-
Medical journals
- Career
Thrombolysis as a treatment for transplant renal artery thrombosis – a report of three unsuccessful cases and an overview of reported cases
Authors: J. Kristek 1,2; M. Tavlaridis 1; R. Novotný 1; R. Janousek 3; L. Janousek 1,4; J. Maluskova 5; J. H. Peregrin 6; J. Chlupac 1,2; D. Kachlík 2; J. Froněk 1,2,3
Authors‘ workplace: Department of Transplantation Surgery, Institute for Clinical and Experimental Medicine, Prague 1; Department of Anatomy, 2nd Faculty of Medicine, Charles University, Prague 2; Palas Athena Clinic, Prague 3; 1st Faculty of Medicine, Charles University, Prague 4; AeskuLab Patologie, k. s., Prague 5; Department of Diagnostic and Interventional Radiology, Institute for Clinical and Experimental Medicine, Prague 6
Published in: Rozhl. Chir., 2021, roč. 100, č. 9, s. 445-451.
Category: Case Report
doi: https://doi.org/10.33699/PIS.2021.100.9.445–451Overview
Introduction: Thrombolysis has been suggested as a feasible method to treat arterial renal transplant thrombosis under conditions of short duration of ischemia. Data on maximal duration of ischemia that are still feasible to treat are scarce.
Methods: We retrospectively analysed our experience involving three attempts to utilize thrombolysis to treat transplant renal artery thrombosis. We searched through literature on PubMed and compared the data we found with our own experience.
Results: In case number 1 of our cohort, thrombolysis was initiated 12 hours after the onset of thrombosis and had to be ceased after five hours due to the formation of a haematoma. Perfusion of the graft was restored but it did not regain function, most likely due to long ischemia time. In case number 2, an attempt to use thrombolysis was unsuccessful due to failure to cross the graft artery occlusion with a guidewire. Thrombosis was most likely caused by chronic rejection of the graft. In case number 3, thrombolysis restored arterial patency but, due to an onset of ischemia, which lasted 2 to 3 days, did not lead to restoration of graft function. The prolonged ischemia period in case three occurred, at least in part, due to failure to perform an ultrasound scan when the patient was first admitted.
Conclusion: We can confirm that thrombolysis for transplant renal artery thrombosis seems to be feasible only when the condition has a short duration. In the event of sudden deterioration of graft function, the absence of perfusion must always be ruled out by ultrasound scan.
Keywords:
kidney transplantation – thrombolytic therapy – thrombosis – tissue plasminogen activator
INTRODUCTION
Arterial thrombosis or embolism in a transplanted renal graft is a severe complication that frequently results in loss of the graft. Transplant renal artery thrombosis has a reported incidence of 0.5–3.5% [1,2]. Two types of arterial renal transplant thrombosis are distinguished with respect to onset: (i) early (<1 month after transplantation), and (ii) late (≥1 month). In 80% of cases, thrombosis occurs within the first month after transplantation [3]. Predisposing factors for acute thrombosis include the following: (i) stenotic artery and/or stenotic anastomosis, (ii) renal artery trauma (e.g., dissection of the wall), (iii) kinking or torsion of the artery, (iv) external compression of the donor artery by haematoma/lymphocele, etc. [2,4,5]. Generally, the most significant cause for early transplant renal artery thrombosis is an erroneous surgical technique. Late arterial renal transplant thrombosis is less frequent. Traditionally, treatment of transplant renal arterial thrombosis is based on surgical thrombectomy with/without repair of the anastomosis [5]. Outcome is usually dismal, involving loss of the graft. A possible alternative to surgical thrombectomy might be catheter directed thrombolysis (CDT). Although CDT is a well-established and effective treatment option in other indications, there is very limited experience using CDT in the treatment of transplant renal artery thrombosis; there have only been a few reported cases (Tab. 1). The largest series was published by Rouvière et al. [2]. Their case series reports 4 patients, only 2 of whom were treated successfully. [2] According to experiments on native kidneys, the maximum normothermic warm ischemia time that kidneys can completely tolerate is 30–45 minutes. [6,7]. According to various published experience, thrombolysis as a treatment of thrombosed transplanted renal artery is potentially feasible if started less than 24 hours from the onset of thrombosis [2]. Considering the lack of studies/research focusing on renal artery graft thrombosis as an indication for CDT, we would like to present our small case series involving three patients. However, thrombolysis was started in only two cases due to technical failure in the third patient.
1. Reported cases of thrombolysis for thrombosed renal artery of a kidney transplant 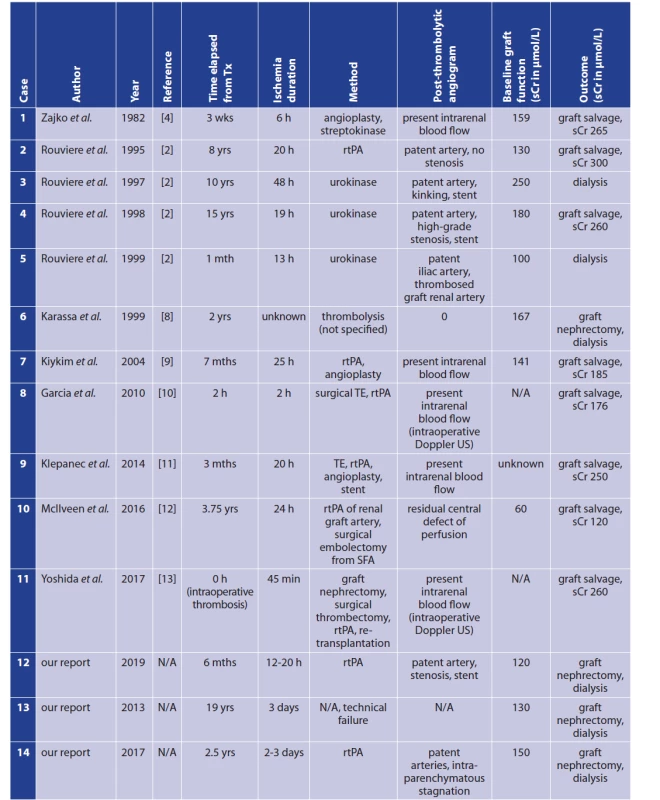
Notes: h − hour(s); L − litre; mth/mths, month(s); N/A − not applicable; rtPA − recombinant tissue plasminogen activator; sCr − serum creatinine; SFA − superficial femoral artery; TE − thrombectomy; Tx − transplantation; US − ultrasound; wk/wks − week(s); yr/yrs − year(s). METHODS
We retrospectively analysed three cases of transplant renal artery thrombosis. Two of these cases were treated with thrombolysis. We searched through literature on PubMed and compared the data we found with that of our own case reports. This search took place on January 31, 2020.
CASE REPORT 1
A 55-year-old male with end-stage renal disease underwent a deceased-donor kidney transplantation in late 2018. Morphology characteristics of the graft were as follows: right-sided kidney, one artery with early branching, 2 veins (1 implanted into the main stem), and a ureter anastomosed with a JJ stent. Cold ischemia time was 23 hours 54 minutes. The graft became fully functional immediately. Serum creatinine levels stabilized at 130 μmol/L, with an estimated glomerular filtration rate of 0.95 mL/s. A per-protocol biopsy conducted at three months post-transplantation revealed focal interstitial fibrosis and tubular atrophy grade I (approximately 15%), and did not reveal any rejection. Over the next 3 months, serum creatinine levels remained stable.
At 6 months post-transplantation and prior to a second per-protocol biopsy, graft function rapidly deteriorated. The patient passed urine for the last time before going to sleep at 9 p.m. He woke up earlier than usual (i.e., 5 a.m.) due to “a strange emotional experience as well as a feeling of tension in the graft region.” He immediately found out that he was unable to pass urine. Upon admission to the hospital, a Doppler ultrasound (US) indicated no arterial perfusion of the graft, while blood flow in the iliac arteries remained intact (Fig. 1). Thrombolysis with a recombinant tissue plasminogen activator (rtPA) was initiated approximately 12.5 hours after the onset of subjective symptoms. Due to the development of inguinal haematoma, a follow-up angiography was performed earlier than planned (i.e., 5 hours after the initiation of thrombolysis). The patient received 14 mg of rtPA. The angiography revealed that graft perfusion had been restored; however, it was reduced and non-homogenous. Perfusion of the upper pole remained reduced (Fig. 2A,B). Due to a small residual thrombus in the arterial anastomosis, an additional stent was placed into the take-off of the renal artery (Fig. 2C,D). We felt the need to cease thrombolysis due to a progressive haematoma surrounding the sheath. In retrospect, we believe that we could have proceeded with thrombolysis if bleeding had been stopped by the introduction of a sheath with a thicker calibre. Graft function and diuresis did not resume over the course of the next 14 days. The graft was removed due to sepsis, which was most likely brought on by a necrotic and/or infected graft. A histological examination of the graft revealed pyelonephritis, focal haemorrhagic necrosis, and rejection of unknown duration. In this case, thrombolysis was in essence partially successful (perfusion was restored) but did not salvage the graft.
Fig. 1: A Doppler ultrasound scan showing absent perfusion of the renal transplant –perfusion of the iliac vessels is preserved 
Fig. 2: Thrombolytic catheter introduced into the thrombosed graft artery 
Poor patchy renal parenchymal filling in the lower part of the kidney (A, B). Thrombus (arrow) in the proximal part of the graft artery (C). Stent placed in the proximal part of the graft artery (arrowheads) (D). Branches of the graft artery for both lower and upper kidney poles are patent, but no clear parenchymal phase is present. CASE REPORT 2
A 54-year-old male patient with chronic kidney disease underwent a deceased-donor kidney transplantation in 1994. The graft maintained a stable function for 19 years (i.e., a serum creatinine level of 130 μmol/L, and an estimated glomerular filtration rate of 0.74 mL/s). In 2013, the patient was referred to our department with a 3-day history of dyspnoea, decreased diuresis, and overhydration. The baseline Doppler US scan that had been performed at another institution had allegedly indicated perfusion of the graft and dilatation of the renal sinus. The C-reactive protein levels were above 300 mg/L. Taking these facts into consideration, the physician assumed the patient was suffering from urine sepsis that was brought about by infra-vesical stenosis. However, soon after the patient was admitted to our hospital, a contrast - enhanced Doppler US scan revealed a near-total absence of renal perfusion. Iliac arteries remained patent. Within 3 hours of admitting the patient, we performed an angiography and confirmed arterial thrombosis of the graft, a patent lower polar artery and occluded main renal artery (Fig. 3). However, we did not initiate thrombolysis since we failed to cross the occluded transplant renal artery with a guidewire despite multiple attempts with various types of guidewires. Three days later, the patient underwent graft nephrectomy. The postoperative course was uneventful from a surgical point of view. A histological examination of the graft showed acute necrosis of the renal parenchyma and grade I interstitial fibrosis/ tubular atrophy, which were caused by chronic humoral rejection along with severe transplant glomerulopathy (C4d1). In this case, thrombolysis was not initiated for reasons previously mentioned.
Fig. 3: Proximal part of the graft artery is patent (arrowhead) including the polar branch (arrow) – the remainder of the graft arterial bed is thrombosed 
CASE REPORT 3
An 80-year-old man suffering from chronic tubulointerstitial nephritis underwent a deceased-donor kidney transplantation in 2014. The patient was at high immunologic risk. In the early post-transplant period, he developed acute humoral rejection with de novo donor-specific antibodies (DSA) and was treated with pulses of corticosteroids (cumulative dose of 1.5 g), plasmapheresis, intravenous immunoglobulins (cumulative dose of 1.2 g/kg), and immunoadsorption (8 courses in total). Over the course of the next weeks, creatinine levels decreased to approximately 145 μmol/L. After some time, renal function became stable and remained so for two and half years after transplantation. Luminex testing for DSA at 2 years after transplantation was negative.
Two and half years after transplantation, the patient suffered from a concurrence of diarrhoea, pneumonia, and type A influenza virus. He received treatment at another institution and was discharged with what was perceived to be normal renal function. Two days after his discharge, he sought medical help due to fever, diarrhoea, and a decrease in diuresis. Upon readmission, his serum creatinine levels were 980 μmol/L. The physicians at that institution did not carry out a US scan, and renal failure was attributed to dehydration brought on by diarrhoea. The patient was later transferred to our hospital, where, once again, no immediate US scan was performed. The first US examination took place after 2 to 3 days from the onset of symptoms. The contrast-enhanced US showed a near total absence of graft perfusion. Thrombolysis was initiated within 2 hours. After 5 hours of thrombolysis (total administration of 8.5 mg of rtPA), an angiography showed a perfectly patent renal artery as well as patent intrarenal arterial branches. However, there was a marked stagnation of the contrast media in the parenchyma, which was an indicator of an intra-parenchymatous obstruction (Fig. 4 A, B, C, D). Due to complete anuria and sepsis that developed over the next 2 days, we performed a graft nephrectomy. Histological examination of the graft showed subtotal acute ischemic necrosis with no signs of acute rejection (C4d was negative).
Fig. 4: Only the stump of the graft artery is seen (arrow) 
A – after thrombolysis, a major part of the transplanted kidney arterial bed is patent but no parenchymal or venous phase is seen – B, C, D. DISCUSSION
The outcome of a renal graft thrombosis is generally dismal. In most cases, thrombosis results in the loss of renal function and necrosis of the graft. Human kidney tolerance to ischemia is a somewhat controversial topic and data remain inconsistent [14]. Traditionally, the maximum warm ischemia time tolerable for surgical procedures on human kidneys was said to be 20 to 30 minutes. [15]. A recent prospective study on native human kidneys (n=40) by Parekh et al. suggests that human kidneys may safely tolerate up to 60 minutes of clamp ischemia time [14]. A review by Mir et al. reports that the tolerance of the native human kidney to ischemia seems to depend on the quality and quantity of renal parenchyma rather than on ischemia time [16]. It is well established that subjecting a kidney to warm ischemia causes a decline in function in the long term [17]. If warm ischemia time exceeds 60 minutes, severe kidney damage is to be expected. Damage severity is in direct proportion to the length of ischemia time [18]. Most authors report that a maximum duration of warm ischemia that can be treated by thrombolysis is 24 hours (Tab. 1). Data on this therapeutic approach and the maximum time of ischemia are scarce. It must be considered that anuria may also be present in highgrade arterial stenosis (incomplete occlusion), which may be an explanation for successful thrombolysis in cases of extended duration of ischemia [19].
In this report, we present 2 cases in which thrombolytic therapy was technically successful in the treatment of transplant renal artery thrombosis. However, thrombolysis did not lead to graft salvation in any of the 2 cases in our report. In case number 1, the patient underwent thrombolysis approximately 12 hours after the onset of clinical symptoms. However, it seemed very difficult to determine the precise length of ischemia time. In this particular case, it is questionable whether the patient awakened immediately upon the onset of thrombosis or the thrombosis had been present for some time when he awakened. This case was limited by the insufficient duration of thrombolysis. Thrombolysis had to be ceased prematurely due to a growing haematoma in the groin area. Typically, CDT as a treatment for other conditions (e.g., acute limb ischemia) takes from 12 to 24 hours. In our first case, the thrombolysis took 5 hours. In total, 14 mg of rtPA was administered. Although the renal artery appeared patent, it is possible that micro-emboli were still present, albeit invisible on the follow-up angiography (considering decreased peripheral/parenchymal perfusion). If we could have used thrombolysis for a longer period of time and applied more rtPA, the graft might have been saved. In retrospect, we feel this might have been achieved by introducing a sheath with a thicker calibre, which could have stopped the bleeding.
In our second case, thrombolysis could not be essentially initiated due to a technical failure/inability to cross what might have been a chronic total occlusion of the transplanted renal artery (which most likely would not have benefitted from thrombolysis anyway). A histological examination of the graft indicated chronic humoral rejection and severe transplant glomerulopathy (C4d1). In other words, graft thrombosis was more a manifestation of chronic rejection. We therefore assume that not all cases of kidney graft thrombosis are treatable with thrombolysis.
Our third case involved a high immunologic risk patient with a history of acute humoral rejection in the early post-transplant period. Thrombosis lingered for 2 to 3 days as a consequence of an incorrect initial diagnosis. Although thrombolysis was successful in restoring blood flow to the main renal artery as well as to the intrarenal arterial branches, stagnation of the contrast solution signalized obstruction at the level of renal parenchyma. Since histological examination revealed no rejection, the final diagnosis pointed towards subacute necrosis attributed to a delay in diagnosis of graft thrombosis. In this case, thrombolysis led to restoration of graft perfusion, yet it was unable to salvage the graft due to the long ischemia time.
It cannot be overemphasised that an accurate Doppler ultrasound scan is essential in cases where graft function abruptly deteriorates. A timely and accurate diagnosis might have led to salvation of the graft in case three. Moreover, if thrombolysis had continued in case 1, the graft might have been salvaged. If thrombosis occurs as a result of intra-parenchymatous damage (e.g., chronic humoral rejection, severe interstitial fibrosis), thrombolysis may not be effective regardless of how early it is initiated.
CONCLUSION
Thrombosis and embolism to a renal transplant artery is a rare but severe condition with a dismal outcome for the graft. Thrombolysis for transplant renal artery thrombosis seems to be feasible only when the condition has a short duration. A timely Doppler ultrasound scan is necessary in cases where graft function suddenly deteriorates. Only early detection and rapid treatment of thrombosis can lead to salvation of the graft. It is important to keep in mind that thrombolysis, even as a timely treatment, will not always ensure graft salvation (e.g., cases in which thrombosis is a symptom of an underlying intra-parenchymatous condition).
Abbreviations:
CDT – catheter directed thrombolysis
DSA – donor-specific antibodies
rtPA – recombinant tissue plasminogen activator
US – ultrasound
Conflict of interests
The authors declare that they have not conflict of interest in connection with this paper and that the article has not been published in any other journal, except congress abstracts and clinical guidelines.
Jakub Kristek
Department of Transplantation Surgery,
Institute for Clinical and Experimental Medicine,
Videnska 1958/9
140 21 Prague
e-mail: krsj@ikem.cz
ORCID: 0000-0002-3758-2273
Sources
1. Jordan ML, Cook GT, Cardella CJ. Ten years of experience with vascular complications in renal transplantation. J Urol. 1982;128(4):689–692. doi: 10.1016/s0022 - 5347(17)53136-5.
2. Rouviere O, Berger P, Beziat C, et al. Acute thrombosis of renal transplant artery: graft salvage by means of intra-arterial fibrinolysis. Transplantation 2002;73(3):403–409. doi: 10.1097/00007890-200202150-00014.
3. Groggel GC. Acute thrombosis of the renal transplant artery: a case report and review of the literature. Clin Nephrol. 1991;36(1):42–45.
4. Zajko AB, McLean GK, Grossman RA, et al. Percutaneous transluminal angioplasty and fibrinolytic therapy for renal allograft arterial stenosis and thrombosis. Transplant. 1982;33(4):447-450.
5. Dimitroulis D, Bokos J, Zavos G, et al. Vascular complications in renal transplantation: a single-center experience in 1367 renal transplantations and review of the literature. Transplant Proc. 2009;41(5):1609–1614. doi: 10.1016/j. transproceed.2009.02.077.
6. Shabanah F, Connolly J, Martin DC. Acute renal artery occlusion. Surg Gynecol Obstet. 1970;131(3):489–494.
7. Collins GM, Taft P, Green RD, et al. Adenine nucleotide levels in preserved and ischemically injured canine kidneys. World J Surg. 1977;2(1):237–243. doi: 10.1007/BF01665093.
8. Karassa FB, Avdikou K, Pappas P, et al. Late renal transplant arterial thrombosis in a patient with systemic lupus erythematosus and antiphospholipid syndrome. Nephrol Dial Transplant. 1999;14(2):472 – 474. doi: 10.1093/ndt/14.2.472.
9. Kiykim AA, Ozer C, Yildiz A, et al. development of transplant renal artery thrombosis and signs of haemolytic-uraemic syndrome following the change from cyclosporin to tacrolimus in a renal transplant patient. Nephrol Dial Transplant. 2004;19(10):2653–2656. doi: 10.1093/ ndt/gfh375.
10. Garcia A, Vedula G, Nowygrod R, et al. Recombinant tissue-type plasminogen activator in the treatment of acute renal artery thrombosis after kidney transplantation. Am J Transplantation 2010;10(8):1931–1933. doi: 10.1111/j.1600-6143.2010.03183.x.
11. Klepanec A, Balazs T, Bazik R, et al. Pharmacomechanical thrombectomy for treatment of acute transplant renal artery thrombosis. Ann Vasc Surg. 2014;28(5):1314.e1311–1314. doi: 10.1016/j.avsg.2013.09.016.
12. McIlveen E, Jackson A, Bowie J, et al. A unique case of acute embolus in a renal transplant with salvage by catheter-directed thrombolysis. Scott Med J. 2016;61(2):106–110. doi: 10.1177/0036933016635402.
13. Yoshida T, Yanishi M, Nakamoto T, et al. Successful treatment of transplant renal artery thrombosis with systemic infusion of recombinant-tissue-plasminogen activator after renal transplant. Exp Clin Transplant. 2017;15(5):571–573. doi: 10.6002/ect.2015.0099.
14. Parekh DJ, Weinberg JM, Ercole B, et al. Tolerance of the human kidney to isolated controlled ischemia. J Am Soc Nephrol. 2013;24(3):506–517. doi: 10.1681/ASN.2012080786.
15. Thompson RH, Lane BR, Lohse CM, et al. Every minute counts when the renal hilum is clamped during partial nephrectomy. Eur Urol. 2010;58(3):340–345. doi: 10.1016/j.eururo.2010.05.047
16. Mir MC, Pavan N, Parekh DJ. Current paradigm for ischemia in kidney surgery. J Urol. 2016;195(6):1655–1663. doi: 10.1016/j.juro.2015.09.099.
17. Zabell J, Isharwal S, Dong W, et al. Acute kidney injury after partial nephrectomy of solitary kidneys: Impact on longterm stability of renal function. J Urol. 2018;200(6):1295–1301. doi: 10.1016/j. juro.2018.07.042.
18. Madden JL. Renal artery and suprarenal aortic occlusion. An experimental study. Arch Surg. 1968;97(6):853–858. doi: 10.1001/archsurg. 1968.01340060031001.
19. Juvenois A, Ghysels M, Galle C, et al. Successful revascularization for acute renal allograft thrombosis after 32 hours of ischaemia. Nephrol Dial Transplant. 1999;14(1):199–201. doi: 10.1093/ ndt/14.1.199.
Labels
Surgery Orthopaedics Trauma surgery
Article was published inPerspectives in Surgery

2021 Issue 9-
All articles in this issue
- Atestace
- Komentář k editorialu pana profesora Pavla Pafka
- Prehabilitation, improving postoperative outcomes
- Acute appendicitis during the spring COVID-19 pandemic in 2020 – a comparative retrospective study
- Regenerative abilities of a nanofiber wound dressing based on polylactide
- Laparoscopic versus open hernia repair in patients with incarcerated inguinal hernia
- Synchronous tumor duplicity of a neuroendocrine tumor of Meckel’s diverticulum with liver metastases and colorectal carcinoma
- Omental torsion as a possible cause of acute abdomen
- Thrombolysis as a treatment for transplant renal artery thrombosis – a report of three unsuccessful cases and an overview of reported cases
- Perspectives in Surgery
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Prehabilitation, improving postoperative outcomes
- Laparoscopic versus open hernia repair in patients with incarcerated inguinal hernia
- Omental torsion as a possible cause of acute abdomen
- Atestace
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

