-
Medical journals
- Career
NOD2/CARD15 mutations and the risk of reoperation in patients with Crohn’s disease
Authors: L. Martínek 1; T. Kupka 2; J. Simova 3; P. Klvaňa 2; M. Bojková 2; Magdalena Uvírová 3; Petr Dítě 2; J. Dvorackova 4; J. Hoch 1; P. Zonca 5
Authors‘ workplace: Department of Surgery, nd Faculty of Medicine, Charles University Prague and Motol University Hospital Prague, Department Head: prof. J. Hoch, MD, PhD. 1; Department of Gastroenterology, Metabolism and Nutrition, Clinic of Internal Medicine, University Hospital Ostrava Department Head: ass. prof. A. Martinek, MD, PhD 2; Educational and Research Institute AGEL, CGB laboratory Inc, Laboratory for Molecular Genetics and Pathology Ostrava, Department Head: J. Simova, M. Sc. 3; Institute of Pathology, University Hospital Ostrava, Department Head: ass. prof. J. Dvorackova, MD, PhD 4; Department of Surgery, University Hospital Ostrava and Faculty of Medicine, University of Ostrava, Department Head: ass. prof P. Zonca, MD, PhD, FRCS 5
Published in: Rozhl. Chir., 2015, roč. 94, č. 6, s. 242-246.
Category: Original articles
Overview
Introduction:
Three NOD2/CARD15 gene variants (3020insC, R702W, G908R) have been identified as genetic risk factors for Crohn’s disease patients. However the diagnostic and therapeutic relevance for clinical practice remains limited. The aim of this study was to evaluate the association between these variants, the risk of reoperation and disease phenotype.Methods:
In 76 Crohn’s disease patients (41 female, 35 male) with a minimum 5 year follow-up, three polymorphisms of the NOD2/CARD15 gene (R702W, G908R, 3020insC) were tested. Detailed clinical and medical history including surgical procedures and reoperations were obtained by reviewing the medical charts and completed prospectively. Association between the need for reoperation, disease phenotypes and gene variants were analyzed.Results:
24 patients (32%) showed at least one NOD2/CARD15 mutation. 25 patients (33%) required reoperation, 51 (67%) represented the control group. The expected trend that patients with NOD2/CARD15 variants have a higher frequency of reoperations was not confirmed to a level of statistical significance (p=0.2688). Two of the four patients (50%) with the 3020insC variant required further surgery. We did not confirm any association between NOD2/CARD15 mutations and age at diagnosis (p=0.4356), behavior (p=0.6610), or localization (p=0.4747) according to the Montreal classification.Conclusion:
NOD2/CARD15 polymorphisms did not significantly affect the reoperation rate. Homozygosity for the 3020insC variant in the NOD2/CARD15 gene is associated with a high risk of reoperation. NOD2/CARD15 gene variants are not significantly associated with specific disease phenotypes.Key words:
Crohn’s disease – NOD2/CARD15 – reoperationsINTRODUCTION
Crohn’s disease is a chronic relapsing inflammatory disease which may affect any part of the gastrointestinal tract, most often the terminal ileum and the ileocecal valve. A varied clinical presentation is characteristic, with an unforeseeable course due to complicated interactions between the organism of a genetically predisposed individual and the surrounding environment. Genetic factors have therefore become a focus of intensive study.
Studies on twins [1,2] and works studying the familial incidence of Crohn’s disease [3,4] initiated interest in the genetics of this disease. One of the first specific results was establishing an increased incidence of Crohn’s disease in association with variants of the CARD15 gene located on chromosome 16q12 coding NOD2 published in 2001 [5]. After that other genes have been associated with Crohn’s disease. To date, more than 100 gene mutations associated with Crohn’s disease have been identified [6]. Despite intense research, the most significant genetic risk factors remain variants of the NOD2/CARD15 gene and the strongest association with the development of Crohn’s disease is the trio of mutations/variants: 3020insC, R702W and G908R [6,7,8]. Carriers of one mutated allele (heterozygote) of the NOD2/CARD15 gene have a 2−4x increased risk of developing Crohn’s disease, mutations of two alleles (homozygotes, compound heterozygotes) present an increased risk of 20−40x [7,9].
Based on these findings, other possible associations have been sought. Several studies have analyzed mutations in the NOD2/CARD15 gene and their association with an earlier onset of the disease [6,10], specific localization and behaviour of the disease [6,18,10,11] or complicated disease course [6,12]. Attention has been paid to the prediction of therapeutic response to a specific treatment, which takes into account an individual’s genetic variability, aiming to move away from rigid therapeutic regimens to personalized treatment of Crohn’s disease [9,13]. The obtained results, however, are inconsistent and do not enable valid and reliable conclusions.
From a surgical viewpoint, it is significant that 70−80% of patients with Crohn‘s disease undergo surgical intervention in their lifetime [14] and 25−38% of patients require repeated surgical intervention [15]. A number of studies describes an association between NOD2/CARD15 mutations and surgical intervention [6,8,11,12]. The most significant association seems to be with the 3020insC mutation (also termed 1007fs) [8,16,17,18]. The association between mutations in the NOD2/CARD15 gene and repeated surgical interventions has only rarely been studied [11,19]. The possibility of identifying patients with an increased risk for repeated surgical interventions could represent a theoretical basis for future work in tailoring therapy to the individual, either in the sense of primarily aggressive regimens or in the so-called top-down approach (early application of biological therapy).
This work is primarily aimed to analyze the incidence of disease recurrence requiring repeated surgical intervention in association with the selected mutations in the NOD2/CARD15 gene. The secondary goal is to evaluate the association between the studied polymorphisms (3020insC, R702W, G908R) and characteristics of the disease as defined by the Montreal classification.
METHODS
After signing an informed consent form, 76 patients were included in the study, 41 women (54%) and 35 men (46%). Diagnosis of Crohn’s disease was based on the results of standard clinical, radiological, endoscopic and histological examinations. The study had a retrospective-prospective character. The data were collected retrospectively from medical documentation of patients diagnosed with Crohn’s disease and prospectively supplemented according to the study protocol. The parameters included gender, age, body mass index, comorbidities, smoking (smoker, non-smoker, ex-smoker), disease presentation (primary presentation, recurrence), duration of symptoms, extraintestinal manifestations, Montreal classification for Crohn’s disease, CDAI (Crohn’s disease activity index), endoscopic findings, medication (5-ASA, corticoids, azathioprine, methotrexate, infliximab, adalimumab), operations (none, acute, elective), type of surgical procedure, operation technique, intraoperative complications, postoperative complications (Clavien-Dindo classification), reoperations, mortality, length of hospital stay, histological findings (macroscopic, microscopic), genetic examination (NOD2/CARD15 – R702W, G908R, 3020insC).
25 patients (33%) underwent more than one surgical intervention. The patients without surgery (24 patients) and patients with only one surgical procedure (27 patients) in the least five years of follow-up represented the control group. The primary surgical procedure included a bowel resection or stricturoplasty. Repeated surgical interventions included any kind of subsequent intervention associated with the primary disease including anorectal procedures with histological confirmation of Crohn’s disease. Surgical procedures due to surgical complications (bowel obstruction, bleeding...) or associated with the primary procedure (stoma reversal…) were logically not included into the group of disease recurrence and reintervention. Minimal follow-up for inclusion in the study was five years. Patients with incomplete data were excluded from the study.
A peripheral blood sample was collected from all the patients to test for single nucleotide polymorphisms (SNPs) p.R702W (rs139104022) and p.G908R (rs2066845) in the NOD2/CARD15 gene, which lead to a change in the protein‘s amino acid sequence and a single base insertion of cytosine in the p.L1007fs sequence (rs2066847, also termed c.3019_3020insC, p.Leu1007fsX1008) leading to the creation of a stop codon and development of a shortened protein. Analysis of these sequence variants was performed from a sample of deoxyribonucleic acid (DNA) isolated from approximately 5 ml of venous blood collected into a sterile test tube containing anticoagulant (EDTA – ethylenediaminetetraacetic acid). The method used to detect the above-mentioned SNP was by amplification of the targeted areas of the NOD2/CARD15 gene located on chromosome 16q12 using polymerase chain reaction (PCR) followed by cleavage of the created PCR products using restriction enzymes and separation of the resulting fragments in polyacrylamide gel. The group of patients with the studied variants (24 patients) was represented by patients who had at least one mutation of the three studied variants in the NOD2/CARD15 gene. Results of the clinical study, including the course of the disease and possible recurrences, were blinded in relation to the genetic testing.
The results were presented using descriptive statistic methods – average, standard deviation, median, range for continuous variable, absolute values and relative frequency for categorical variables. Categorical variables were compared using the Fisher exact test, continuous variables were analyzed by the Mann-Whitney test. All tests were two-sided and a p value less than 0.05 was considered statistically significant. Analyses were performed using the STATISTICA program, version 8.
RESULTS
76 patients fulfilled the inclusion criteria and were included in the study. The average age of the included patients was 34 years (standard deviation ±11, median 33 years, range 18−70 years). There were 24 (32%) patients without surgery, 27 (36%) patients underwent one operation based on the study methodology (bowel resection, stricturoplasty). There were 25 (33%) patients with reoperation. Thirteen patients underwent two surgical procedures, 5 patients underwent three procedures, 2 patients underwent four procedures, and 5 or more procedures were required in five patients.
At least one variant of the three studied variants in the NOD2/CARD15 gene was found in 24 patients (32%) with a confirmed diagnosis of Crohn’s disease. Four patients (5%) were compound heterozygotes, meaning patients who had mutations on both alleles of the gene (R702W + 3020insC and 3020insC + G908R), four patients (5%) were homozygotes for the 3020insC mutation, one patient (1%) was homozygote for the R702W mutation.
Demographic and clinical data comparing the group of patients required reoperation with the control group (without or one surgery) are shown in Tab. 1.
1. Demografic and clinical variables of patients with reoperations and control group 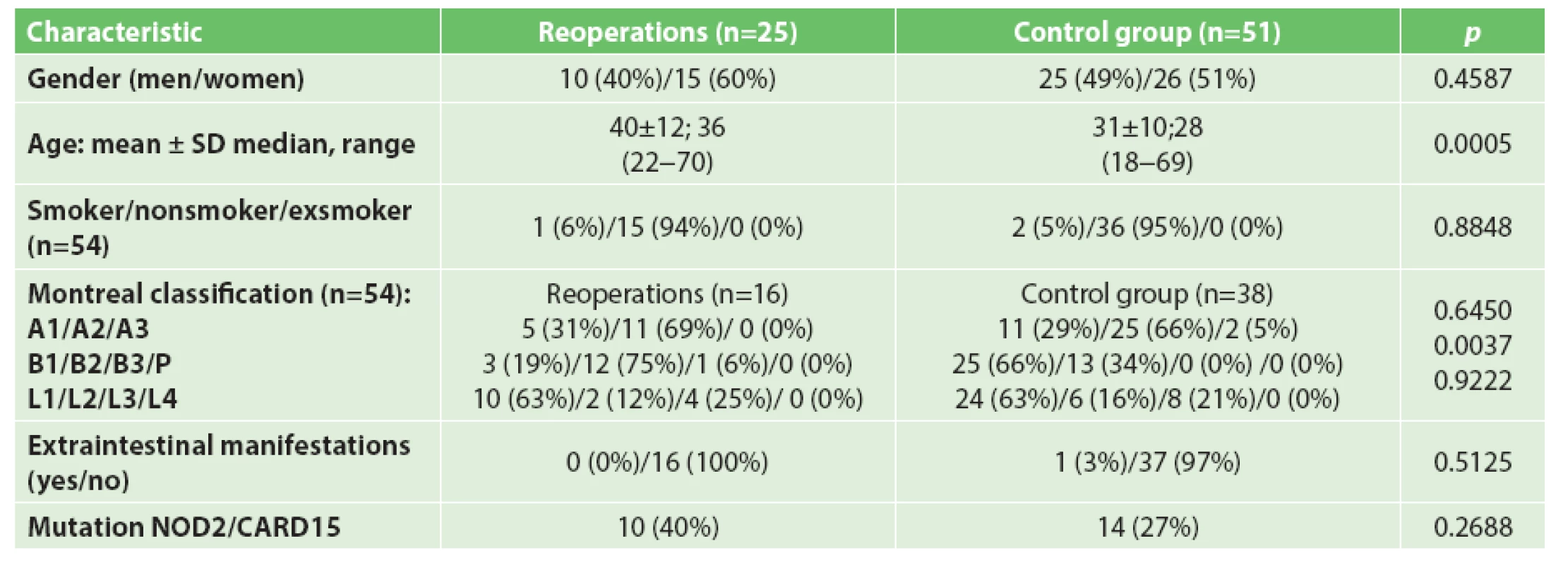
The group of patients required further operation was significantly older. There were no differences observed concerning smoking habits and extraintestinal manifestations between the groups Data from the Montreal classification did not confirm a difference in the age at diagnosis of Crohn’s disease or a difference in the localization of the disease in association with disease recurrence and repeated surgical interventions. A significant difference was seen in the disease behaviour with a predominance of stricturing form in the group with need for further surgery.
A trend of a higher relative frequency of the studied variants in the NOD2/CARD15 gene (R702W, G908R, 3020insC) in the group of reoperated patients did not reach statistical significance at the level of significance p=0.05.
Two of the four homozygotes (50%) for the 3020insC variant were reoperated.
Results analyzing the parameters of the Montreal classification, smoking and the presence of extraintestinal manifestations were obtained from a subgroup of 54 patients with complete data. Of this subgroup, 16 patients (30%) need reoperation and 38 patients (70%) represented the control group.
Disease characteristics based on the variants in the NOD2/CARD15 gene are shown in Tab. 2.
2. Montreal classification and NOD2/CARD15 variants 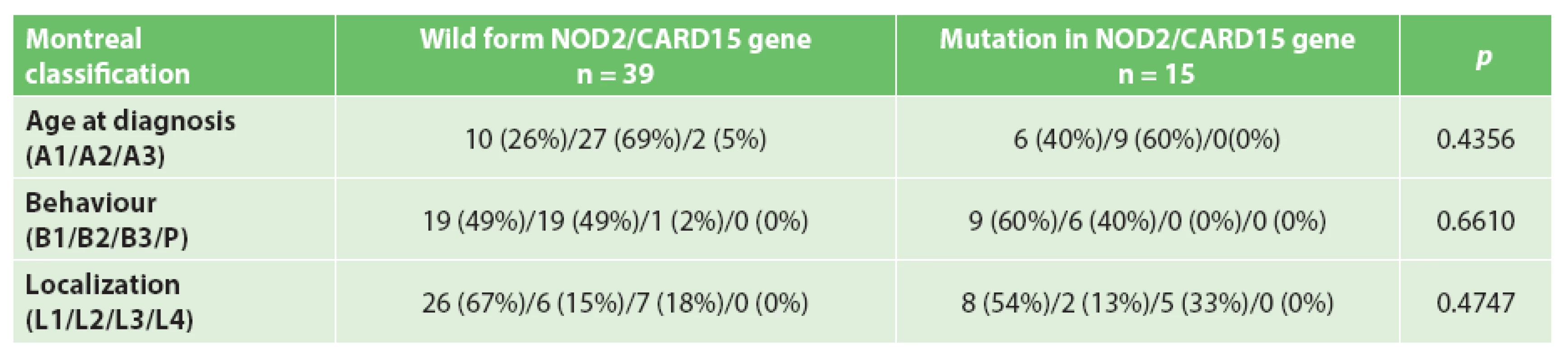
Analysis was performed on the set of 54 patients with complete data; 39 patients (72%) had a non-mutated wild-type form of the NOD2/CARD15 gene and 15 (28%) had at least one of the three variants/mutations (R702W, G908R, 3020insC) in the NOD2/CARD15 gene. There was no significant association between the studied polymorphisms in the NOD2/CARD15 gene and the age at diagnosis, behavior or localization of the disease. Analysis of the results shows a tendency towards earlier disease onset and a more frequent incidence of the ileocolic form in patients with Crohn’s disease with NOD2/CARD15 mutation.
DISCUSSION
Crohn’s disease is an illness with a complex pathogenesis, which is a reflection of the complicated interaction of many factors, especially genetic predisposition, congenital and acquired immunity, microbial triggers and the surrounding environment. This results in a disease with a highly unpredictable course and with a great risk of recurrence. Research regarding the association between the studied variants in the NOD2/CARD15 gene and Crohn’s disease was conducted in two stages. First, the incidence of Crohn’s disease and its association with the specific genetic variants was studied. Subsequently, associations between the genetic variants in the NOD2/CARD15 gene and the course of the disease, including response to treatment aiming at possibilities of personalized therapy were analyzed. Although there are a number of known variants significantly associated with the risk of Crohn’s disease, the exact mechanisms remain unclear to date. Among those mentioned are dysregulations of the antibacterial response, changes in bowel permeability, and changes in the regulation of congenital and acquired immunity.
In our set of patients, at least one of the three studied variants (R702W, G908R, 3020insC) in the NOD2/CARD15 gene were found in 24 patients (32%) with a confirmed diagnosis of Crohn’s disease. In general, there are great ethnic and geographical differences in the incidence of the individual mutations. The relative incidence in European countries ranges from 36−50% [7]; contrarily, these polymorphisms are rarer in Japan, China or Korea [7]. Our result seems to be at the lower end of the range of presented incidence in Europe. A higher incidence in the Czech population was described by authors Hradsky et al. who presented an incidence of at least one polymorphism of the NOD2/CARD15 gene in 46% of adults affected with Crohn’s disease [20].
Surgical treatment of Crohn’s disease represents a treatment of disease´s complications [21,22]. In the literature, the risk of repeated surgical procedures for patients with Crohn’s disease ranges from 11–32% within 5 years, 20–44% within 10 years and 46–55% within 20 years [23]. Our group of patients with repeated surgical procedures was significantly older than the control group, which may be naturally explained by a longer follow-up in these patients. A significantly more frequent incidence of the stricturing form in reoperated patients may also be expected, since stenosis is the most common indication for surgical intervention in Crohn’s disease and is a strong predictor of repeated interventions [19,24].
With respect to the presence of the studied variants in the NOD2/CARD15 gene in our group of patients, the expected trend of a higher relative frequency of mutations in the NOD2/CARD15 gene in the group of reoperated patients did not reach a level of statistical significance. This may be due to a type II error ( sample size is not large enough to detect a difference). Despite this fact, the tendency towards a more complicated disease course was confirmed [8,16,17,18]. In three independent studies [11,19,25], patients with mutation in the NOD2/CARD15 gene presented an increased risk for repeated surgical interventions and operations were performed earlier. A higher risk of reoperations would theoretically support an indication for more aggressive medication; on the other hand, reliable information regarding the association between genetic risk factors and the response to specific treatment regimes is sparse [6,9,13]. In contrast, in a study published by Maconi et al., where out of 253 patients operated for complications of Crohn’s disease 35% of patients underwent at least one reoperation, mutations in the NOD2/CARD15 gene were not associated with a higher risk of reoperation [19].
A comparison of patients - carriers of any of the studied variants in the NOD2/CARD15 gene with patients with a non-mutated (wild-type) form did not show a statistically significant difference in the age at diagnosis, in the behaviour or in the localization of the disease. The only observed trend was earlier incidence and ileocolic localization in patients with the mutation, which, however, did not reach statistical significance. There is a great variability in the literature regarding these findings. The incidence of Crohn’s disease at an earlier age, a predominant localization in the ileum, and a stricturing form in patients carrying the mutation in the NOD2/CARD15 gene have been published [6,12,26,27]. In contrast, a meta-analysis by Adler et al. concludes that the NOD2/CARD15 gene is a fairly unreliable predictor of a complicated course including surgical interventions [12]. Similarly a meta-analysis by authors Solon et al., 2013 did not confirm an increased risk of repeated surgical interventions in patients with Crohn’s disease and variants in the NOD2/CARD15 gene [28].
A significant limitation of the study is the small number of patients and therefore the possibility of a type II error. Another limitation is the retrospective character of the study. The control group consisting patients without surgery and with only one surgical procedure is also questionable. The purpose of this grouping was to gain a sufficient number of patients for a more objective analysis of the results. The mandatory minimal follow-up interval of five years should eliminate this potential misrepresentation. Another limitation may also be the fact that most studies, including ours, analyze only the selected three sequence variants (3020insC, R702W and G908R) in the NOD2/CARD15 gene. Only a few authors have attempted to fully sequence the NOD2/CARD15 gene, although it seems that complete sequencing may not be beneficial for predicting complications [7]. On the other hand, it may be expected that only the combination of clinical and genetic factors together with consideration of the surrounding environment may provide clinicians with guidelines for personalizing therapy for patients with Crohn’s disease. To date, despite the importance of genetics in the pathogenesis of inflammation, they have not yet played a significant role in clinical practice, neither in the diagnostic process nor in the prediction of the course of the disease and response to medication and surgical treatment.
CONCLUSION
In our group of patients, the studied sequence variations in the NOD2/CARD15 gene were not a definite predictor of a complicated course of Crohn’s disease with surgical recurrence - reoperation; however, the results are limited by the small number of patients. Homozygotes with a mutation in the 3020insC sequence in the NOD2/CARD15 gene represent a high risk group of patients who could profit from personalized aggressive therapy. Prospective multicentric studies with large numbers of patients and a clearly defined unified protocol may help understand the influence of genetic factors on the course of Crohn’s disease.
List of abbreviations
5-ASA 5 aminosalicylic acid
CDAI Crohn’s Disease Activity Index
DNA deoxyribonucleic acid
EDTA ethylenediaminetetraacetic acid
NOD2/CARD15 nucleotide-binding oligomerization domain-containing protein 2 (NOD2)/caspase recruitment domain-containing protein 15 (CARD15)
PCR polymerase chain reaction
SNP single nucleotide polymorphism
Conflict of Interests
The authors declare that they have not conflict of interest in connection with the emergence of and that the article was not published in any other journal.
MUDr. Lubomír Martínek, Ph.D.
Odboje 1164
739 32 Vratimov
e-mail: lubomir.martinek@fnmotol.cz
Sources
1. Thompson NP, Driscoll R, Pounder RE, et al. Genetics versus environment in inflammatory bowel disease: results of a British twin study. BMJ 1996;312 : 95−6.
2. Spehlmann ME, Begun AZ, Burghardt J, et al. Epidemiology of inflammatory bowel disease in a German twin cohort: results of a nationwide study. Inflamm Bowel Dis 2008;14 : 968−76.
3. Kyle J. Crohn’s disease in the northeastern and northern Isles of Scotland: an epidemiological review. Gastroenterology 1992;103 : 392−9.
4. Lapidus A. Crohn’s disease in Stockholm County during 1990-2001: an epidemiological update. World J Gastroenterol 2006;12 : 75−81.
5. Hugot JP, Chamaillard M, Zouali H, et al. Association of NOD2 leucinerich repeat variants with susceptibility to Crohn’s disease. Nature 2001;411 : 599−603.
6. Cleynen I, González JR, Figueroa C, et al. Genetic factors conferring an increased susceptibility to develop Crohn’s disease also influence disease phenotype: results from the IBDchip European Project. Gut 2013;62 : 1556−65.
7. Bhullar M, Macrae F, Brown G1, et al. Prediction of Crohn’s disease aggression through NOD2/CARD15 gene sequencing in an Australian cohort. World J Gastroenterol 2014;20 : 5008−16.
8. Seiderer J, Brand S, Herrmann KA, et al. Predictive value of the CARD15 variant 1007fs for the diagnosis of intestinal stenoses and the need for surgery in Crohn’s disease in clinical practice: results of a prospective study. Inflamm Bowel Dis 2006;12 : 1114−21.
9. Niess JH, Klaus J, Stephani J, et al. NOD2 polymorphism predicts response to treatment in Crohn’s disease -first steps to a personalized therapy. Dig Dis Sci 2012;57 : 879−86.
10. Lesage S, Zouali H, Cézard JP, et al. CARD15/NOD2 mutational analysis and genotype-phenotype correlation in 612 patients with inflammatory bowel disease. Am J Hum Genet 2002;70 : 845−57.
11. Alvarez-Lobos M, Arostegui JI, Sans M, et al. Crohn’s disease patients carrying Nod2/CARD15 gene variants have an increased and early need for first surgery due to stricturing disease and higher rate of surgical recurrence. Ann Surg 2005;242 : 693−700.
12. Adler J, Rangwalla SC, Dwamena BA, et al. The prognostic power of the NOD2 genotype for complicated Crohn’s disease: a meta-analysis. Am J Gastroenterol 2011;106 : 699−712.
13. Freire P, Portela F, Donato MM, et al. CARD15 mutations and perianal fistulating Crohn’s disease: correlation and predictive value of antibiotic response. Dig Dis Sci 2011;56 : 853−9.
14. Sachar DB. Recurrence rates in Crohn’s disease: predicting the future and predicting the past. Gut 2006;55 : 1069−70.
15. Vannozzi G, Fontana R, Milla M, et al. Disease history in 382 Italian patients with Crohn’s disease. Ital J Gastroenterol Hepatol 1997;29 : 525−32.
16. Hampe J, Cuthbert A, Croucher PJ, et al. Association between insertion mutation in NOD2 gene and Crohn’s disease in German and British populations. Lancet 2001;357 : 1925−8.
17. Annese V, Lombardi G, Perri F, et al. Variants of CARD15 are associated with an aggressive clinical course of Crohn’s disease--an IG-IBD study. Am J Gastroenterol 2005;100 : 84−92.
18. Ferreira AC, Almeida S, Tavares M, et al. NOD2/CARD15 and TNFA, but not IL1B and IL1RN, are associated with Crohn’s disease. Inflamm Bowel Dis 2005;11 : 331−9.
19. Maconi G, Colombo E, Sampietro GM, et al. CARD15 gene variants and risk of reoperation in Crohn’s disease patients. Am J Gastroenterol 2009;104 : 2483−91.
20. Hradsky O, Lenicek M, Dusatkova P, et al. Variants of CARD15, TNFA and PTPN22 and susceptibility to Crohn’s disease in the Czech population: high frequency of the CARD15 1007fs. Tissue Antigens 2008;71 : 538-47.
21. Šerclová Z. Historie chirurgické léčby nespecifických zánětů střevních. Vnitř Lék 2014;60 : 645−8.
22. Kala Z, Marek F, Válek VA et, al. Chirurgická léčba Crohnovy choroby. Vnitř Lék 2014;60 : 617−23.
23. Yamamoto T. Factors affecting recurrence after surgery for Crohn’s disease. World J Gastroenterol 2005;11 : 3971−9.
24. Huťan M, Huťa M., ml. Úloha chirurgického výkonu v liečbě Crohnovej choroby. Rozhl Chir 2009;88 : 185−8.
25. Büning C, Genschel J, Bühner S, et al. Mutations in the NOD2/CARD15 gene in Crohn’s disease are associated with ileocecal resection and are a risk factor for reoperation. Aliment Pharmacol Ther 2004;19 : 1073−8.
26. Newman B, Siminovitch KA. Recent advances in the genetics of inflammatory bowel disease. Curr Opin Gastroenterol 2005;21 : 401–7.
27. Radford-Smith G, Pandeya N. Associations between NOD2/CARD15 genotype and phenotype in Crohn’s disease - Are we there yet? World J Gastroenterol 2006;12 : 7097−103.
28. Solon JG, Burke JP, Walsh SR, et al. The effect of NOD2 polymorphism on postsurgical recurrence in Crohn’s disease: a systematic review and meta-analysis of available literature. Inflamm Bowel Dis 2013;19 : 1099−105.
Labels
Surgery Orthopaedics Trauma surgery
Article was published inPerspectives in Surgery

2015 Issue 6-
All articles in this issue
- Individualized medicine
- Acute abdominal surgery in pregnancy – as viewed by the surgeon
- Current standards of care in the management of patients with abdominal sepsis
- A novel method of endovascular aneurysm sealing (EVAS) in patients with abdominal aortic aneurysm
- Ganglioneuroma, a rare cause of soft neck tissues tumor in adult age
- Synchronous cancer duplicities of pancreas and stomach/kidney and their surgical treatment
- Using a stent graft in the treatment of hepatic artery bleeding after pancreatoduodenectomy
- NOD2/CARD15 mutations and the risk of reoperation in patients with Crohn’s disease
- Perspectives in Surgery
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Acute abdominal surgery in pregnancy – as viewed by the surgeon
- Current standards of care in the management of patients with abdominal sepsis
- Ganglioneuroma, a rare cause of soft neck tissues tumor in adult age
- A novel method of endovascular aneurysm sealing (EVAS) in patients with abdominal aortic aneurysm
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

