-
Medical journals
- Career
Benefits and limitations of physical activity in pediatric patients with inflammatory bowel diseases
Authors: P. Dvoran; D. Kosorinova; P. Suchá; Z. Havlíčeková; Z. Michnová; P. Bánovčin Jr.
Authors‘ workplace: Department of Children and Adolescents, Jessenius Faculty of Medicine in Martin, Commenius University in Bratislava, University Hospital in Martin
Published in: Gastroent Hepatol 2023; 77(6): 484-494
Category:
doi: https://doi.org/10.48095/ccgh2023484Overview
Introduction: Inflammatory bowel diseases (IBDs) are a group of diseases including Crohn’s disease, ulcerative colitis and unclassified IBD. They are chronic, immune-mediated inflammatory diseases of the gastrointestinal tract. Due to their extraintestinal manifestation, they are considered to be systemic diseases. IBD has a negative impact on health-related quality of life. The treatment is life-long and may be accompanied by side effects. Low-intensity activity of moderate duration is sufficient to elicit improvements in fitness, decrease stress, and improve symptoms. In this study, the relationship between physical activity and IBD development and course is described. Aims: To assess the relationship between physical activity and exercise capacity and the severity and course of IBD. Methods: Probands with IBD and healthy controls underwent pulmonary function testing (PFT) and cardiopulmonary exercise testing (CPET). The results were compared between these two groups; in the IBD group, the results were correlated with disease activity parameters as well. In the IBD group, the probands were encouraged to perform regular physical activity. After 0.5–1 year, they underwent another CPET. Their exercise capacity development was objectively assessed and it was correlated with their IBD development. Results: Forty-one children were included in the study. Twenty of them were healthy controls, while 21 comprised the IBD patient group. These probands underwent the PFT and CPET. When comparing healthy controls and IBD patients, significant differences were found in peak oxygen uptake and peak work rate. In the IBD group, a negative correlation was found between peak work rate and C-reactive protein concentration. In faecal calprotectin, a significant correlation was found only in the subgroup with ulcerative colitis – the negative correlation with peak oxygen uptake and partial pressure of CO2 in exhaled air in the peak of the exercise. PCDAI correlated positively with ventilatory index, peak dead space and tidal volume ratio but negatively with peak work rate, work rate at the time of anaerobic threshold, peak oxygen uptake and oxygen uptake efficacy slope. In PUCAI, we found a positive correlation with CO2 ventilatory uptake at the time of anaerobic threshold and negative correlations with peak oxygen uptake and oxygen uptake efficacy slope. In further CPET in the IBD group, a significant decrease was found in faecal calprotectin and PCDAI or PUCAI; there were also positive correlations between PCDAI/PUCAI changes and resting heart rate changes. The negative correlation was found in the correlation with the changes in peak oxygen uptake, metabolic index and ventilatory equivalent for CO2 at the time of anaerobic threshold. Conclusion: These results suggest that regular recreational physical exercise positively impacts IBD. When adhering to treatment recommendations, doing sport professionally is also possible.
Keywords:
inflammatory bowel diseases – physical activity
Introduction
Inflammatory bowel diseases are a group of chronical diseases characterised by recurring episodes of relapse and remission. In this group, two main units are described: ulcerative colitis (UC) and Crohn’s disease (CD). Unclassified colitis (IBD-U), an illness with an atypical phenotype that does not properly fit any previous units, is also considered to be a type of IBD. Although inflammatory bowel diseases mainly affect the gastrointestinal tract, due to their frequent extraintestinal manifestations, they are considered to be systemic illnesses [1–3].
A positive impact of adequate regular physical activity on physical and mental health is generally known. In children and adolescents, physical exercise is the cornerstone for health maintenance and wellbeing; it lowers the risk of cardiovascular diseases and malignancies and it has a positive impact on bone mineral density [4–8]. In addition, it improves cognitive functions as well as the ability to cope with some difficult situations. There are several hypotheses claiming it can reduce the symptoms of anxiety and depressive disorders [9]. In contrast, inactivity is considered to be one of the most important risk factors of cardiovascular diseases, type II diabetes mellitus, obesity, some neoplastic diseases and bone and joint diseases [10]. Generally, mild or moderate intensity physical activity (e. g. jogging, or swimming) for at least 20–30 min (optimum 45 min) 3-times a week is recommended [11].
IBDs severely influence patients’ daily life and may cause several problems at school or work. They require long-term treatment, which may involve side effects, thereby reducing the patients’ treatment adherence [12].
In some chronic inflammation disorders, regular physical activity can be helpful, or could even be the adjuvant part of the therapy [13–16].
Children with IBD generally manage to perform regular aerobic exercise; however, they often state that they are less active than they were before their diagnosis. About 18% of children asked about their physical activity stated that they stopped doing their favourite sports regularly after being diag - nosed with IBD [17]. According to Gatt et al (2019) the tendency for a sedentary lifestyle is observed more in the group of patients with UC than in the group with CD [18].
Regular physical exercise may improve the course of IBD and reduce complications. Regular physical activity of a metabolic equivalent (multiple of quiescent metabolism) of 3–6 may have an immunomodulatory effect. During moderate intensity physical exercise, factors, e. g. interleukin 6, are released, which may lead to the production of glucagon-like peptide – the trophic factor associated with the intestinal mucosa reparation [19]. Animal-model studies showed a decrease in proinflammatory cytokines (e. g. TNFa or interleukin 1b) secretion. Exercise helps to reduce the stress-induced barrier dysfunction and softens the colitis symptoms [20]. On the other hand, short-term high-intensity exercise may lead to transitory elevation of inflammatory markers [21]. Patients with IBD may also profit from the positive impact of physical exercise on bone mineral density, as this also prevents pathological fractures [22,23].
In pediatric IBD patients, there are some limitations of regular physical activity. Children with active disease often feel abdominal discomfort and may be afraid of its worsening during physical exercise. There are also certain extraintestinal complications that may prevent the child from doing some sports.
Inflammatory bowel diseases can affect (inter alia) joints (type I and II enteropathic arthritis), the respiratory system (bronchiectasis, bronchitis) and the cardiovascular system (pericarditis, myocarditis, arrhythmias) which may prevent individuals from performing sports at an amateur or professional level [24,25].
Methods
Study population
In this study, 42 probands were included, aged 7–19 years. We created two groups of probands: 1. a group of IBD patients presenting to the outpatient gastroenterology office at the Department of Children and Adolescents in University Hospital of Jessenius Faculty of Medicine in Martin of Commenius University; and 2. a group of healthy controls, which was recruited from the population of the children who underwent CPET before being admitted to a professional sport club and started doing sports professionally. In the IBD patient group, only probands that were motivated and cooperative were included. Absence of current intestinal or extraintestinal symptomatology, that may limit the physical exercise and CPET, was also necessary. Children with a history of acute illness within the last 3 weeks were excluded from both groups. In the healthy control group, we included healthy probands with a sedentary lifestyle similar to the examined IBD patients. In the IBD group, mostly the newly diagnosed probands were included, however, in some cases, we had to wait because of their inability of performing CPET in the acute phase of the disease. Each proband’s parent signed the written consent for participation in the study. The study was approved by the Ethical Committee of Jessenius Faculty of Medicine.
Testing
All children came to the hospital by car or by bike or walked if their residence was within 10 min of the hospital. Patients were informed to consume a light meal no later than 1 hour before testing and to come properly hydrated. Every athlete underwent a pulmonary function test (PFT, spirometry) (Geratherm Respiratory GmbH, Germany) and 12 lead electrocardiography (ECG) (BTL Cardiopoint, Czech Republic); only those with physiological findings were included. Afterwards, CPET was performed in the upright position using treadmill (ITAM ERT-100, Poland) with a breath-by-breath respiratory gas analysis (Geratherm Respiratory GmbH, Germany). Subjects breathed through a face mask of an appropriate size and through a low impedance turbine volume transducer for the measurement of expiratory volume and expiratory gas concentrations. CPET results were interval averaged (every 30 s); the reported peak value represents the mean value of all data collected during the final stage (if longer than at least 30 s). CPET was performed with the personalised incremental ramp protocol to maximal exhaustion which was achieved between the 8th and 12th minute of exercise [6]. Blood pressure was measured every 2 minutes until peak exercise and then every 2 minutes until full recovery. All participants were verbally encouraged to exercise to exhaustion. Cut-off values for maximal exertion were established as an RER greater than 1.10, breathing rate more than 40/min and/or a plateau in oxygen consumption. Before testing, all equipment was calibrated according to the instructions of the manufacturer.
In the IBD patient group, baseline C-reactive protein level (CRP) (mg × l–1), faecal calprotectin (μg/g of stool) and Pediatric Crohn’s disease Activity Index (PCDAI) or Pediatric Ulcerative Colitis Activity Index (PUCAI) values were established.
After a 0.5–1-year period, IBD patients underwent CPET repeatedly and CRP, faecal calprotectin and PCDAI or PUCAI were set again. After the first CPET, these patients were encouraged and motivated to perform regular recreational physical exercise and improve their physical fitness.
Data and statistical analysis
First, we compared PFT results between IBD patients and healthy controls. We also compared the subgroups with Crohn’s and ulcerative colitis; the same was done with CPET results. In the IBD group, the CPET data were correlated with current CRP, faecal calprotectin and PCDAI or PUCAI. When performing the second CPET, we assessed the improvement in CPET results and correlated it to the improvement in CRP, faecal calprotectin and PCDAI or PUCAI.
The data was processed using the basic descriptive statistics methods. The data distribution was examined by Shapiro-Wilk’s normality test. Results are presented as mean values ± standard deviation and the median and 25th and 75th percentile. For comparison of the data with normal distribution, Student’s t-test was used. Data without normal distribution was compared by the Mann-Whitney U-test. Correlations between the sets of data were examined by correlation analysis with a Pearson correlation coefficient setting. Alterations between the sets of data obtained from repeated tests were examined by Wilcoxon’s pair test.
Results with P values less than 0.05 were considered to be statistically significant.
Results
We examined 41 children and adolescents: 21 with IBD (2 females and 19 males; 13 with CD and 8 with UC) and 20 male healthy controls. Ten of the 21 IBD patients failed to come for repeated CPET, therefore only 11 (8 with CD and 3 with UC) were tested repeatedly. The anthropometric characteristics of all groups are shown in Tab. 1–3. In the IBD group, the disease activity was evaluated as well. The results are shown in Tab. 4. Eleven of the IBD patients underwent repeated CPET with the re-evaluation of the disease activity parameters, which is shown in Tab. 5.
1. Age and anthropometric characteristics in the group of patients with IBD. // Tab. 1. Vek a antropometrické charakteristiky v skupine pacientov s IBD. 
2. Age and anthropometric characteristics in the group of healthy controls. // Tab. 2. Vek a antropometrické charakteristiky v skupine zdravých kontrol. 
3. Age and anthropometric characteristics in the group of IBD patients who came for repeated CPET. // Tab. 3. Vek a antropometrické charakteristiky v skupine pacientov s IBD, ktorí prišli na opakovaný CPET. 
4. Disease activity parameters in the IBD group. // Tab. 4. Parametre aktivity ochorenia v skupine IBD. 
5. Disease activity parameters in IBD patients who came for repeated CPET. // Tab. 5. Parametre aktivity ochorenia u pacientov s IBD, ktorí prišli na opakovaný CPET. 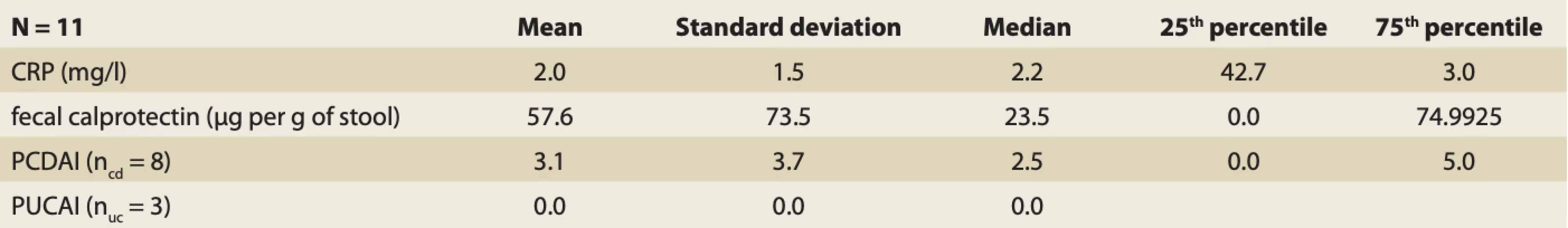
In the IBD group, 8 of the CD patients and 3 of UC patients suffered from mild-active disease. The other (6 with CD and 4 with UC) were in remission. One of the CD patients suffered from perianal disease, another one with CD suffered from erythema nodosum, however, it did not prevent them from doing physical exercise. All of the IBD patients were treated according to the current Guidelines of the Gastroenterology and Nutrition Section of Czech and Slovak Pediatric Society.
6. Differences between PFT findings in IBD patients and healthy controls (Student’s T-test). // Tab. 6. Rozdiely medzi nálezmi PFT u pacientov s IBD a zdravých kontrol (Studentov t-test). 
7. Differences in CPET parameters between IBD patients and healthy controls (Student’s t-test). // Tab. 7. Rozdiely v parametroch CPET medzi pacientmi s IBD a zdravými kontrolami (Studentov t-test). 
8. Differencies in CPET parameters between IBD patients and healthy controls (Mann-Whitney U-test). // Tab. 8. Rozdiely v parametroch CPET medzi pacientmi s IBD a zdravými kontrolami (Mann-Whitney U-test). 
First, we compared PFT results between IBD patients and healthy controls (Tab. 6). Student’s T-test analysis shows a significant difference only in peak expiratory flow (PEF) (P = t = 2.06; P = 0.0457). In PFT, percentages of the values appropriate for sex and age were compared. In the IBD group, PEF was 90.62 ±16.36%, whereas it was 100.5 ±12% in the healthy control group. No significant differences were found between the CD and UC subgroups.
In the CPET data comparison, we found several significant differences between the IBD group and healthy controls (Tab. 7, 8): peak breathing frequency (BF peak) – t = 4.66; P = 0.000036, peak diastolic pressure (Dia BP peak) – t = 2.58; P = 0.0138, breathing frequency at anaerobic threshold (BF at AT) – t = 2.32; P = 0.0257, maximum work rate per kilogram of weight (WR/kg) U = 2.23; P = 0.0259 and oxygen uptake per kilogram of weight (VO2 peak/kg) – U = = 3.22; P = 0.00128. Parameters in healthy controls were significantly higher: BF peak = 53.1 ±5.64 breaths per minute; Dia BP peak = 71.5 ±11.13 mmHg; BF at AT = 34.08 ±8.83 breaths per minute; WR peak /kg = 4.14 ±0.47 W × kg–1; VO2 max/kg = 47.33 ±7.56 ml × kg–1 × min–1. In IBD patients, these parameters were: BF peak = 43.86 ±6.94 breaths per minute; Dia BP peak = 62.86 ±10.32 mmHg; BF at AT = 28.48 ±6.52 breaths per minute; WR peak /kg = 3.50 ±0.88 W × kg–1; VO2 max/kg = 37.59 ±10.01 ml × kg–1 × min–1. The only significant difference found between the CD and UC subgroups was the heart rate at anaerobic threshold (HR at AT) (U = 18.5; P = 0.025). In CD patients, the mean HR at AT was 147.71 ±12.64 beats per minute, while in UC patients it was 134.86 ±11.29 beats per minute.
In the group of probands with IBD, we correlated the CPET parameter with CRP, faecal calprotectin and PCDAI or PUCAI (Tab. 9).
The C-reactive protein value correlates positively with resting heart rate (HR rest) (r = 0.55; P = 0.001), heart rate in the 1st minute of exercise (HR1) (r = 0.51; P = 0.019), the heart rate in the 2nd minute of exercise (HR2) (r = 0.64; P = 0.002) and breath reserve at anaerobic threshold (BR at AT) (r = 0.48; P = 0.027). In addition, it correlates negatively with peak work rate per kilogram of weight (WR peak/kg) (r = –0.44; P = 0.046), peak breathing frequency (BF peak) (r = –0.47; P = 0.031) and respiratory exchange ratio at anaerobic threshold (RER at AT) (r = –0.57; P = 0.007).
Faecal calprotectin concentration correlates negatively with peak diastolic pressure (r = –0.49; P = 0.023).
When dividing IBD patients into the CD and UC subgroups, we found that CRP serum concentrations in CD patients correlated positively with HR rest (r = 0.70; P = 0.053), HR1 (r = 0.67; P = 0.009) a HR2 (r = 0.76; P = 0.002) and negatively with BF rest (r = –0.53; P = 0.049) and RER at AT (r = –0.64; P = 0.013). In UC patients, CRP concentration correlated only with BR at AT (r = 0.80; P = 0.03 – positive correlation). In the UC subgroup, faecal calprotectin correlated positively with BF rest (r = 0.83; P = 0.02) and negatively with VO2max/kg (r = –0.80; P = 0.031) and peak partial CO2 pressure in exhaled air (PET CO2 peak (r = –0.93; P = 0.003).
The Pediatric Crohn’s disease Activity Index correlated positively with: HR2 (r = 0.63; P = 0.016), BR peak (r = 0.73; P = 0.003), ventilatory index (VE/VCO2) slope (r = 0.57; P = 0.032), peak dead space ventilation ratio (r = 0.59; P = 0.027), ventilatory equivalent for CO2 (ratio of minute ventilation and the amount of exhaled CO2) at anaerobic threshold (VE/VCO2) v AT (r = 0.63; P = 0.015) and dead space/tidal volume ratio at anaerobic threshold (Vd/Vt at AT) (r = 0.56; P = 0.036). PCDAI correlated negatively with resting O2 Pulse (r = –0.56; P = 0.036), WR peak (r = –0.68; P = 0.008), WR peak /kg (r = –0.81; P = 0.000), peak oxygen uptake (VO2peak) (r = –0.74; P = 0.002), peak oxygen uptake per kilogram of weight VO2peak/kg (r = –0.66; P = 0.010), peak minute ventilation (VE peak) (r = –0.72; P = 004), peak breathing frequency (r = –0.57; P = 0.033), peak tidal volume (r = –0.60; P = 0.023), peak O2 Pulse (r = –0.73; P = 0.003), peak systolic blood pressure (Sys BP peak) (r = –0.54; P = 0.045), oxygen uptake efficacy slope (OUES) (r = –0.63; P = 0.017), O2 Pulse at anaerobic threshold (r = –0.69; P = 0.006), VE at anaerobic threshold (r = –0.56; P = 0.037) and work rate at anaerobic threshold (r = –0.61; P = 0.021).
9. Correlation analysis between CPET parameters and CRP, faecal calprotectin, PCDAI or PUCAI. // Tab. 9. Korelačná analýza medzi parametrami CPET a CRP, fekálnym kalprotektínom, PCDAI alebo PUCAI 
10. Correlation analysis of the relationship between CPET parameter develop- ment and faecal caprotectin, PCDAI or PUCAI change. // Tab. 10. Korelačná analýza vzťahu medzi vývojom parametra CPET a zmenou fekálneho kaprotektínu, PCDAI alebo PUCAI. 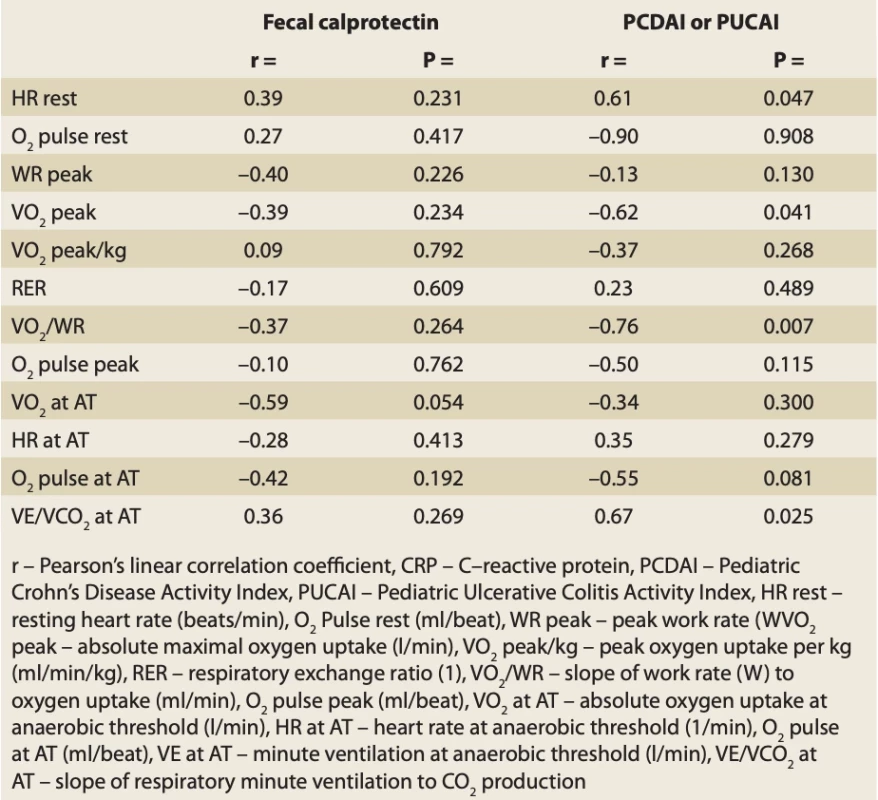
In the Pediatric Ulcerative Colitis Activity Index, positive correlations were found between PUCAI and: BF rest (r = 0.92; P = 0.03) and VE/VCO2 v AT (r = 0.87; P = 0.012). Negative correlations were found in the case of: VO2max/kg (r = –0.84; P = 0.017), PET CO2 peak (r = –0.88; P = 0.009), OUES (r = –0.81; P = 0.026) and O2 Pulse at AT (r = –0.80; P = 0.031).
In 11 IBD patients, we assessed the correlation between CPET parameters development (change of exercise capacity) and the change of CRP, faecal calprotectin and PCDAI or PUCAI. All of these 11 probands were in remission during the repeated CPET. None of them developed any condition that may affect CPET result.
We found that there was a significant change in faecal calprotectin (from 173.00 ±174.07 to 57.64 ±73.47; P = 0.02183) and in PCDAI or PUCAI (from 11.36 ±8.69 to 2.27 ±3.34). In the CPET parameters, a significant change was found for 12 parameters: HR rest (P = 0.0051), O2pulse rest (P = 0.041), WR peak (P = 0.0051), VO2peak (P = 0.040335), VO2 peak/kg (P = 0.01637), RER (P = 0.008049), metabolic index (VO2/WR) (P = 0.00691), O2 pulse peak (P = 0.005847), HR at AT (P = 0.02938), O2 pulse at AT (P = 0.03286), VO2 at AT (P = 0.03286) and VE/VCO2 at AT (P = 0.01637). In 3 of these parameters, a significant decrease was found: HR rest from 104.73 ±9.11 to 96.36±12.65; HR at AT from 145.91 ±15.80 to 138.00 ±12.28 and VE/VCO2 at AT from 28.58 ±4.84 to 26.88 ±3.67. In the other CPET parameters, significant increase was found: O2pulse rest from 2.91 ±0.95 to 3.55 ±0.86; WR peak from 224.73 ±132.89 to 265.31 ±129.10; VO2peak z 2.25 ±1.19 to 2.69 ±1.15; VO2 peak/kg from 36.19 ±10.38 to 38.27 ±10.28; RER from 1.15 ±0.16 to 1.22 ±0.15; VO2/WR from 8.39 ±2.20 to 10.34 ±1.70; O2 pulse peak from 12.25 ±5.85 to 13.50 ±5.22 and VO2 at AT from 1.52 ±0.80 to 1.79 ±0.74.
There was no correlation found between the change in faecal calprotectin and CPET parameters. In the case of PCDAI and PUCAI, we found a positive correlation with HR rest (r = 0.61; P = 0.047) and negative correlations with VO2peak (r = –0.62; P = 0.041) and VO2/WR (r = –0.76; P = 0.007). Complete results are shown in Tab. 10.
During the CPET, in all of the probands (either from IBD group or healthy-controls group), no complications occurred. None of the patients suffered from ectopic cardiac rhythm, there were no ST segment elevations or depressions noted in the ECG records and blood pressure and heart rate reacted normally in all of the individuals. In the recovery phase, the monitored parameters normalised adequately.
Discussion
Inflammatory bowel diseases are a group of diseases that can severely limit a patient’s life. Children and adolescents with IBD are usually more absent from school than the healthy population and IBD symptoms often restrict their daily activities. The diagnosis of a chronic illness is often the reason why the patient forgoes their hobbies. Partially, it is the result of the limitations that come from the disease, but in children and adolescents, there is often not enough time for free-time activities when they are absent from school too much. Leaving previously attended amateur or professional clubs (either sport, art or another type) is a frequently seen phenomenon. IBD patients often tend to lead a sedentary lifestyle more than the healthy population. These factors, amongst others, can lead to a decrease in their quality of life.
The treatment of IBD is long-term and can be accompanied by various side effects. These can, in some cases, lead to patient’s non-compliance with the treatment. According to previously published studies, long-term moderate intensity physical exercise can positively impact chronic inflammation. Therefore, physical exercise can be considered an adjuvant form of therapy in some regards.
In IBD patients, there are several factors that can prevent people from doing physical exercise regularly. The acute phase of the disease and its symptoms prevent the patient from performing sporting activities. On the other hand, during remission, limiting symptoms (e. g. abdominal pain or nausea) are usually not present. Inflammatory bowel disease can also be accompanied by cardiovascular or respiratory impairment; however, in children and adolescents, only case reports and case series of these types of impairments are available [22,24–28].
In our study, probands underwent quiescent electrocardiography, pulmonary function testing and cardiopulmonary exercise testing. In addition, IBD patients also underwent body plethysmography and diffuse lung capacity measurements, either during their regular pneumological dispensary or before being included in this study. All of the results were in the normal reference range for the probands’ sex and age. During CPET, none of the probands suffered from ectopic cardiac rhythm, inadequate blood pressure or heart rate exercise reaction; in the recovery phase, all of the monitored parameters normalised promptly.
At the study outset, the probands underwent pulmonary function testing. Comparing IBD patients and healthy controls, we found a significant difference in peak expiratory flow, but all of the measured parameters were within the normal limits. PFT abnormalities were also studied by Bąk-Drabik et al (2022) and El Amrousy et al (2018) [29,30]. In these studies, significant differences in MEF25 and MEF50 were found; however, all of the tested parameters were, similarly to our study, within normal limits. Subclinical respiratory involvement is quite common in IBD patients, and may be present in 50% of cases in the adult population [31].
In the following, we have compared CPET parameters between IBD patients and healthy controls. We found significant differences in peak breathing frequency, peak diastolic pressure, peak oxygen uptake per kilogram of weight and peak work rate per kilogram of weight.
Lower VO2max, or, in the case of not achieving the oxygen uptake plateau phase, a lower VO2peak, can refer to the lower aerobic capacity of children with IBD. A similar finding was published by Ploeger et al (2011), where a cycle ergometer was used to evaluate anaerobic (Wingate anaerobic cycling test) and aerobic (McMaster All-Out Progressive Continous Cycling Test) capacity. Significant differences in peak work rates were also found [32]. Lower peak work rates refer to a lower exercise capacity in the IBD group.
Paradoxically, we have found significantly higher peak breathing frequency in the healthy control group. During physical exercise, the enlarging of tidal volume is considered to be more effective for achieving a higher work rate, rather than heightening the breathing frequency. However, the VE/VCO2 slope, which is more likely to be used to assess breathing effectiveness [33], was tested in both groups with no significant differences found.
Peak diastolic pressure is the parameter with a low information value. It is only evaluated in the case of pathologically high values.
When comparing CPET parameters with CRP, faecal calprotectin and PCDAI or PUCAI, we have found several correlations. The correlations with VO2peak or WR peak suggest that aerobic capacity may have a positive impact on disease activity and course. We have compared our results with those of Ploeger et al (2011). In this study, the correlations between peak work rate or peak oxygen uptake and disease activity were not verified [32]. At the time of publishing of this article, the study by Vanhelst et al is still in progress. This study explores the relationship between exercise capacity and IBD activity through FitnesGramm and Eurofit tests [34]. Other studies describing the exercise capacity and IBD activity and course have not been published yet.
Finally, we have found that the increase in exercise capacity correlates with the decrease in PCDAI or PUCAI. In patients that were encouraged to perform regular physical exercise, we noted a significant decrease in faecal calprotectin, even though it was without the correlation with concrete CPET parameters. This finding suggests that improvements in sport may moderate the disease its symptoms.
Several studies were published concerning long-term physical exercise and IBD. In the study by Lageret et al (2019), the effect of regular exercise during an 8-week period was described. They have shown that exercise of adequate intensity and duration leads to a decrease in the erythrocyte sedimentation ratio, CRP and thrombocytes. They also tested a single-bout high intensity exercise effect, which lead to the short-term elevation of leukocytes, thrombocytes and CRP; however, their probands did not feel any discomfort [35]. This corresponds to another Ploeger et al study (2012), adding that the increase in inflammatory markers after single-bout high intensity exercise were similar in both IBD patients and healthy controls [36].
Our results show that there is no contraindication for regular recreational physical activity in children with IBD. According to these findings, we can expect that physical exercise not only does not harm the IBD patients but can also decrease disease activity. Even though single-bout high intensity exercise can lead to a short-term inflammatory activity increase, symptom amplification was not observed in our patients. IBD children and adolescents as well as their parents may be encouraged to undertake some form of regular exercise, at least 2 or 3 times a week. If the treatment and dietary recommendations are followed, when adhering a suitable amount of exercise, IBD patients can do sports professionally too.
The weakness of this study is the small size of the tested groups, which may lead to some bias. Another disadvantage is the absence of an exactly defined protocol for long-term moderate intensity exercise; our probands were encouraged to do regular exercise, with several types of physical activity recommended.
On the other hand, we assessed the exercise capacity using CPET, which enables us to objectively quantify the proband’s capabilities; this could be an advantage, because most other studies assessed the exercise frequency and capacity through questionnaires, which can confuse the results due to the subjectivity of the tested proband. Another positive of this study is the fact that we exactly quantified the change in CPET parameters and correlated them with the change in disease activity parameters, while other studies only described the change in disease activity without testing the improvement in exercise capacity.
In the future, similar studies with larger tested groups may be added. Another study with the exact long-term exercise protocol may also be valuable. The disease activity in IBD patients who do sports professionally may be compared to those who practise a sport only recreationally.
Conclusion
This study proved that regular sport activities may be helpful in children and adolescents with inflammatory bowel diseases. Therefore, it is advisable to encourage these patients to lead an active lifestyle as regular mild to moderate intensity physical exercise can improve their disease and its accompanying symptoms. Doing sports professionally is also possible.
Sources
Literature
1. Hills AP, Street SJ, Byrne NM. Physical Activity and Health: “What is Old is New Again”. Adv Food Nutr Res 2015; 75 : 77–95. doi: 10.1016/bs.afnr.2015.06.001.
2. Penedo FJ, Dahn JR. Exercise and well-being: a review of mental and physical health benefits associated with physical activity. Curr Opin Psychiatry 2005; 18 (2): 189–193. doi: 10.1097/00001504-200503000-00013.
3. Kelley GA, Kelley KS, Kohrt WM. Exercise and bone mineral density in men: a meta-analysis of randomized controlled trials. Bone 2013; 53 (1): 103–111. doi: 10.1016/j.bone.2012.11.031.
4. Morris PJ. Physical activity recommendations for children and adolescents with chronic disease. Curr Sports Med Rep 2008; 7 (6): 353–358. doi: 10.1249/JSR.0b013e31818f0795.
5. Poitras VJ, Gray CE, Borghese MM et al. Systematic review of the relationships between objectively measured physical activity and health indicators in school-aged children and youth. Appl Physiol Nutr Metab 2016; 41 (6 Suppl 3): S197–S239. doi: 10.1139/apnm-2015-0663.
6. Strong WB, Malina RM, Blimkie CJ et al. Evidence based physical activity for school-age youth. J Pediatr 2005; 146 (6): 732–737. doi: 10.1016/j.jpeds.2005.01.055.
7. Hind K, Burrows M. Weight-bearing exercise and bone mineral accrual in children and adolescents: a review of controlled trials. Bone 2007; 40 (1): 14–27. doi: 10.1016/j.bone.2006.07.006.
8. Levine A, de Bie CI, Turner D et al; EUROKIDS Porto IBD Working Group of ESPGHAN. Atypical disease phenotypes in pediatric ulcerative colitis: 5-year analyses of the EUROKIDS Registry. Inflamm Bowel Dis 2013; 19 (2): 370–377. doi: 10.1002/ibd.23013.
9. Lubans D, Richards J, Hillman C et al. Physical Activity for Cognitive and Mental Health in Youth: A Systematic Review of Mechanisms. Pediatrics 2016; 138 (3): e20161642. doi: 10.1542/peds.2016-1642.
10. European Association of Cardiovascular Prevention and Rehabilitation Committee for Science Guidelines; EACPR; Corrà U, Piepoli MF, Carré F et al. Secondary prevention through cardiac rehabilitation: physical activity counselling and exercise training: key components of the position paper from the Cardiac Rehabilitation Section of the European Association of Cardiovascular Prevention and Rehabilitation. Eur Heart J 2010; 31 (16): 1967–1974. doi: 10.1093/eurheartj/ehq236.
11. Aune D, Norat T, Leitzmann M et al. Physical activity and the risk of type 2 diabetes: a systematic review and dose-response meta-analysis. Eur J Epidemiol 2015; 30 (7): 529–542. doi: 10.1007/s10654-015-0056-z.
12. Behrens G, Jochem C, Keimling M et al. The association between physical activity and gastroesophageal cancer: systematic review and meta-analysis. Eur J Epidemiol 2014; 29 (3): 151–170. doi: 10.1007/s10654-014-9895-2.
13. Benatti FB, Pedersen BK. Exercise as an anti-inflammatory therapy for rheumatic diseases-myokine regulation. Nat Rev Rheumatol 2015; 11 (2): 86–97. doi: 10.1038/nrrheum.2014.193.
14. Wang Q, Xu KQ, Qin XR et al. Association between physical activity and inflammatory bowel disease risk: A meta-analysis. Dig Liver Dis 2016; 48 (12): 1425–1431. doi: 10.1016/ j.dld.2016.08.129.
15. Marchioni Beery RM, Li E, Fishman LN. Impact of pediatric inflammatory bowel disease diagnosis on exercise and sports participation: Patient and parent perspectives. World J Gastroenterol 2019; 25 (31): 4493–4501. doi: 10.3748/wjg.v25.i31.4493.
16. Gatt K, Schembri J, Katsanos KH et al. Inflammatory Bowel Disease [IBD] and Physical Activity: A Study on the Impact of Diagnosis on the Level of Exercise Amongst Patients With IBD. J Crohns Colitis 2019; 13 (6): 686–692. doi: 10.1093/ecco-jcc/jjy214.
17. Ellingsgaard H, Hauselmann I, Schuler B et al. Interleukin-6 enhances insulin secretion by increasing glucagon-like peptide-1 secretion from L cells and alpha cells. Nat Med 2011; 17 (11): 1481–1489. doi: 10.1038/nm.2513.
18. Saxena A, Fletcher E, Larsen B et al. Effect of exercise on chemically-induced colitis in adiponectin deficient mice. J Inflamm (Lond) 2012; 9 (1): 30. doi: 10.1186/1476-9255-9-30.
19. Engels M, Cross RK, Long MD. Exercise in patients with inflammatory bowel diseases: current perspectives. Clin Exp Gastroenterol 2017; 11 : 1–11. doi: 10.2147/CEG.S120816.
20. Vanhelst J, Vidal F, Turck D et al. Physical activity is associated with improved bone health in children with inflammatory bowel disease. Clin Nutr 2020; 39 (6): 1793–1798. doi: 10.1016/ j.clnu.2019.07.018.
21. Robinson RJ, Krzywicki T, Almond L et al. Effect of a low-impact exercise program on bone mineral density in Crohn’s disease: a randomized controlled trial. Gastroenterology 1998; 115 (1): 36–41. doi: 10.1016/s0016-5085 (98) 70362-2.
22. Lukáš M, Vašáková M. Plicní postižení u idiopatickách střevních zánětů. In: Vašáková M, Bečvéř R, Lukáš M et al. Plicní postižení u systémových nemocí pojiva, vaskulitid a idiopatických zánětů v gastroenterologii. Praha: Mladá fronta 2016 : 177–192.
23. Bunu DM, Timofte CE, Ciocoiu M et al. Cardiovascular Manifestations of Inflammatory Bowel Disease: Pathogenesis, Diagnosis, and Preventive Strategies. Gastroenterol Res Pract 2019; 2019 : 3012509. doi: 10.1155/2019/3012509.
24. Curione M, Barbato M, Amato S et al. Atrioventricular block associated with Crohn’s relapsing colitis in a 12-year-old child. Inflamm Bowel Dis 2010; 16 (3): 373–374. doi: 10.1002/ibd.21006.
25. Erolu E, Polat E. Cardiac Repolarization Properties in Children with Inflammatory Bowel Disease. Cyprus J Med Sci 2020; 5 (2): 126–130. doi: 10.5152/cjms.2020.1390.
26. Hensel KO, Abellan Schneyder FE, Wilke L et al. Speckle Tracking Stress Echocardiography Uncovers Early Subclinical Cardiac Involvement in Pediatric Patients with Inflammatory Bowel Diseases. Sci Rep 2017; 7 (1): 2966. doi: 10.1038/s41598-017-03255-1.
27. Massart A, Hunt DP. Pulmonary Manifestations of Inflammatory Bowel Disease. Am J Med 2020; 133 (1): 39–43. doi: 10.1016/j.amjmed. 2019.07.007.
28. Vadlamudi NB, Navaneethan U, Thame KA et al. Crohn’s disease with pulmonary manifestations in children: 2 case reports and review of the literature. J Crohns Colitis 2013; 7 (3): e85–e92. doi: 10.1016/j.crohns.2012.05.007.
29. Bąk-Drabik K, Malik M, Gwoździewicz K et al. Pulmonary Function in Pediatric Patients with Inflammatory Bowel Disease. J Clin Med 2022; 11 (20): 6095. doi: 10.3390/jcm11206095.
30. El Amrousy DM, Hassan S, El-Ashry H et al. Pulmonary Function Tests Abnormalities in Children With Inflammatory Bowel Disease: Is It Common? J Pediatr Gastroenterol Nutr 2018; 67 (3): 346–350. doi: 10.1097/MPG.0000000000001989.
31. Barfield E, Deshmukh F, Slighton E et al. Pulmonary Manifestations in Adolescents With Inflammatory Bowel Disease. Clin Pediatr (Phila) 2020; 59 (6): 573–579. doi: 10.1177/0009922 820910821.
32. Ploeger HE, Takken T, Wilk B et al. Exercise capacity in pediatric patients with inflammatory bowel disease. J Pediatr 2011; 158 (5): 814–819. doi: 10.1016/j.jpeds.2010.10.020.
33. Olekšák F, Dvoran P, Jakušová Ľ et al. Reference Values for Cardiopulmonary Exercise Testing in Young Male Slovak Athletes. Acta Medica (Hradec Kralove) 2021; 64 (2): 119–124. doi: 10.14712/18059694.2021.20.
34. Vanhelst J, Beghin L, Coopman S et al. Physical fitness in children and adolescents with inflammatory bowel disease: protocol for a case-control study. BMJ Open 2022; 12 (10): e063403. doi: 10.1136/bmjopen-2022-063403.
35. Legeret C, Mählmann L, Gerber M et al. Favorable impact of long-term exercise on disease symptoms in pediatric patients with inflammatory bowel disease. BMC Pediatr 2019; 19 (1): 297. doi: 10.1186/s12887-019-1680-7.
36. Ploeger H, Obeid J, Nguyen T et al. Exercise and inflammation in pediatric Crohn’s disease. Int J Sports Med 2012; 33 (8): 671–679. doi: 10.1055/s-0032-1304323.
Labels
Paediatric gastroenterology Gastroenterology and hepatology Surgery
Article was published inGastroenterology and Hepatology
2023 Issue 6-
All articles in this issue
- Pediatric gastroenterology and hepatology
- Editorial
- Thanks to reviewers
- Benefits and limitations of physical activity in pediatric patients with inflammatory bowel diseases
- Hepatobiliary complications in autosomal recessive polycystic kidney disease
- Evaluation of the association between red cell distribution width (anisocytosis) and degree of liver fibrosis in children with chronic liver diseases
- Practical clinical recommendations for perioperative care in bariatric surgery 2023: adaptation of ERAS (Enhanced Recovery After Surgery) recommendations with consensual voting of working group of Joint Section of Bariatric and Metabolic Surgery of Czech Surgery Society and Czech Obesitology Society
- New perspectives in bariatric-metabolic treatment
- Pancreatic cancer surveillance in high-risk individuals Position statement of professional societies
- Pharmacological characteristics of subcutaneously administered monoclonal antibodies
- Answer to the quiz
- The selection from international journals
- Laudačný príhovor jubilantovi Moja rola v profesnej výchove prof. MUDr. Tibora Hlavatého, PhD.
- Report from the 37th Slovak and Czech Congress of Gastroenterology
- First Congress of the Benin Society of Hepato-Gastroenterology
- Report from the 37th Slovak and Czech Congress of Gastroenterology
- Autodidaktický test | Self-educated test
- Gastroenterology and Hepatology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Pancreatic cancer surveillance in high-risk individuals Position statement of professional societies
- Practical clinical recommendations for perioperative care in bariatric surgery 2023: adaptation of ERAS (Enhanced Recovery After Surgery) recommendations with consensual voting of working group of Joint Section of Bariatric and Metabolic Surgery of Czech Surgery Society and Czech Obesitology Society
- Hepatobiliary complications in autosomal recessive polycystic kidney disease
- Report from the 37th Slovak and Czech Congress of Gastroenterology
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

