague for the testing of lead-acid defibrillator batteries. An original ZOLL tester function was analyzed and its weakness was described.
A testing routine which simulates the cyclic defibrillation was described and designed. The comparison between ZOLL tester and our testing routine for lead-acid acu-pack show Tab. 2.
2. The comparison between ZOLL tester and our testing routine for lead-acid acu-pack ZOLL. 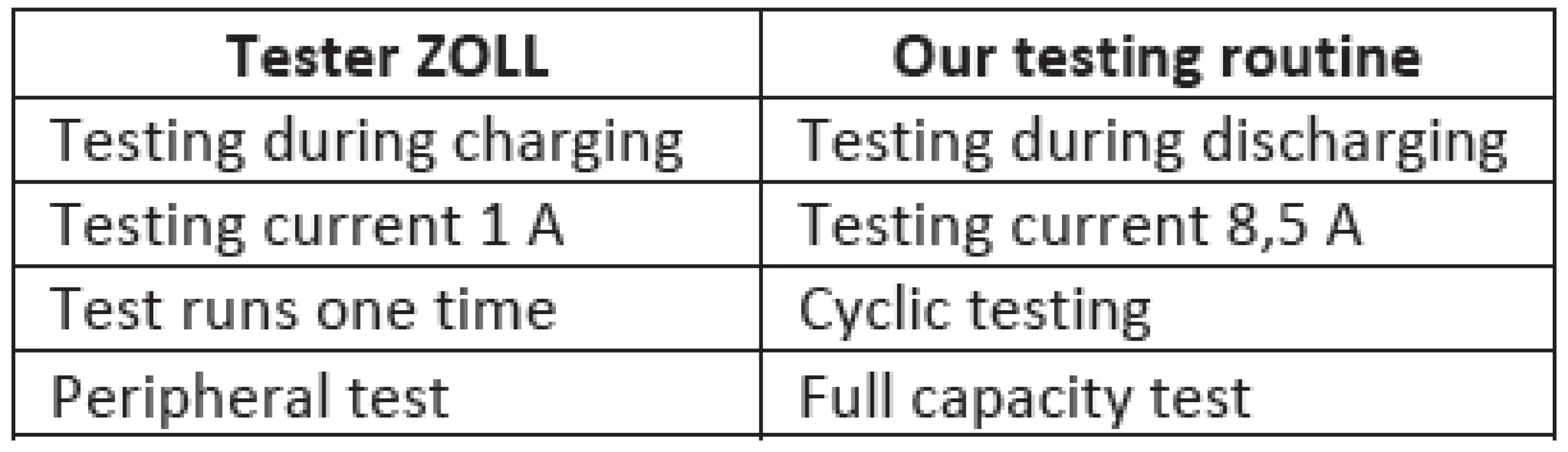
A new testing device was created. It’s construction corresponding to the designed testing routine.
Preliminary results show that periodic testing of batteries using our testing routine and our device could be beneficial for the lead-acid batteries. The main reason is periodic charging and discharging of the batteries using a very similar current as the defibrillator using during the charging for the defibrillation.
Whole device was designed and constructed as a requirement of VFN v Praze. Its purpose of application is to give brief information about the condition of lead-acid batteries for the ZOLL M-series defibrillators in VFN v Praze. There is no metrological defined merit determined by this device. Only brief information about the condition of the acu-pack represented by the number of achievable defibrillation shocks that can be given. After the evaluation of the results of a half year testing period, there will be made a decision about the possible upgrading of the testing device and its validation by a ZOLL company.
Acknowledgement
The work was supported by Department of medical devices, General faculty hospital in Prague and OMS – ZOLL s.r.o. Authors would like to thank to Department of biomedical technology CTU FBME.
Jan Suchomel
Department of Biomedical Technology
Faculty of Biomedical Engineering
Czech Technical University in Prague
Náměstí Sítná 3105, CZ-272 01 Kladno
E-mail: jan.suchomel@fbmi.cvut.cz
Phone: +420 224 355 049
Sources
[1] Cenek, M., et al. Akumulátory: Od principu k praxi. FCC Public. Praha. 2003. ISBN 80-86534-03-0.
[2] Kaválek, J., 555C: Příručka pro konstruktéry. Epsilon. Paha. 1994. ISBN 90-900035-5-9.
[3] ZOLL M-Series configuration guide. ZOLL Medical Corporation. 2007.
[4] Precision Low Power Instrumentation Amplifier INA 128. Datasheet. Texas Instruments. 2011.
Labels
Biomedicine
Article was published inThe Clinician and Technology Journal

2012 Issue 4-
All articles in this issue
- Difficulties of thermographic measurements in medicine
- Vliv poruch sondy sonografu na kvalitativní parametry ultrazvukového B-obrazu
- Vliv ultrazvuku na účinnost fotodynamické terapie – in vitro studie
- Fantom pro diagnostický ultrazvuk a dopplerovské vyšetření
- A performance tester of defibrillator accumulators for clinical purposes
- Methodology of thermographic atlas of the human body
- Home measurement of blood pressure: present problems and perspective improvements
- The Clinician and Technology Journal
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Difficulties of thermographic measurements in medicine
- Fantom pro diagnostický ultrazvuk a dopplerovské vyšetření
- Vliv poruch sondy sonografu na kvalitativní parametry ultrazvukového B-obrazu
- Vliv ultrazvuku na účinnost fotodynamické terapie – in vitro studie
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

