-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Malignant tumours of the duodenum
Maligní nádory duodena
Ucelený přehled duodenálních nádorů se v současné literatuře prakticky nevyskytuje. S jednotlivými typy zhoubných nádorů se lze seznámit v rámci přehledu malignit tenkého střeva, popsanými soubory kazuistik či jednotlivých případů. Výjimkou jsou nádory ampulární oblasti, které jsou podrobně uvedeny v poslední WHO histologické klasifikaci nádorů trávicího ústrojí. Z domácích ani zahraničních literárních pramenů nelze vyhledat ani ucelený náhled na léčbu nádorů duodena. Obdobně je tomu i při vyhledávání chirurgických postupů. Resekční výkony na duodenu by proto měly být prováděny na pracovištích, které mají dostatečnou zkušenost s výkony v oblasti hepato-pankreato-biliární.
Klíčová slova:
maligní nádory duodena – diagnostika – chirurgická terapie
Authors: M. Ryska 1,3; P. Hrabal 2
Authors place of work: Surgery Department of 2nd Faculty of Medicine Charles University and Central Military Hospital, Prague Head of Department: Professor M. Ryska, MD, Ph. D. 1; Pathology Department of the Military University Hospital Prague, Head of Department: P. Hrabal, MD. 2; Faculty of Health Care and Social Work, Trnava University, Trnava, Dean of Faculty: Professor J. Slaný, MD, Ph. D. 3
Published in the journal: Rozhl. Chir., 2015, roč. 94, č. 12, s. 497-503.
Category: Souhrnné sdělení
Summary
No comprehensive knowledge of duodenal tumours exists in the current literature; individual types of malignant tumours may be described within malignancies of the small bowel, sets of case reports, or individual cases. Ampullary carcinomas are the exception and they are detailed in the current WHO histological classification of tumours of digestive system. Neither national nor international literature sources provide a comprehensive review of their therapy. The situation is similar when searching for surgical procedures. Resection procedures on the duodenum should thus be performed in specialized centres with sufficient experience with hepato-pancreato-biliary surgery.
Key words:
malignant tumours of the duodenum – diagnostics – surgical therapyIntroduction
No comprehensive knowledge of duodenal tumours exists in the current literature. Individual types of malignant tumours may be described within malignancies of the small bowel, sets of case reports, or individual cases. Ampullary carcinomas are the exception and they are detailed in the current WHO histological tumour classification of digestive system [1]. Neither national nor international literary sources provide a comprehensive review of their treatment [2,3]. The situation is similar when researching surgical procedures [4]. But in fact every surgeon who specializes in treating diseases of the upper GIT approaches surgery of the duodenum with great apprehension of possible complications in the early postoperative course [5].
This review focuses the reader’s attention on the most commonly occurring malignant tumours in the area of the duodenum, their effective diagnosis and treatment.
Anatomical remark
From the tumour viewpoint, the most important region is the descending part of the duodenum, whose left side borders the head of the pancreas and where the common bile duct runs dorsally in a wedge between this part of the duodenum and the pancreas, and along with the pancreatic duct pierces the duodenal wall and opens on the major duodenal papilla (papilla of Vater) (Fig. 1). Tumours which develop in the head of the pancreas, in the terminal portion of the common bile duct and in the area of the papilla of Vater may seem like tumours of the duodenum due to their localisation and as a result of tumour invasion.
Fig. 1. Anatomical relations in the duodenal region, distal portion of the common bile duct and head of the pancreas 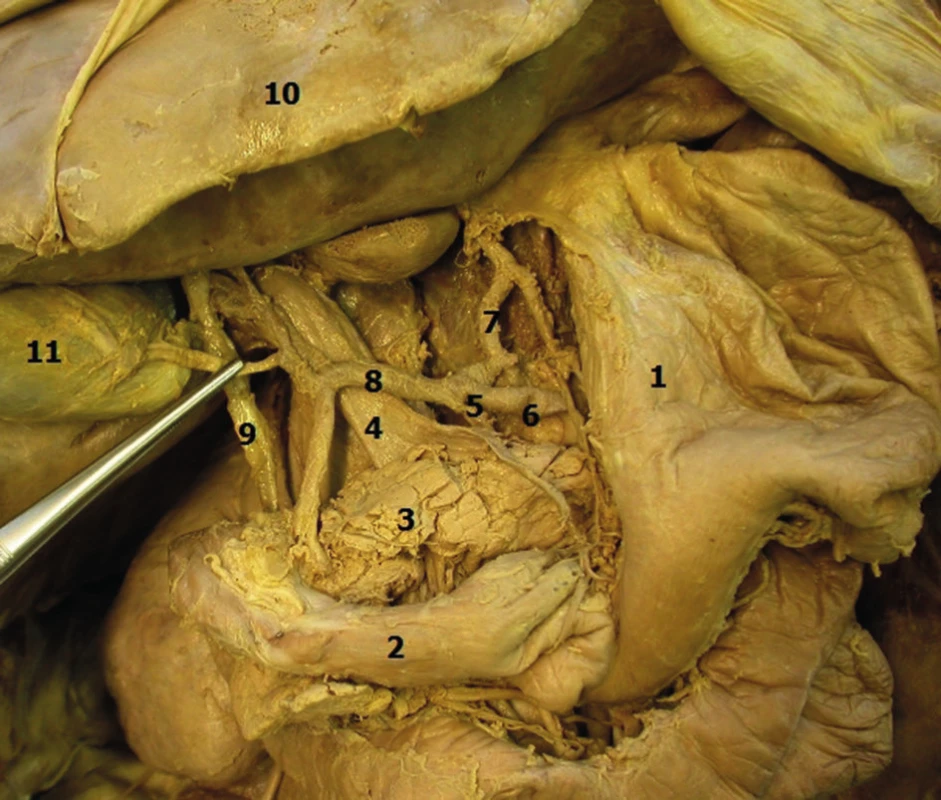
Legenda: 1 − stomach, 2 − duodenum, 3 − pancreas, 4 − portal vein, 5 − celiac trunk, 6 − splenic artery, 7 − gastric artery, 8 − hepatic artery, 9 − bile duct, 10 − liver, 11 − gallbladder. The incidence of malignant tumours in the small bowel, which includes the duodenum, jejunum and ileum, is very low, despite the fact that its length of 5 − 6 meters represents approximately 75% of the length of the gastrointestinal tract and 90% of the absorptive surface of the esophagogastrointestinal system: however, the incidence is only 2% (in comparison malignancies of the colorectum with a length of 1.5 m is 57%). The highest incidence of adenocarcinoma in the small bowel is in the duodenum, especially in the periampullary region − 65% [6]. Most primary adenocarcinomas of the duodenum are sporadic tumours and only a small number has a hereditary basis (familial adenomatous polyposis - FAP, Lynch syndrome, juvenile polyposis syndrome, Peutz - Jeghers syndrome, etc.). Celiac disease is considered to be a predisposition for adenocarcinoma; however, this disease is much more significantly associated with the development of malignant lymphomas.
In the duodenum, adenomas of the small bowel in all their histological variations (tubular, tubulovillous, villous) are more common than adenocarcinomas. Here the incidence is also sporadic, or rare, such as multiple adenomas as part of FAP. Just as adenomas of the colon, they have the potential for malignant transformation.
The second most frequent group of duodenal tumours are neuroendocrine neoplasms (NENs). Three percent of all of these tumours located in the GIT are found in the duodenal region. They are more common in males (median – 59 years). These tumours may be hormonally active (produce gastrin, somatostatin) as well as inactive. Hormonally active tumours are more common in young individuals and may be localised in any part of the duodenum. Hormonally inactive tumours are most often found in the duodenal bulb.
Based on their biological characteristics, NENs are classified based on the current WHO classification from 2010 [1] as neuroendocrine tumours (NETs) G1 or G2, neuroendocrine carcinoma (NEC) which is exclusively G3 and mixed adenoneuroendocrine carcinomas (MANECs).
The GIT is the most common extranodal site for malignant lymphomas. Primary intestinal lymphomas mainly include diffuse large B-cell lymphomas and MALT-type (mucosa associated lymphoid tissue) tumours. T-cell malignant lymphomas may arise as a complication of celiac disease (EATL – enteropathy associated T-cell lymphoma).
The most significant mesenchymal tumours are gastrointestinal stromal tumours (GISTs). Between 25−40% of all GISTs of the GIT develop in the small bowel; 10−20% of these are located in the duodenum. Based on their biological characteristics, these tumours are classified as benign, of uncertain malignant potential, or malignant.
Other mesenchymal tumours of the duodenum include submucosal lipoma, and leiomyocellular and angiogenic tumours, which may be benign or malignant. An extremely rare tumour recently described in the literature is the gastrointestinal clear cell sarcoma.
Tumours of the ampullary region are classified as a separate group. Both intestinal and pancreatobiliary morphological types of adenocarcinomas are most significant, as well as neuroendocrine tumours with the above-mentioned spectrum of biological characteristics.
Surgical remark
The fact that mobilization of the duodenum is very limited is unfavourable for the surgeon. The duodenum is fixed by the hepatoduodenal ligament, by the insertion of the terminal choledochus, the opening of the pancreatic duct at the papilla of Vater, and by its close proximity to the pancreas. After local resection, it is often only possible to bring together the edges of the duodenal wall under tension, unlike suturing free small bowel loops of the duodenum, and this usually leads to dehiscence, which may not only be the cause of repeated operations and prolonged therapy, but also be associated with a high mortality. Resecting part of the duodenum that lies in close proximity to the pancreas, while attempting to preserve both the parenchyma of the gland as well as the duodenum may be technically difficult or even impossible. It is often safer to perform a more extensive procedure - pancreatoduodenectomy.
The most common indication for surgery for a malignant tumour of the duodenum and the role of the multidisciplinary team when determining the treatment plan
The NOR data show that in the Czech Republic, the incidence of these tumours in 1977 and 2012 was 0.37/100.000 inhabitants and 0.91/100.000 respectively, with a mortality of 0.18/100.000 inhabitants and 0.38/100.000 respectively. This means that in the last 40 years, our country has seen more than a two-fold increase in both incidence and mortality [7]. This is in contrast to the United States, where there is only a 50% increase [8]. The presented increase in incidence is mainly due to adenocarcinoma, NEN (carcinoid) and lymphoma in males and NEN (carcinoid) and lymphoma in females, with the finding that adenocarcinoma and lymphoma can be expected in patients over the age of 30 years. Contrarily, sarcomas are present in older decades. It is interesting to note that the increasing incidence of adenocarcinomas of the small bowel directly correlates with the increasing incidence of colorectal cancer.
In the duodenal region, tumours include adenocarcinomas, which are most frequent, especially in the area of the papilla of Vater, gastrointestinal stromal tumours (GISTs) of mesenchymal origin, neuroendocrine tumours, non-Hodgkin lymphoma, rare malignant forms of paragangliomas and other seldom occurring tumours [9].
1 - Adenocarcinoma
In adenocarcinomas occurring in the form of polypoid, infiltrative or annular lesions, and the development from adenoma is similar as in the large bowel. Adenomas are histologically classified as tubular (least aggressive), villous (most aggressive) and tubulovillous (Fig. 2, 3).
Fig. 2. Tubular adenoma of the duodenum, HE, 40x 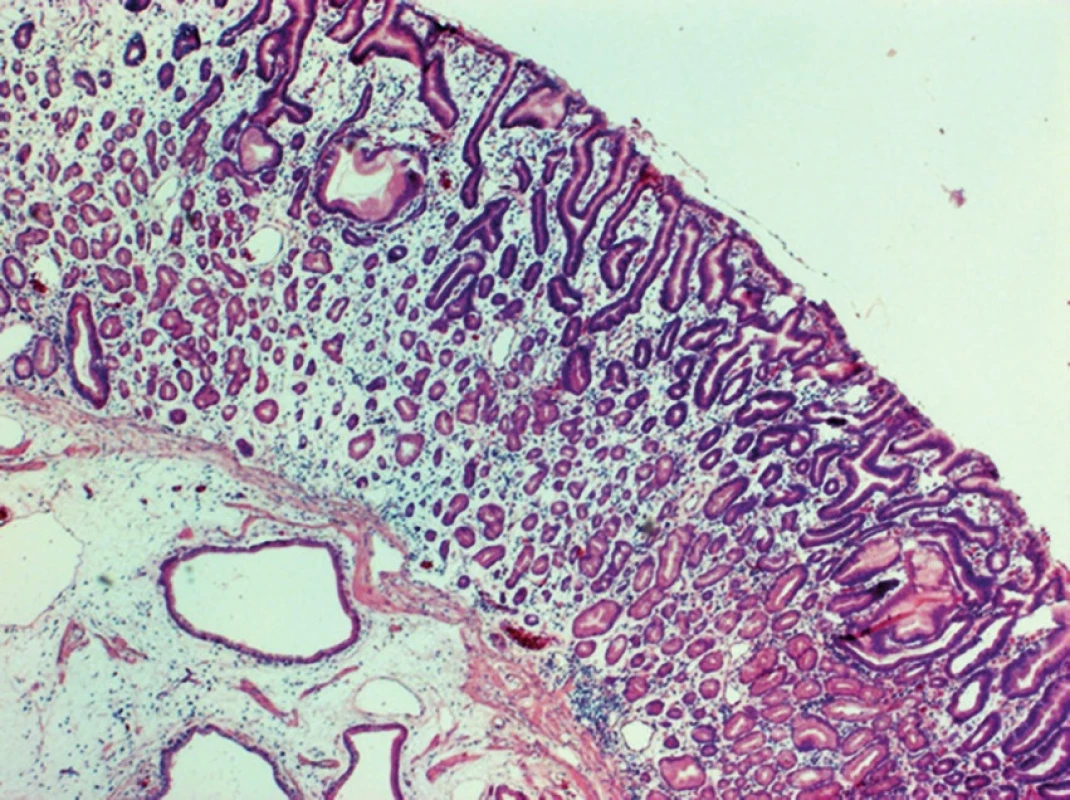
Fig. 3. Tubulovillous adenoma of the duodenum, HE, 40x 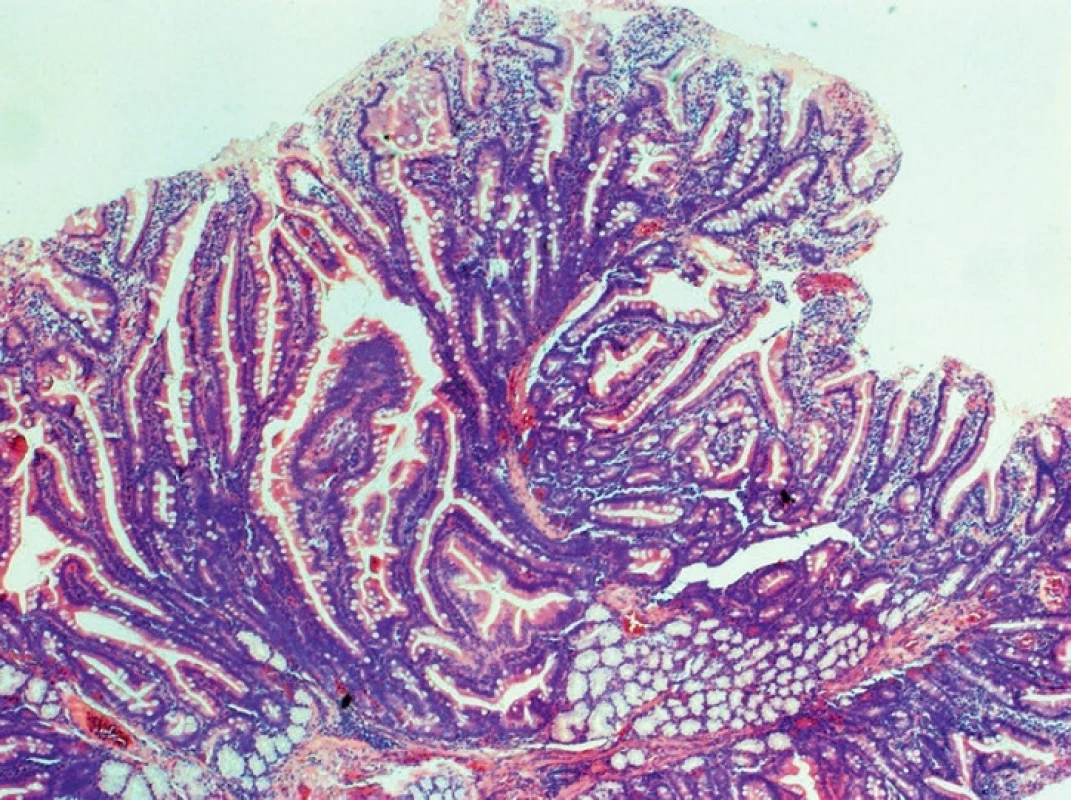
Villous adenomas are localized predominantly in D2. They exhibit high grade dysplasia more often with highest risk of development of adenocarcinoma in about 45%. This is also true for carcinomas in patients with familial adenomatous polyposis (FAP). Recently, an increase in the prevalence of adenomas in the duodenum has been reported in this group [8], thus increasing the risk of adenocarcinoma development with 10–15% microsatellite instability, low cellular differentiation, lymphocytic infiltration and mucin production. Duodenal carcinoma is a primary cause of death in patients with FAP who undergo colectomy. Patients with Peutz – Jeghers syndrome may have hamartomatous polyps in the duodenum with adenomatous lesions, which may develop into malignancies.
Duodenal adenocarcinomas are most often located in the ampullary region. It is necessary to note that these tumours are often preoperatively referred to as pancreaticoduodenal carcinomas [10]. This term includes adenocarcinomas of the duodenum, papilla of Vater, distal bile duct, and pancreatic head. It is not always possible to definitely establish the origin of the cancer prior to the operation. This is determined only by the pathologist. The percentage of resectability and prognosis of a patient with ampullary or distal bile duct adenocarcinoma is higher and more favourable than that of a patient with adenocarcinoma of the pancreatic head.
The widespread term ampulloma refers to adenocarcinoma of the papilla of Vater in 2/3 of cases. This is the most common malignancy of the duodenal region. In certain cases, it grows exophytically from the epithelium of the papilla of Vater, in others, the epithelium of the papilla is covered and growth is intra-ampullary (Fig. 4−6).
Fig. 4. Intraampullary adenocarcinoma of the papilla of Vater – resected specimen 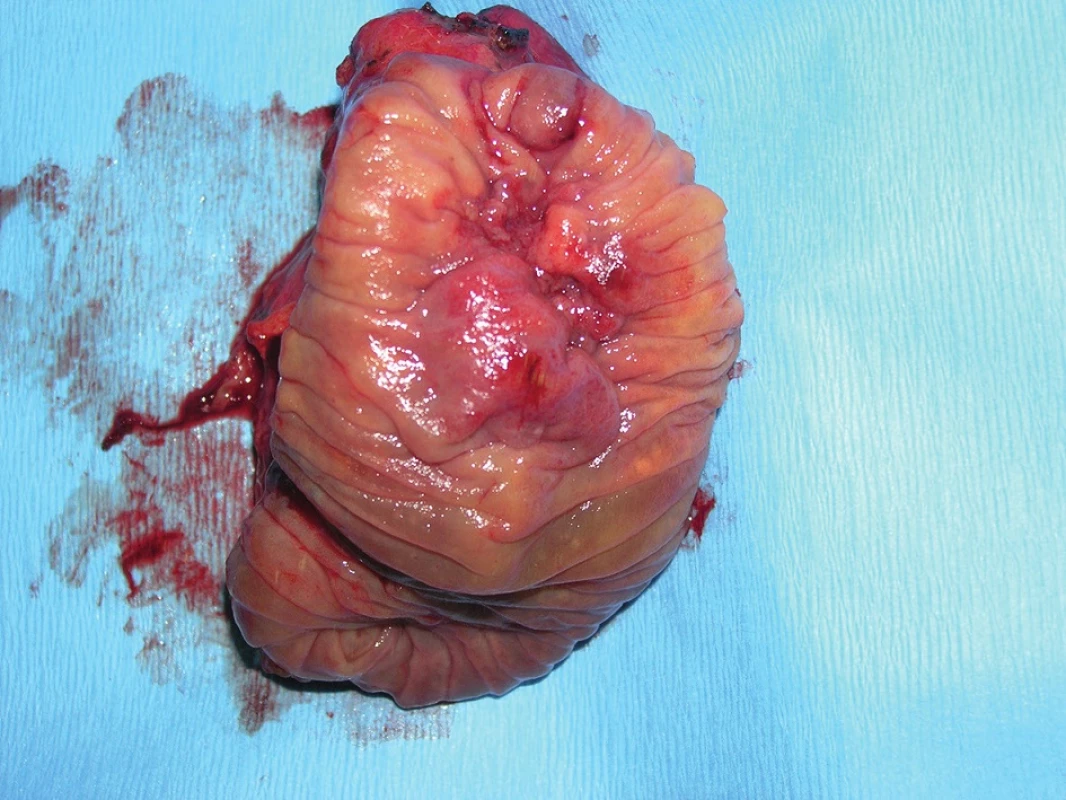
Fig. 5. Exophytically growing adenocarcinoma of the papilla of Vater – resected specimen 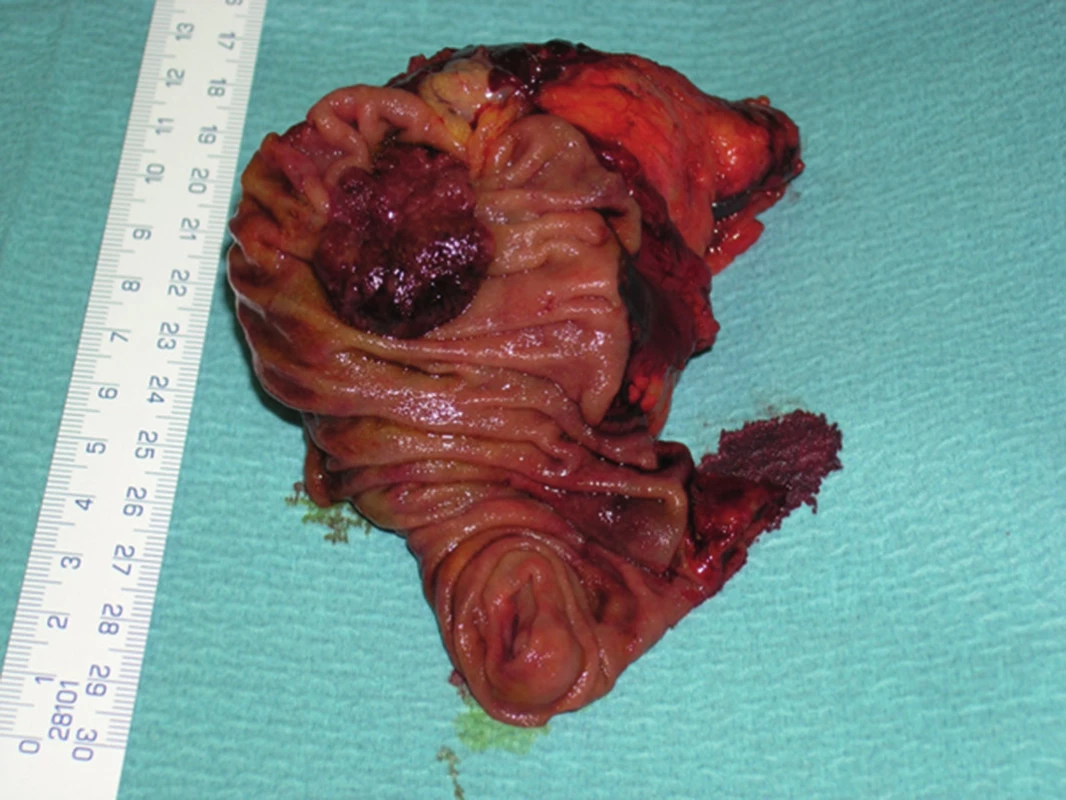
Fig. 6. Adenocarcinoma of the ampullary region with invasion to the pancreas, HE, 100x 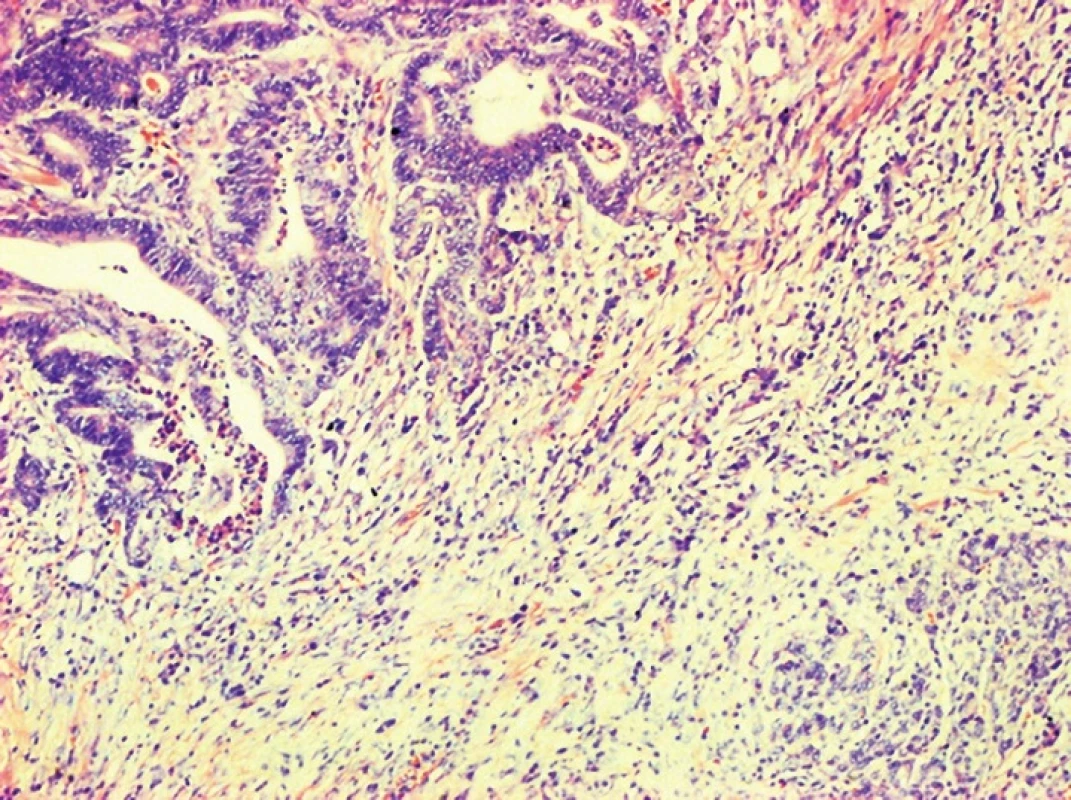
High grade intraepithelial neoplasia (carcinoma in situ) and non-invasive papillary carcinoma are significantly less common. They affect the intra-ampullary portion of the duct, are not directly visible during endoscopy and they deform the entire papilla of Vater. Ampullary adenocarcinomas may be ulcerated or may present with a central defect. They are usually only diagnosed once they reach a size which causes biliary obstruction.
A localised adenocarcinoma of the duodenum without signs of generalisation is indicated for radical removal. The rapidly developing method of endoscopic submucosal dissection (ESD) may only be considered for small non-ampullary carcinomas [11]. ESD performance is always dependent on the exceptional dexterity of the endoscopist. The situation is different for small ampullary tumours, where after endoscopic removal, the histological character of the tumour is clarified and based on the findings, the subsequent course of action is determined. Most adenocarcinomas are indicated for surgical treatment.
Local excision – ampullary resection – performed by a longitudinal duodenotomy, is indicated very selectively, primarily for benign tumours or for patients in whom a pancreatoduodenectomy is considered too risky. In most patients with adenocarcinoma of the papilla of Vater, pancreatoduodenectomy is indicated.
However, the selected approach and type of procedure depends on the preoperatively determined extent of tumorous changes, on the patient’s overall health condition, on the evaluation by the multidisciplinary team, and on the decision of the surgeon.
2 – Mesenchymal tumours
As mesenchymal tumours occurring in the small bowel, GISTs are more commonly seen in females than in males. Out of the overall incidence in the GIT, the duodenum is affected in about of 5% [9]. GIST tumour cells arise from interstitial cells of Cajal, and are localized in the area between the circular and longitudinal muscle layers of the GIT wall and may present with bleeding or obstruction after a long asymptomatic period. Mitotic index and tumour size are the main criteria for tumour classification into prognostic group defining biological behaviour. Duodenal GIST is much stricter assessment than in GISTs of the stomach due to a higher probability of progression [14].
Currently, no unequivocal criterion exists, which could exclude the possibility of malignant behaviour of this tumour. Diagnosing a duodenal GIST is very difficult, not only because of problematic collection of a relevant bioptic sample during endoscopy, but also during evaluation of the CT or MRI scan. The scan often shows an undefined hypervascular structure, which if it is large may include a cystic or necrotic component. And thus often only a suspicion of GIST is pronounced based on the imaging findings. Cytological examination of tissue collected during endosonography by fine needle aspiration provides the correct diagnosis in up to 80% (cited according to [14]).
A breakthrough in GIST therapy was the introduction of imatinib mesylate (Gleevec) into clinical practice in 2002 as a competitive inhibitor of the KIT receptor responsible for cell proliferation (cited according to [12]). Subsequently, 10 years later it became possible to use regorafenib in patients in which therapy with imatinib and sunitinib failed [13]. Despite this success, surgical tumour removal remains justifiable (Fig. 7,8).
Fig. 7. GIST of the duodenum – resected specimen 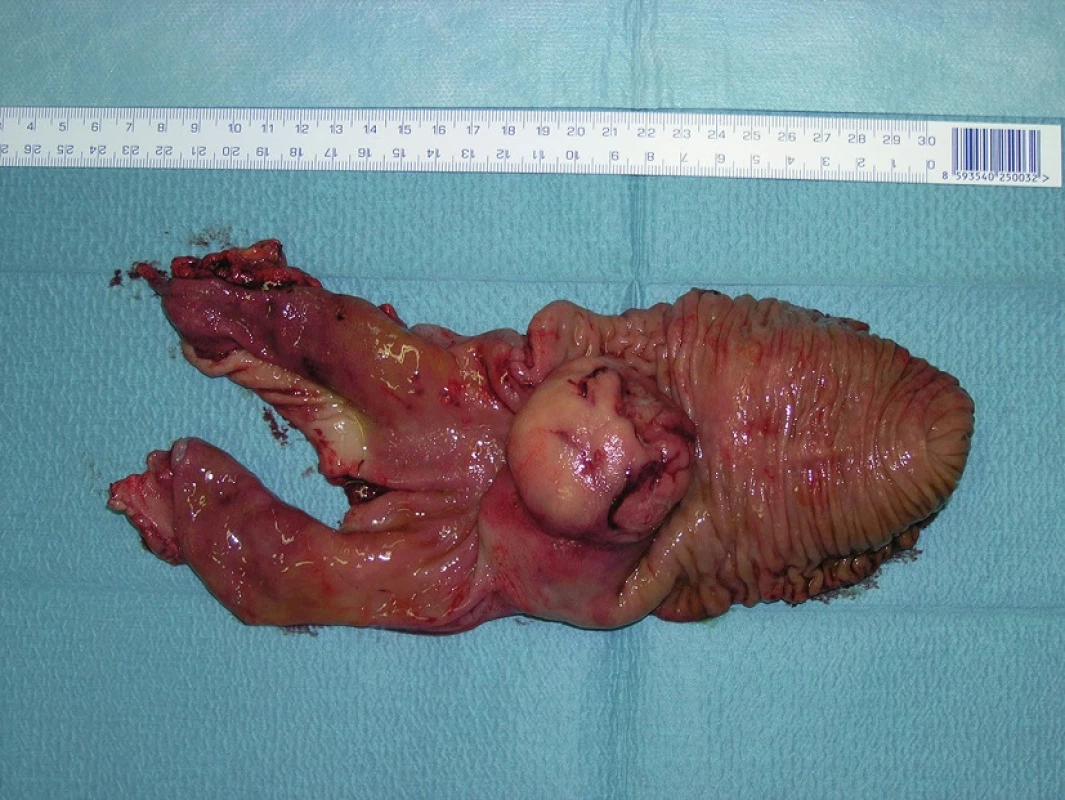
Fig. 8. GIST of the duodenum, HE, 100x 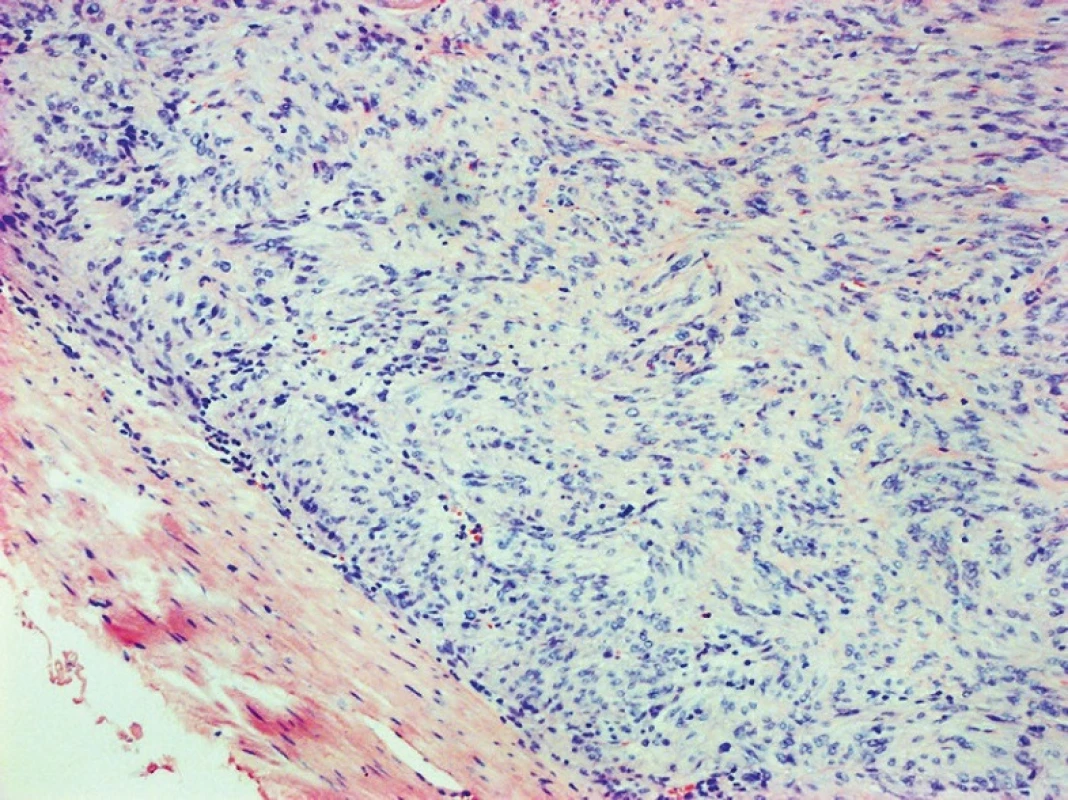
Tumour resection is primarily indicated in patients without signs of disease dissemination, and where the tumour is removable. The only potentially curative method is an R0 resection. To date, there is no unanimous view regarding the optimal method to remove a GIST of the duodenum [14,15]. Thus the procedures may be very diverse, from local excisions and enucleations to a pancreatoduodenectomy. A lymphadenectomy is not performed. Limiting factors in selecting the optimal procedure are mainly tumour size, and whether there is infiltration to surrounding structures. An important factor is whether it is possible to preserve the papilla of Vater in situ. For GISTs up to 2 cm in size with margins at least 2 cm from the papilla of Vater, it is appropriate to perform an excision while ensuring a safe suture of the wall of the duodenum or of the anastomosis. An R0 resection is required. Even in the case of larger well bordered GISTs in the D3−4 region, it is possible to avoid a pancreatoduodenectomy by performing a segmental duodenectomy with an end-to-end or side-to-end duodenojejunal anastomosis. In the remaining cases, a pancreatoduodenectomy is indicated. The main argument against local procedures is a theoretically greater risk of recurrence. Because the degree of malignancy of the tumour is not determined at the time of the operation, the surgeon’s decision regarding the adequacy of the procedure is complicated. Literary data are inconclusive. An unequivocally positive resection margin, not the type of procedure, is considered to be the primary cause of recurrence [14].
3 – Neuroendocrine neoplasms (NEN) of the duodenum
In the duodenum, the most common neuroendocrine tumours, which may be indicated for surgical treatment, are serotonin-producing neuroendocrine tumour – NET (carcinoids). Multifocal localization is seen in one-third of cases. Another possible type of tumour is gastrin-producing NET, and between 40−50% are located in the duodenum when associated with Zollinger-Ellison syndrome (ZES). In 25% of cases it is associated with autosomal dominant Multiple Endocrine Neoplasia type 1 (MEN 1). Therefore in patients with sporadic ZES NET of the duodenum, it is appropriate to search for other possible tumours in the pancreas [9]. The type of surgical procedure must therefore be adjusted according to the localization as well as possible multiplicity. The aim of the procedure is to minimize the resection and preserve both the duodenum as well as the pancreatic parenchyma.
4 − Non-Hodgkin malignant lymphoma
Non-Hodgkin malignant lymphoma includes a wide spectrum of lymphoproliferative neoplasms from B, T and NK cells. Surgical procedures most often include collecting a sufficient amount of tissue for histological and other special analyses, which are necessary for determining the optimal systemic treatment. If the lymphoma is localized to only a segment of the duodenum and is causing obstruction problems for the patient, then a resection procedure may be considered, not only a gastroenteroanastomosis. There is need to provide of PET after successful surgical resection for exclusion of lymphoma dissemination and to assess of adjuvant therapy (combined chemo - and target therapy) with hematooncologists.
Surgical procedures for malignancies localized in the duodenum and their complications
Surgical procedures may be divided into local tumour excisions and resection procedures, where the fundamental aim of both groups is to perform an R0 resection, which means to remove the tumour with a negative resection margin. Selecting the appropriate surgical procedure depends not only on the premise of R0 resection, but also on the potential risk of complications, and on the patients overall medical condition - Performance Status (PS).
A – Local excision of duodenal tumours
In cases of smaller malignant or potentially malignant duodenal lesions, which are not removable endoscopically, usually high grade adenomas of GISTs or NETs, a local excision may be performed. A necessary condition for this procedure is maximally decreasing the risk of dehiscence of the duodenal suture or of the duodenoduodenal or duodenojejunal anastomosis. It is important to avoid iatrogenic injury to the papilla of Vater or deformation of this area by a close or interrupted suture. The most serious complication is suture dehiscence in the early postoperative course. Leakage of gastric juice, bile and pancreatic juice may total more than 3–4 litres/24 hours and leads to the development of diffuse peritonitis and sepsis as well as rapid metabolic collapse. Treatment of the dehiscence is usually very difficult. It is extremely important to try to prevent this complication and minimize its risk by respecting the rule of tension-free suture of the duodenal wall and duodenal decompression by nasoduodenal tube. Some authors recommend the introduction of a temporary transparietal intraduodenal T-tube drain.
Postoperative pancreatitis is the second possible serious complication. It is associated with intraoperative manipulation or instrumentation in the area of the papilla of Vater or head of the pancreas.
Complete local resection of duodenal carcinoma leads to 5-year survival in 50–60% patients. In patients with non-metastasizing NET, this number increases to 75–95%. Radical removal of GISTs up to 5 cm in size with low mitotic index of tumour cells (<5/50 HPF) cures most patients.
B – Ampullary resection
Ampullary resection is usually indicated in small NET tumours or T1 adenocarcinomas in high-risk patients and it may be considered a more extensive endoscopic sphincterotomy. During the procedure, it is necessary to identify and preserve the terminal opening of the biliary and pancreatic ducts. After mobilization of the duodenum, a longitudinal duodenotomy is performed on the anterior wall at the location where we can palpate the probe introduced to the biliary tract through the cystic duct which exits through the papilla into the lumen of the duodenum. The next step is identifying the biliary opening and introducing a stent into the pancreatic duct. After tumour resection and verification of the resection margin, reconstruction of both ducts ensues, then the removal of the probe from the biliary tract and closure of the cystic duct, removal of the stent from the pancreatic duct and a transverse suture of the duodenum.
During the early postoperative course, it is necessary to consider the possibility that postoperative pancreatitis may develop. Dehiscence of the duodenal suture after this procedure is less common. Because tumour excision may not always be considered R0, early tumour recurrence is a threat. Stenosis of the duodenum after closure by transverse suture does not occur.
C − Pancreatoduodenectomy
During the operation, the extent of the pathological findings is compared to preoperative staging and a perioperative staging is determined. Evaluation of resectability during surgery means ruling out metastatic dissemination, arterial angioinvasion to the superior mesenteric artery and to the celiac trunk and excluding tumour spread to surrounding organs. A radical resection includes a pancreatoduodenectomy with standard lymphadenectomy (Fig. 9).
Fig. 9. Post pancreatoduodenectomy and standard lymphadenectomy (intraoperative photo) 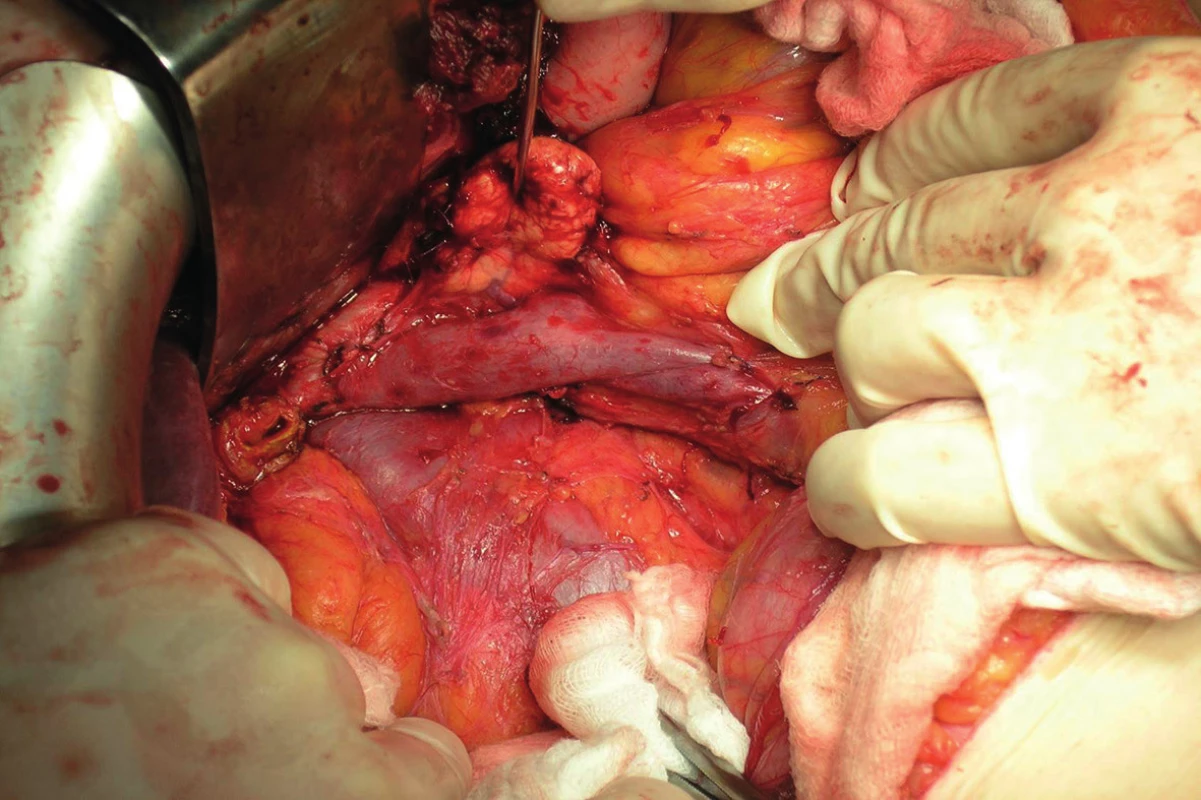
A condition of radicality is the confirmation of negative resection margins. Operation findings differ from preoperative staging in almost one-third of patients. In most cases this means a higher staging than expected. In this group of patients, an unplanned exploration or a palliative procedure instead of a planned resection, especially biliodigestive anastomosis, may be expected. In patients with advanced carcinoma of the ampullary region, it is not erroneous to perform an anastomosis, since endoscopic replacement of a biliary stent is not easy and sometimes may even be technically impossible. Collection of a bioptic sample using a Tru-cut needle is indicated in patients in whom the malignancy was not confirmed by FNA preoperatively.
A gastrojejunoanastomosis is performed in patients with stenosis of the duodenum; however, its patency does not ensure passage renewal. This is due to a motility disorder of the stomach and small bowel caused by the primary disease. We do not perform a preventative gastroenteroanastomosis. Removal of liver metastases in patients with duodenal cancer is not indicated as part of standard therapy.
A pancreatoduodenectomy is considered curative in 80% of patients with ampullary carcinoma with negative lymph node involvement (T1−3, N0). If the patient survives the first three years after the operation, he has a chance at long-term survival [17]. In patients with advanced disease, meaning staging T3−4, N1−2 and M0−1, palliative therapy is indicated. In certain young patients, or older patients in good biological condition, a palliative resection may be performed, which has mainly a psychological effect.
Although early mortality after pancreatic resection has significantly decreased over the last 25 years, and does not exceed 3–5% at specialized centres, the morbidity reaches up to 60% [16]. The most serious complications include dehiscence of the pancreatodigestive anastomosis with the development of a pancreatic and often also enteric fistula, bleeding, and intraabdominal infection. Leakage of pancreatic juice is seen in 6–24%, which requires early reoperation with prolonged hospital stay. Postoperative pancreatitis is fatal in up to 80 %. Prevention involves a careful operating technique and maintaining an adequate blood supply in the splanchnic area during surgery. Bleeding into the GIT may be caused by peptic ulcer, bleeding from the anastomosis, by an eroded arterial stump with rupture into the small bowel or due to digestion of a blood vessel wall by pancreatic juice. Delayed gastric emptying disorders following proximal resections of the pancreas may be seen in 25–70% of patients. Although they are not associated with a higher mortality, they are the cause of prolonged hospital stay and thus increase treatment expenses. Early enteral feeding seems to be suitable in the prevention of this complication.
Factors influencing survival after resection
Independent factors which influence survival of patients with adenocarcinoma of the papilla of Vater are its size (T >2 cm), lymph node involvement and angioinvasion. Other negative factors include higher tumour grading and positive resection surface. Perineural tumour spread is unequivocally a very unfavourable factor. Perioperative blood transfusion decreases the patient’s chance of long-term survival. Jaundice (bilirubin >100 µmol/l) does not have a negative effect on long - term survival. Preoperative weight loss and performing an extended surgical procedure does not significantly affect long-term survival of the patient. The biological character of the tumour has an undeniable effect: cell differentiation and the presence of mutated oncogenes and suppressor genes. The surgeon is an independent treatment factor, who directly affects the survival of the patient as well as his prognosis. The association between the number of resection procedures performed at the workplace and perioperative mortality and morbidity is clear. This is also an argument for centralization of these patients.
The role of the multidisciplinary team
Patients with a suspect or histologically confirmed malignancy of the duodenum are rarely presented at meetings of the oncological multidisciplinary team. Restricted personal experience of the surgeon and limited literature sources may cause doubts when determining optimal treatment, especially in at-risk patients. Determining principles based on effective diagnosis and preoperative disease staging, selecting optimal therapy for the specific patient and subsequent evaluation of further treatment based on the definitive staging with assessment of adjuvant therapy after radical resection, either in the case of a local endoscopic or surgical procedure or pancreatoduodenectomy, is identical in all oncological patients, including long-term follow-up.
Conclusion
No comprehensive review of duodenal tumours exists in the current literature; individual types of malignant tumours may be described within reviews of malignancies of the small bowel, sets of case reports, or individual cases, with the exception of ampullary carcinomas. Neither national nor international literature sources provide a comprehensive review of their therapy. The situation is similar when researching surgical procedures. Resection procedures on the duodenum should thus be performed in specialized centres with sufficient experience with hepato-pancreato-biliary surgery.
Supported by MO 1012.
Conflict of Interests
The authors declare that they have not conflict of interest in connection with the emergence of and that the article was not published in any other journal
Prof. Miroslav Ryska, MD, Ph.D.
Surgery Department, 2nd Faculty of Medicine,
Charles University and Central Military Hospital, Prague
U Vojenské nemocnice 1200
169 02 Prague 6
e-mail: miroslav.ryska@uvn.cz
Zdroje
1. Bosman FT, Carneiro F, Hruban RH, et al. WHO classification of tumours of the digestive system, World Health Organization International histological classification of tumours. Lyon: IARC, 2010.
2. Krška Z, Hoskovec D, Petruželka L, a kol. Chirurgická onkologie. Praha, Grada 2013.
3. Fischer JE, Kirby MD, Bland I. Mastery of Surgery. 5th edition. Wolters Kluwe, Lippincott Williams and Wilkins 2007.
4. Scott-Conner EH. Essential operative techniques and anatomy. 4th edition, Wolters Kluwe, Lippincott Williams and Wilkins 2014.
5. Schein M, Rogers PN, Leppäniemi A, et al. Prevention and management of surgical complications. Gutenberg Press Ltd., 2014 : 116–28.
6. Dilworth HP. Neoplasms of the small intestine. In: Iacobuzio-Donahue, Christine A., and Elizabeth Montgomery. Gastrointestinal and liver pathology. Philadelphia, Churchill Livingstone/Elsevier 2005.
7. Niederle B a kol. Neodkladné speciální operace. Avicenum 1984.
8. Schottenfeld D, Beebe-Dimmer JL, Vigneau FD. The epidemiology and pathogenesis of neoplasia in the small intestine. Ann Epidemiol 2000;19 : 58–69.
9. Odze RD, Goldblum JR. Surgical patology of the GI tract, liver, biliary tract and pancreas. Sauders 2015.
10. Yamaguchi K. Pancreatoduodenectomy for bile duct and ampullary cancer. J Hepatobiliary Pancreat Sci 2012;19 : 210 – 5.
11. Matsumoto S, Miyatani H, Yoshida Y. Future directions of duodenal endoscopic submucosal dissection. World J Gastrointest Endosc 2015;7 : 389–95.
12. Somaiah N, von Mehren M. New drugs and combinations for the treatment of soft-tissue sarcoma: a review. Cancer Manag Res 2012;4 : 397–411.
13. Demetri GD, Reichardt P, Kang YK, et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib: an international, multicentre, prospective, randomised, placebocontrolled phase 3 trial (GRID). Lancet 2013;381 : 295–302.
14. Cavallaro G, Polistena A, D‘ Ermo G, et al. Duodenal gastrointestinal stromal tumors: Review on clinical and surgical aspects. Int J Surg 2012;10 : 463–5.
15. Fiala L, Šefr R, Kocáková I, et al. Léčba gastrointestinálních stromálních tumorů – komplexní pohled chirurga. Rozhl Chir 2015;94 : 189–192.
16. Hariharan D, Saied A, Kocher HM. Analysis of mortality rates for pancreatic cancer across the word. HPB 2008;10 : 58–62.
17. Brown KM, Tompkins AJ, Young S, et al. Pancreaticoduodenectomy is curative in the majority of patients with node-negative ampullary cancer. Arch Surg 2005;140 : 529–33.
Štítky
Chirurgie všeobecná Ortopedie Urgentní medicína
Článek vyšel v časopiseRozhledy v chirurgii
Nejčtenější tento týden
2015 Číslo 12- Metamizol jako analgetikum první volby: kdy, pro koho, jak a proč?
- Nejlepší kůže je zdravá kůže: 3 úrovně ochrany v moderní péči o stomii
- Stillova choroba: vzácné a závažné systémové onemocnění
- Metamizol v léčbě různých bolestivých stavů – kazuistiky
-
Všechny články tohoto čísla
- Desetileté jubileum Chirurgické kliniky 2. LF UK a ÚVN, Praha
- Malignant tumours of the duodenum
- Irreversible electroporation in the treatment of locally advanced pancreatic cancer
- Surgery of the hiatal hernia and gastroesophageal reflux dinase, Nissen or Toupet?
- Třetí vydání monografie o štítné žláze
- Bile leakage after liver resection: A retrospective cohort study
- Oznámení
- “Liver fist approach“ in the management of synchronous liver metastases from colorectal cancer: Preliminary non-randomized study results
- Da Vinci assisted surgery for rectal cancer – preliminary results of nonrandomized trial
- Urgent surgical treatment of gastric volvulus related to upside-down stomach syndrome
- Za profesorem Larsem Påhlmanem
- Rare situations in treatment of polytraumatised patients – case reports
- Komentář
- XIX. Národní kongres ČSOT 2015
- Rozhledy v chirurgii
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Surgery of the hiatal hernia and gastroesophageal reflux dinase, Nissen or Toupet?
- Urgent surgical treatment of gastric volvulus related to upside-down stomach syndrome
- Malignant tumours of the duodenum
- Bile leakage after liver resection: A retrospective cohort study
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



