-
Medical journals
- Career
Laparoscopic pancreaticoduodenectomy for ampullary adenocarcinoma – a case report
Authors: L. Kunovsky 1,2; Z. Kala 2; V. Procházka 2; M. Dastych 1; I. Novotný 3; J. Trna 1; J. Mazanec 4; T. Andrasina 5; M. Eid 6; M. Potrusil 2; P. Moravčík 2
Published in: Gastroent Hepatol 2018; 72(5): 401-407
Category: Gastrointestinal Oncology: Review Article
Overview
Ampullary adenocarcinoma (AA) is rare disease, accounting for less than 1% of all digestive cancers, although it is the second most common cancer arising from the periampullary region (after pancreatic cancer). Treatment of ampullary neoplasia varies from endoscopic resection, transduodenal surgical ampullectomy to a pancreaticoduodenectomy. At the time of diagnosis, the curative surgical resection rate of AA is higher than that of pancreatic cancer (80% to 20%) and the 5-year survival rate is also significantly longer. Laparoscopic procedures in colorectal surgery became the standard and the benefit of early post-operative outcomes is well proven. In this article, we present a 68-year-old patient with AA treated by laparoscopic pancreaticoduodenectomy (LPD). We consider the pros and cons of LPD and open pancreaticoduodenectomy, discuss possible treatment options of AA (including endoscopy treatment), and present a current review of literature.
Key words:
adenocarcinoma of the ampulla of Vater – pancreaticoduodenectomy – surgery – periampullary tumors – pancreatic cancer – laparoscopy – endoscopy
Introduction
Ampullary adenocarcinoma (AA) is quite rare, accounting for less than 1% of all digestive cancers [1,2], with a reported incidence of fewer than 1 person per 100 000 [3,4]. However, it is the second most common cancer arising from the periampullary region, comprising 6–20% of periampullary tumours [5,6]. Premalignant lesions of ampulla of Vater are generally treated by endoscopic resection. According to some authors, endosco-pic treatment is also feasible in selected patients with early carcinoma limited to mucosa [4,7]. The first endoscopic papillectomy was performed by Suzuki in 1983 [8]. Transduodenal surgical ampullectomy (TSA) is considered if premalignant lesions cannot be endoscopically resected [9,10] and in the early stage of cancer in polymorbid patients [11,12]. American surgeon, William Halsted, first performed this procedure in 1898 [11,13]. Nevertheless, in AA pancreaticoduo-denectomy (PD) is the preferred therapeutic method. PD can be performed by open surgery, which was first performed by an Italian surgeon, Alessandro Codivilla, in February 1898 (although he never published his surgery) [14]. A German surgeon, Walter Kausch, performed and first published PD for AA in 1909 [14]. Later the PD procedure was improved by an American surgeon, Allen Whipple, in 1935 [15] and is often called Whipple’s procedure. With the current increasing role of mini invasive procedures, PD is also possible to carry out laparoscopically. Laparoscopic pancreaticoduodenectomy (LPD) was first described and performed almost 100 years later after an open procedure by Canadian surgeons Gardner and Pomp in 1994 [16]. From that time laparoscopic technology and instruments have been improved, however, LPD remains one of the most advanced and challenging laparoscopic surgeries. PD is an extensive surgical procedure with high morbidity and mortality even in high volume centres. Surgical post-operative complications as well as non-surgical post-operative complications appear after PD. After laparoscopic colorectal surgery, a lower incidence of respiratory infections is observed compared to open procedures. Improved post-operative outcomes, including lower morbidity, are reasons why minimally invasive surgery is also more expandent in hepatopancreatobiliary surgery.
Case report
Our patient is a 68-year-old woman. Her case history includes hypertension and cholecystectomy in 2006 (solitary cholecystolithiasis). In 2014, the patient underwent endoscopic retrograde cholangiopancreatography (ERCP) for liver enzyme elevation and suspicion of choledocholithiasis with findings of slight stenosis of papilla (histologically chronic inflammation with fibrosis). There were no gallstones present in the bile duct and papillosphincterotomy was performed. In 2018, the patient was examined after a weight loss, liver enzyme and bilirubin elevation (alanine aminotransferase 4.9 µkat/L, aspartate transaminase 3.4 µkat/L, alkaline phosphatase 15.0 µkat/L, gamma-glutamyltransferase 21.7 µkat/L, bilirubin 33.1 µmol/L, amylase 0.34 µkat/L, carbohydrate antigen 19-9 6.7 U/ml). Due to a dilation of the biliary tract and ultrasound suspicion of a choledocholithiasis, ERCP was indicated. ERCP revealed a stenosis of ampulla of Vater and there were no lithiasis present in the biliary tract. A biopsy from the papilla and the papillosphincterotomy was performed. The biopsy confirmed adenocarcinoma. The staging CT scan has added findings of dilation of the bile and pancreatic duct (double duct sign) (Fig. 1 A, B). Due to a double duct sign we suspected invasion of the ducts. The patient was presented at a multidisciplinary committee and was indicated for surgery.
1. A, B. Portal venous phase of contrast-enhanced CT – image A shows the dilatation of pancreatic duct (arrow) and pancreatic head atrophy (arrowheads), image B reveals marked dilatation of extrahepatic bile ducts, so-called double duct sign refers to the presence of obstruction of both ducts, frequently due to tumour invasion. Source: Department of Radiology, Hospital Uherské Hradiště.
Obr. 1. A, B. Portovenózní fáze kontrastního CT – obrázek A ukazuje dilataci pankreatického duktu (šipka) a atrofii hlavy pankreatu (větší šipky), obrázek B ukazuje výraznou dilataci extrahepatálních žlučových cest, tzv. double duct sign poukazuje na přítomnost obstrukce obou vývodů, často kvůli nádorové invazi. Zdroj: Radiologické oddělení, nemocnice Uherské Hradiště.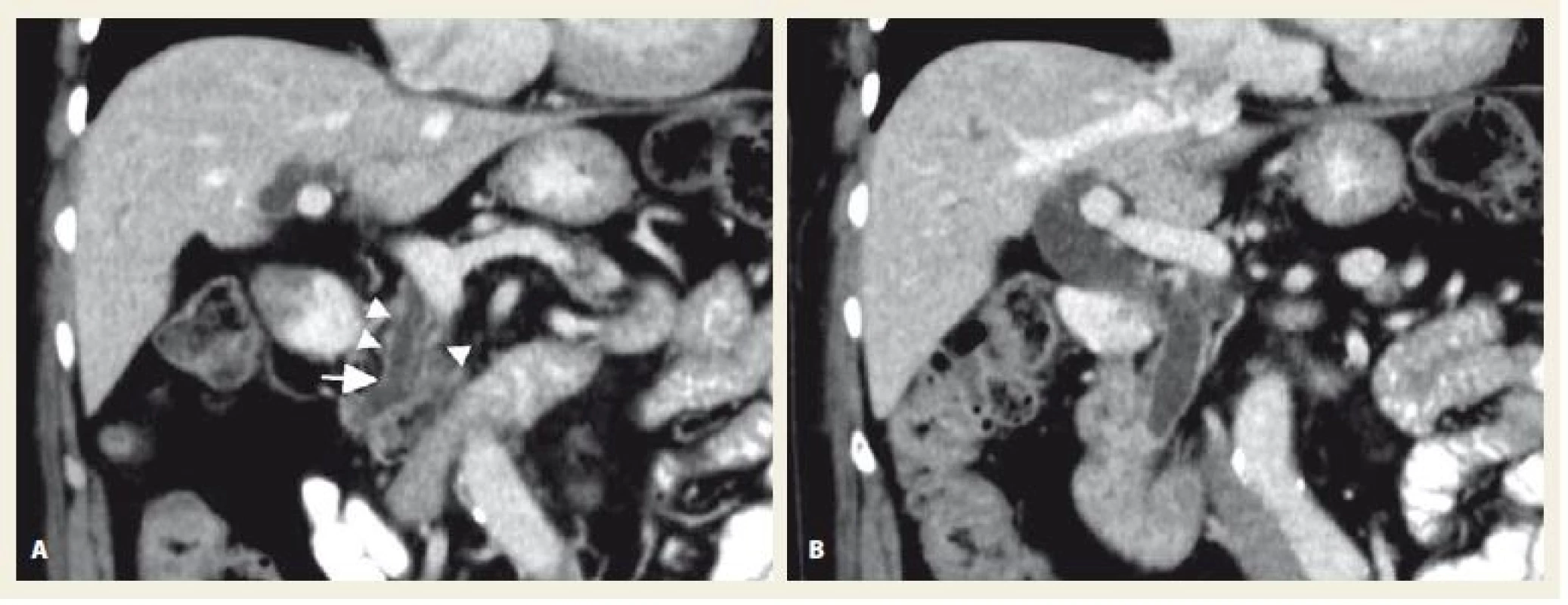
Surgery procedure
The operation started with a staging laparoscopy. After exclusion of liver metastasis, six ports for the laparoscopy procedure were placed. First, the lesser sac was opened through the gastrocolic ligament and then the lower edge of the pancreas was released; the superior mesenteric vein (SMV) was found and at its level the pancreas body was liberated from the SMV surface. After this, the pancreas was interrupted by Thunderbeat® (Fig. 2A) and the drain was inserted into the dilated pancreatic duct. Near the upper pancreatic edge, the common hepatic artery was located and a lymphadenectomy from her detachment from the celiac trunk to the hepatoduodenal ligament was performed. The gastroduodenal artery was ligated and the common dilated hepatic duct was interrupted (Fig. 2B). The duodenum was cut below the pylorus, released from retroperitoneum, followed by a dissection of the uncinate process from SMV and a radical resection was finished by dissection from superior mesenteric artery. The reconstruction phase differs from the colorectal laparoscopic procedures as most of the anastomoses are possible to carry out by stapler. In this procedure all anastomoses were done by laparoscopic suture (however, there are some authors, who prefer to perform the reconstruction phase from minilaparotomy). Our department prefers to construct the pancreatico-gastric anastomosis (PGA) to the poste-rior wall of the stomach, as was also done in this laparoscopic procedure. First, the posterior portion of PGA was performed with single stitches between the remnants of the pancreas and posterior gastric wall (Fig. 2C,D), followed by anterior and posterior gastrotomy. The pancreatic body was pulled through the posterior gastrotomy into the stomach. The anterior portion of PGA was finished by single stitches (through the anterior gastrotomy) (Fig. 2E) and finally the anterior gastrotomy was sutured (Fig. 2F). The reconstruction phase was completed by Y-Roux hepaticojejunal anastomosis (HJA) and gastrojejunal anastomosis. The specimen was extracted in a protective bag by a Pfannenstiel incision. Two drains were placed into the abdomen cavity (in proximity to HJA and PGA). PGA and HJA were augmented by autologous platelet-rich fibrin sealant Vivostat® (Fig. 2G). The patient was discharged 10 days after the surgical procedure without any post-operative complications. Definitive histology (Fig. 3A) established a diag-nosis of AA (grade 2) with invasion of duodenal wall and pancreas (T3). Resection margins, evaluated according to a Leeds pathology protocol [17,18], were negative, 2 of 11 removed lymph nodes were positive (N1) (Fig. 3B) and perineural invasion was present. Considering these risk factors we prescribed adjuvant treatment with gemcitabine for 6 months.
2. A. Interruption of common dilated hepatic duct, B. interruption of pancreas (in the area of neck) with visible superior mesenteric vein and confl uence, C, D. posterior gastric wall and remnant of pancreas with inserted stent to the pancreatic duct, E. suturing of pancreatogastro-anastomosis through anterior gastrotomy, at the bottom remnant of pancreas with inserted stent to the pancreatic duct, F. suture of the anterior gastrotomy, G. application of autologous platelet-rich fi brin sealant Vivostat® for anastomosis augmentation.
Obr. 2. A. Přerušení dilatovaného společného jaterního vývodu, B. Přerušení slinivky v místě krčku s patrnou dolní mezenterickou žílou a konfluens, C, D. zadní stěna žaludku a přerušená slinivka se stentem v pankreatickém vývodu, E. našívání pankretogastro- anastomózy přes přední gastrotomii, na spodině patrna slinivka se stentem v pankreatickém vývodu, F. sutura přední gastrotomie, G. aplikace autologního destičkového fibrinem obohaceného lepidla Vivostat® k augmentaci anastomóz.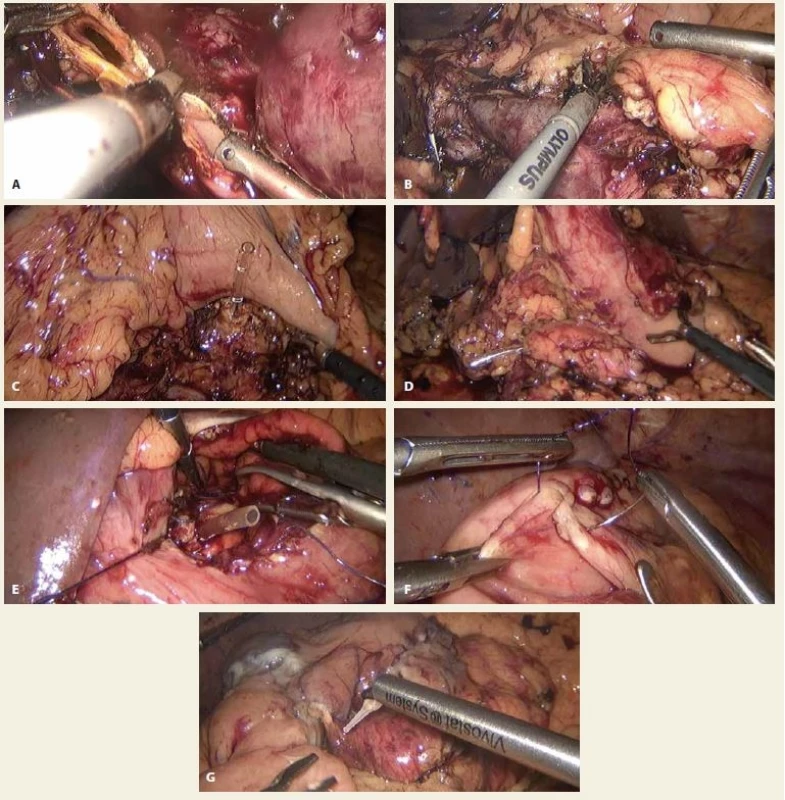
3. A, B. Histological section – ampullary adenocarcinoma. Tumour glands invade the duodenum wall. Haematoxylin-eosin staining 10× (A). Histological section – regional lymph node with metastasis of the adenocarcinoma. Haematoxylin-eosin staining 40× (B).
Obr. 3. A, B. Histologické vyšetření – ampulární adenokarcinom. Nádorové žlázky invadují stěnu duodena. Barvení hematoxylin-eozinem 10× (A). Histologické vyšetření – regionální lymfatická uzlina s metastázou adenokarcinomu. Barvení hematoxylin-eozinem 40× (B).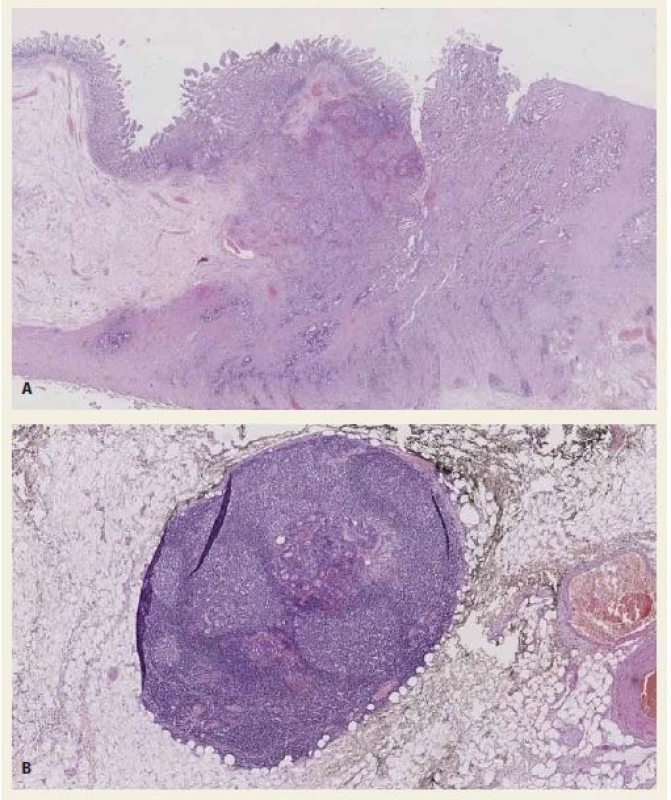
Discussion
Endoscopic papillectomy is globally recognised as the first method of choice in premalignant ampullary lesions [10,19]. The possibility of endoscopic therapy of early adenocarcinoma of the ampulla of Vater bounded to mucosa, without a deeper invasion to submucosa, is stated by some authors [4,7,20]. In cases where the premalignant lesions cannot be resected endoscopically [9,10] or in select patients within the early carcinoma stage (without a lymph node invasion and in polymorbid patients) [11,12,17], the TSA should be considered. However, PD remains a method of choice in AA. Due to its anatomical location, AA usually presents early with jaundice or pancreatitis [21] and due to an earlier clinical presentation, resection rates (possibility of resection) for most patients are much higher than for pancreatic cancer. Curative surgery is possible in up to approximately 80% of cases at the time of diagnosis of ampullary cancer compared to pancreatic adenocarcinoma, where the resectability rate is around 20% [2,22,23]. Easier feasibility of laparoscopic resection in the case of ampullary tu-mours compared to pancreatic cancer can be due to its earlier clinical manifestation and so, at time of diagnosis, the surrounding organs, arteries (superior mesenteric artery, celiac trunk) and veins (portal vein) are not infiltrated by tumour. In comparison with other periampullary cancers, AA has better surgical outcomes in treatment and long-term survival rate. The 5-year survival rates following resection range from 38% to 67% [24,25]. Choi et al. [26] in 2011 reported a 59.9% overall 5-year survival rate after PD (R0/R1 resection). Sudo et al. [24] even published a 64% overall 5-year survival rate after PD resection for AA. The reason seems to be due to earlier clinical diagnosis and so a higher resectability rate and less aggressive tumour characteristics compared to other periampullary cancers (pancreatic cancer, distal bile duct carcinoma) [2,24,25]. The advantages and benefits of laparoscopy for patients have been well described in other kinds of abdominal surgery (primarily in colorectal and inflammatory bowel disease surgery), such as shortened hospital stay, quicker recovery, lower morbidity, better cosmetic outcome and lower percentage of incisional hernias [27–31]. However, in hepatopancreatobiliary surgery, it still remains a challenge for surgeons and represents one of the most difficult laparoscopic procedures. What also must be considered is that in oncologic curative surgery, the radicality and complete resection of the tumour is the primary goal. Many studies have been comparing the surgical oncologic results and survival rate after laparoscopic and open pancreaticoduodenectomy (OPD). Long-term overall survival after LPD is comparable to and also appears to be true for pancreatic cancer as well [32–35]. Studies that also compared pathology results did not demonstrate any significant difference and achieved similar negative resection margins and number of lymph node harvest in LPD and OPD (in AA and also pancreatic cancer) [32,34,36–38]. PD is associated with high mortality and morbidity rate even in high volume centres. The post-operative mortality rate is between 3 and 5% [39,40]. The morbidity ranging from 40 up to 60%, primarily due to pancreatic fistula, post-operative bleeding and delayed gastric emptying [11,39,41]. Just Dokmak et al. [39] reported higher morbidity in cases of LPD. Otherwise, the same morbidity rate has been recorded in comparison of LPD with OPD [32,35,37,38,42] and some studies and systematic meta-analysis have even reported a lower occurrence of post-operative complications in LPD [33,43,44]. Adjuvant oncologic therapy should be considered after surgery resection if risk factors occur (positive lymph nodes, positive resection margins, unfavourable grading, perineural, angio or lymphovascular invasion) [17,22,45]. As presented in our case, because of positive lymph nodes and perineural invasion, the adjuvant therapy was indicated. Typical advantages of laparoscopy were confirmed in LPD, such as less abdomen pain, less blood loss, shorter hospital stay and faster post-operative recovery [32,38,42,43]. Comparison of incisions and cosmetic outcome between LPD and OPD can be seen in figure 4. On the contrary, the length of the operative time has been described as shorter in OPD in some comparative studies [35,42–44], but it is dependent on the surgeon’s learning curve of this highly advanced technical laparoscopic procedure [46]. It has to be said that most studies performed LDP on select patients. Inclusion criteria in cohorts were tumours which had not spread to the main vessels or smaller tumour size (up to 2–3 cm); excluded were patients with high cardiopulmonary morbidity or relative exclusion criterion for laparoscopy with previous history of major surgery [32,36,37,47].
4. A, B. Comparison of cosmetic outcome in laparoscopic (A) and open (B) pancreaticoduodenectomy.
Obr. 4. A, B. Srovnání výsledného kosmetického efektu po laparoskopické (A) a otevřené (B) pankreatoduodenektomii.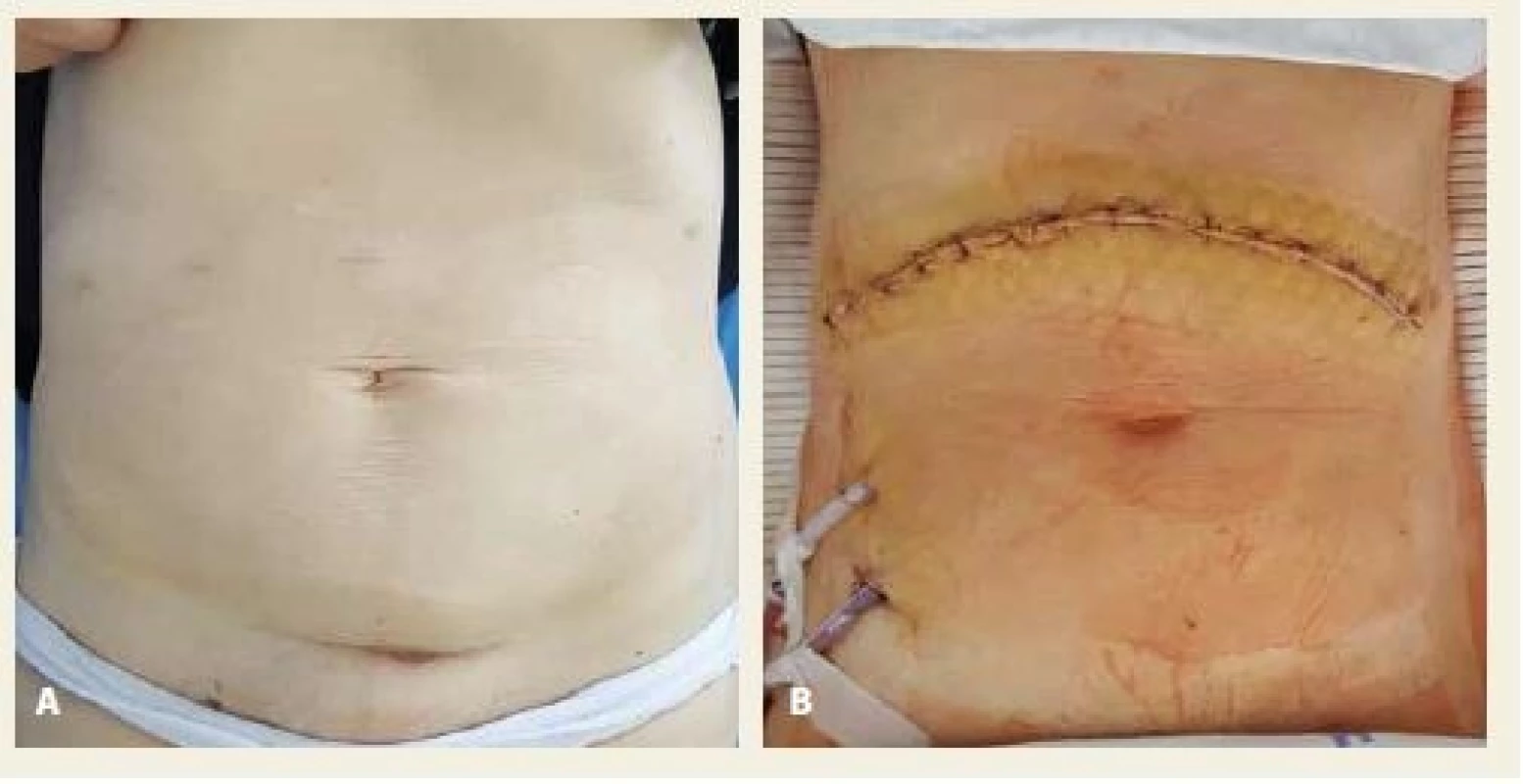
Conclusion
We present a 68-year-old woman with adenocarcinoma of ampulla of Vater that successfully underwent LDP. Patients with periampullary adenocarcinoma treated by LPD have a similar long-term survival rate compared to OPD and similar or lower post-operative complications, with shorter length of hospital stay and faster post-operative recovery (and other more positive surgical outcomes). However, future prospective studies are needed because most of the current published studies are retrospective. LPD is performed optimally on select patients (smaller tumour, no vessels infiltration, as mentioned above) due to the procedure’s technical difficulties. LPD is an extremely challenging and difficult procedure with a slow learning curve and should be performed by experienced laparoscopic surgeons. Due to this, AA has a higher resectability rate than pancreatic carcinoma, and in the early stage of AA where there is usually no spreading of the tumour to mesenteric vessels and portal vein, it seems to be suitable for a minimally invasive procedure.
Supported by Ministry of Health, CZ – conceptual development of research organisation (FNBr, 65269705). Submitted/Doručeno
The Editorial Board declares that the manuscript met the ICMJE „uniform requirements“ for biomedical papers.
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.
Submitted: 10. 9. 2018
Accepted: 16. 10. 2018
MUDr. Vladimír Procházka, Ph.D.
Department of Surgery, University Hospital
Brno Jihlavská 20 625 00 Brno
Sources
1. Rostain F, Hamza S, Drouillard A et al. Trends in incidence and management of cancer of the ampulla of Vater. World J Gastroenterol 2014; 20 (29): 10144–10150. doi: 10.3748/wjg.v20.i29.10144.
2. Klein F, Jacob D, Bahra M et al. Prognostic factors for long-term survival in patients with ampullary carcinoma: the results of a 15-year observation period after pancreaticoduodenectomy. HPB Surg 2014; 2014 : 970234. doi: 10.1155/2014/970234.
3. Askew J, Connor S. Review of the investigation and surgical management of resectable ampullary adenocarcinoma. HPB 2013; 15 (11): 829–838. doi: 10.1111/hpb.12038.
4. Panzeri F, Crippa S, Castelli P et al. Management of ampullary neoplasms: A tailored approach between endoscopy and surgery. World J Gastroenterol 2015; 21 (26): 7970–7987. doi: 10.3748/wjg.v21.i26.7970.
5. Amini A, Miura JT, Jayakrishnan TT et al. Is local resection adequate for T1 stage ampullary cancer? HPB 2015; 17 (1): 66–71. doi: 10.1111/ hpb.12297.
6. Feng J, Zhou X, Mao W. Prognostic analysis of carcinoma of the ampulla of Vater: pancreaticoduodenectomy versus local resection. Hippokratia 2012; 16 (1): 23–28.
7. De Palma GD. Endoscopic papillectomy: indications, techniques, and results. World J Gastroenterol 2014; 20 (6): 1537–1543. doi: 10.3748/wjg.v20.i6.1537.
8. Suzuki K, Kantou U, Murakami Y. Two cases with ampullary cancer who underwent endoscopic excision. Prog Dig Endosc 1983; 23 : 236–239.
9. Kim J, Choi SH, Choi DW et al. Role of transduodenal ampullectomy for tumors of the ampulla of Vater. J Korean Surg Soc 2011; 81 (4): 250–256. doi: 10.4174/jkss.2011.81.4.250.
10. Schneider L, Contin P, Fritz S et al. Surgical ampullectomy: an underestimated operation in the era of endoscopy. HPB 2016; 18 (1): 65–71. doi: 10.1016/j.hpb.2015.07.004.
11. Song J, Liu H, Li Z et al. Long-term prognosis of surgical treatment for early ampullary cancers and implications for local ampullectomy. BMC Surg 2015; 15 : 32. doi: 10.1186/s12893-015-0019-z.
12. Gao Y, Zhu Y, Huang X et al. Transduodenal ampullectomy provides a less invasive technique to cure early ampullary cancer. BMC Surg 2016; 16 (1): 36. doi: 10.1186/s12893-016-0156-z.
13. Halsted WS. Contributions to the surgery of the bile passages, especially of the common bile duct. Boston Med Surg J 1899; 141 : 145–654. doi: 10.1056/NEJM189912281412601.
14. Howard JM, Hess W (eds.). History of the pancreas: Mysteries of a hidden organ. New York: Kluwer Academic/Plenum Publishers 2002.
15. Whipple AO, Parsons WB, Mullins CR. Treatment of carcinoma of the ampulla of Vater. Ann Surg 1935; 102 (4): 763–779.
16. Gagner M, Pomp A. Laparoscopic pylorus-preserving pancreatoduodenectomy. Surg Endosc 1994; 8 (5): 408–410.
17. Kunovský L, Kala Z, Procházka V et al. Surgical treatment of ampullary adenocarcinoma – single center experience and a review of literature. Klin Onkol 2017; 31 (1): 46–52. doi: 10.14735/amko201846.
18. Hlavsa J, Procházka V, Mazanec J et al. Standardization of pancreatic cancer specimen pathological examination. Rozhl Chir 2014; 93 (3): 132–138.
19. Gaspar JP, Stelow EB, Wang AY. Approach to the endoscopic resection of duodenal lesions. World J Gastroenterol 2016; 22 (2): 600–617. doi: 10.3748/wjg.v22.i2.600.
20. Napoleon B, Gincul R, Ponchon T et al. Endoscopic papillectomy for early ampullary tumors: long-term results from a large multicenter prospective study. Endoscopy 2014; 46 (2): 127–134. doi: 10.1055/s-0034-1364875.
21. Pittayanon R, Imraporn B, Rerknimitr R et al. Advances in diagnostic endoscopy for duodenal, including ampullary, adenoma. Dig Endosc 2014; 26 (Suppl 2): 10–15. doi: 10.1111/den.12244.
22. Ahn DH, Bekaii-Saab T. Ampullary cancer: an overview. Am Soc Clin Oncol Educ Book 2014; 112–115. doi: 10.14694/EdBook_ AM.2014.34.112.
23. Kunovsky L, Tesarikova P, Kala Z et al. The use of biomarkers in early diagnostics of pancreatic cancer. Can J Gastroenterol Hepatol 2018; 2018 : 53898202018. doi: 10.1155/2018/5389820.
24. Sudo T, Murakami Y, Uemura K et al. Prognostic impact of perineural invasion following pancreatoduodenectomy with lymphadenectomy for ampullary carcinoma. Dig Dis Sci 2008; 53 (8): 2281–2286. doi: 10.1007/s10620-007-0117-6.
25. Qiao QL, Zhao YG, Ye ML et al. Carcinoma of the ampulla of Vater: factors influencing long-term survival of 127 patients with resection. World J Surg 2007; 31 (1): 137–143. doi: 10.1007/s00268-006-0213-3.
26. Choi SB, Kim WB, Song TJ et al. Surgical outcomes and prognostic factors for ampulla of Vater cancer. Scand J Surg 2011; 100 (2): 92–98. doi: 10.1177/145749691110000205.
27. Kunovský L, Marek F, Kala Z et al. Possibilities of minimally invasive surgery in patients with Crohn’s disease and ulcerative colitis. Gastroent Hepatol 2017; 71 (1): 29–35. doi: 10.14735/amgh201729.
28. Maartense S, Dunker MS, Slors JF et al. Laparoscopic-assisted versus open ileocolic resection for Crohn’s disease. Ann Surg 2006; 243 (2): 143–149. doi: 10.1097/01.sla.00001973 18.37459.ec.
29. Clinical Outcomes of Surgical Therapy Study Group, Nelson H, Sargent DJ et al. A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med 2004; 350 (20): 2050–2059. doi: 10.1056/NEJMoa032651.
30. Veldkamp R, Kuhry E, Hop WC et al. Laparoscopic surgery versus open surgery for colon cancer: short-term outcomes of a randomised trial. Lancet Oncol 2005; 6 (7): 477–484. doi: 10.1016/S1470-2045 (05) 70221-7.
31. Hemandas AK, Abdelrahman T, Flashman KG et al. Laparoscopic colorectal surgery produces better outcomes for high risk cancer patients compared to open surgery. Ann Surg 2010; 252 (1): 84–89. doi: 10.1097/SLA.0b013e 3181e45b66.
32. Song KB, Kim SC, Hwang DW et al. Matched case-control analysis comparing laparoscopic and open pylorus-preserving pancreaticoduodenectomy in patients with periampullary tumors. Ann Surg 2015; 262 (1): 146–155. doi: 10.1097/SLA.0000000000001079.
33. Delitto D, Luckhurst CM, Black BS et al. Oncologic and perioperative outcomes following selective application of laparoscopic pancreaticoduodenectomy for periampullary malignancies. J Gastrointest Surg 2016; 20 (7): 1343–1349. doi: 10.1007/s11605-016-3136-9.
34. Kantor O, Talamonti MS, Sharpe S et al. Laparoscopic pancreaticoduodenectomy for adenocarcinoma provides short-term oncologic outcomes and long-term overall survival rates similar to those for open pancreaticoduodenectomy. Am J Surg 2017; 213 (3): 512–515. doi: 10.1016/j.amjsurg.2016.10.030.
35. Stauffer JA, Coppola A, Villacreses D et al. Laparoscopic versus open pancreaticoduodenectomy for pancreatic adenocarcinoma: long-term results at a single institution. Surg Endosc 2017; 31 (5): 2233–2241. doi: 10.1007/s00464-016-5222-1.
36. Hakeem AR, Verbeke CS, Cairns A et al. A matched-pair analysis of laparoscopic versus open pancreaticoduodenectomy: oncological outcomes using Leeds Pathology Protocol. Hepatobiliary Pancreat Dis Int 2014; 13 (4): 435–441.
37. Meng LW, Cai YQ, Li YB et al. Comparison of laparoscopic and open pancreaticoduodenectomy for the treatment of nonpancreatic periampullary adenocarcinomas. Surg Laparosc Endosc Percutan Tech 2018; 28 (1): 56–61. doi: 10.1097/SLE.0000000000000504.
38. Asbun HJ, Stauffer JA. Laparoscopic vs open pancreaticoduodenectomy: overall outcomes and severity of complications using the accordion severity grading system. J Am Coll Surg 2012; 215 (6): 810–819. doi: 10.1016/j.jamcollsurg.2012.08.006.
39. Dokmak S, Ftériche FS, Aussilhou B et al. Laparoscopic pancreaticoduodenectomy should not be routine for resection of periampullary tumors. J Am Coll Surg 2015; 220 (5): 831–838. doi: 10.1016/j.jamcollsurg.2014.12.052.
40. Hariharan D, Saied A, Kocher HM. Analy-sis of mortality rates for pancreatic cancer across the world. HPB 2008; 10 (1): 58–62. doi: 10.1080/13651820701883148.
41. Ryska M, Hrabal P. Malignant tumours of the duodenum. Rozhl Chir 2015; 94 (12): 497–503.
42. Chen K, Pan Y, Liu XL et al. Minimally invasive pancreaticoduodenectomy for periampullary disease: a comprehensive review of literature and meta-analysis of outcomes compared with open surgery. BMC Gastroenterol 2017; 17 (1): 120. doi: 10.1186/s12876-017-06 91-9.
43. Wang S, Shi N, You L et al. Minimally invasive surgical approach versus open procedure for pancreaticoduodenectomy: A systematic review and meta-analysis. Medicine (Baltimore) 2017; 96 (50): e8619. doi: 10.1097/MD.0000 000000008619.
44. Zhang H, Wu X, Zhu F et al. Systematic review and meta-analysis of minimally invasive versus open approach for pancreaticoduodenectomy. Surg Endosc 2016; 30 (12): 5173–5184. doi: 10.1007/s00464-016-4864-3.
45. Hlavsa J, Cecka F, Zaruba P et al. Tumor grade as significant prognostic factor in pancreatic cancer: validation of a novel TNMG staging system. Neoplasma 2018; 65 (4): 637–643. doi: 10.4149/neo_2018_171012N650.
46. Liao CH, Wu YT, Liu YY et al. Systemic review of the feasibility and advantage of minimally invasive pancreaticoduodenectomy. World J Surg 2016; 40 (5): 1218–1225. doi: 10.1007/s00268-016-3433-1.
47. Palanivelu C, Jani K, Senthilnathan P et al. Laparoscopic pancreaticoduodenectomy: technique and outcomes. J Am Coll Surg 2007; 205 (2): 222–230. doi: 10.1016/j.jamcollsurg.2007.04.004.
Labels
Paediatric gastroenterology Gastroenterology and hepatology Surgery
Article was published inGastroenterology and Hepatology
2018 Issue 5-
All articles in this issue
- Gastrointestinal oncology
- Evaluation of colorectal cancer screening centers in the Czech Republic
- Pancreatic cancer from the patients’s point of view
- Therapy of colorectal carcinoma liver metastases
- Comparison of sulfate-based solution and polyethylene glycol solution for bowel cleansing before colonoscopy – randomized, single-blind study
- Self-expanding duodenal stents, palliative treatment of gastric outlet obstruction in malignant disease
- Six years of the National program for colorectal cancer screening in Slovakia
- Proton pump inhibitors in the light of clinical studies and the safety profile of long-term use
- Gastrointestinal tract involvement in hereditary kidney diseases
- The selection from international journals
- Laparoscopic pancreaticoduodenectomy for ampullary adenocarcinoma – a case report
- А case of fulminant hepatitis against a background of paucity of the interlobular bile ducts
-
Novel developments in small bowel diseases and intestinal microbiota,
Sherine Khater (France) – Gastro Update Europe 2018, Prague
- Gastroenterology and Hepatology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Proton pump inhibitors in the light of clinical studies and the safety profile of long-term use
- Self-expanding duodenal stents, palliative treatment of gastric outlet obstruction in malignant disease
- Laparoscopic pancreaticoduodenectomy for ampullary adenocarcinoma – a case report
- Pancreatic cancer from the patients’s point of view
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

