-
Medical journals
- Career
Redistribution of acute traumatic infratentorial subdural hematoma to the spinal subdural space
Authors: Silvia Farkašová Iannaccone 1; Alžbeta Ginelliová 2; Ivana Šantová 3; Marián Šanta 4; Daniel Farkaš 2; Radoslav Morochovič 5; Lucia Fröhlichová 6; Vladimír Balik 7
Authors‘ workplace: Department of Forensic Medicine, Faculty of Medicine, Pavol Jozef Šafárik University, Košice, Slovak Republic 1; Medico-Legal and Pathological-Anatomical Department of the Health Care Surveillance Authority, Košice, Slovak Republic 2; Department of Radiology, Hospital of St. Barbora, Rožňava, Slovak Republic 3; Department of Emergency Healthcare, Catholic University in Ružomberok, Ružomberok, Slovak Republic 4; Department of Traumatology, Faculty of Medicine, Pavol Jozef Šafárik University and Louis Pasteur University Hospital, Košice, Slovak Republic 5; Department of Pathology, Louis Pasteur University Hospital, Košice, Slovak Republic 6; Department of Neurosurgery, University Hospital Olomouc and Faculty of Medicine and Dentistry, Palacký University Olomouc, Olomouc, Czech Republic 7
Published in: Soud Lék., 63, 2018, No. 3, p. 25-28
Category: Original Article
Overview
Spinal subdural hematoma is a rare and potentionally life-threatening condition associated with trauma and other pathological conditions. In this paper we report the autopsy findings of a 64 year old male who was repeatedly hospitalized with traumatic head injuries in the past. In this case spinal subdural hematoma was diagnosed post-mortem and later comfirmed by ante-mortem CT scan revaluation.
Keywords:
intracranial subdural hematoma – recurrent spinal subdural hematoma – diffuse axonal injury – autopsy findings
In contrast to spinal subdural hematoma (SSH) which is a very rare condition, intracranial subdural hematoma (ISH) is a well-known entity in the field of neuropathology (1,2) and forensic pathology (3). SSH can be associated with spinal puncture (4), drug administration for medical purposes (5), low-molecular-weight heparin use (6,7), vascular malformations (8) and post-traumatic redistribution of ISH to the spinal subdural space (9). Spontaneous SSH with no underlying pathology has been also described (10-12). SSH has been described in every region of the spinal canal. The thoracolumbal and lumbal regions are the most commonly affected areas (4). Clinical manifestation of SSH may include sudden back pain radiating into upper or lower extremities or to the trunk, sensory, motor and autonomic deficits (12), headache (13) and an extremely rare Pourfour du Petit syndrome which is characterized by mydriasis, widening of the palpebral fissure, exophthalmos, or pale and cool facial skin with increased sweating (9). The treatment of SSH is conservative or surgical depending on the patient’s initial neurological condition, the severity and the position of the hematoma (5).
CASE REPORT
We report the case of a 64 year old chronic alcoholic with a history of repeated hospital admissions. His medical history included hypertension with no previous history of coagulopathy. His past history also included fracture of the petrous pyramid in 2007, untreated secondary epilepsy since 2012 and decompressive craniectomy with duroplasty due to subdural hematoma in the right anterior fossa in 2013. Four months prior to his death he was admitted to the hospital following head trauma with cerebral contusions, occipital bone fracture and fracture of the anterior arch of the C1 vertebra on the left side, left posterior fossa hemorrhage and SSH (Fig. 1). The treatment was conservative. One month after the injury, cranial computed tomography (cCT) of the head revealed severe cerebral and cerebellar atrophy, remote cerebral contusions and complete resolution of the previous subdural hematoma in the posterior fossa (Fig. 2). He was admitted to the hospital again in a drunken state four months later following a fall on his back. On admission he was deeply comatose with non-reactive pupils, did not respond to a verbal or painful stimuli (GCS=3) and had a laceration in the left occipital region of his head. An initial brain CT scan revealed contusions of the inferior portion of the right and left frontal lobe and left temporal lobe, intraventricular hemorrhage in the right lateral ventricle and fourth ventricle, hematoma spanning the subdural space from the posterior fossa to the cervical spine and remote cerebral injury. Severe cerebellar edema with subdural and subarachnoid hemorrhage was also evident (Fig. 3). Follow-up CT scan on the second hospital day showed no further progression of the supratentorial hemorrhage. CT angiography comfirmed irreversible brain damage. He died 36 hours after admission to the hospital due to traumatic cerebral and cerebellar edema complicating traumatic brain injury.
1. A – CT scan (transverse plane), intraspinal subdural hemorrhagic collection around the medulla. B – occipital bone fracture and fracture of the anterior arch of the C1 vertebra on the left side. C – CT scan (transverse section), subdural hematoma in the left posterior fossa and subarachnoid hemorrhage around the left cerebellar hemisphere. D – CT scan (sagittal section), infratentorial and spinal subdural hematoma. 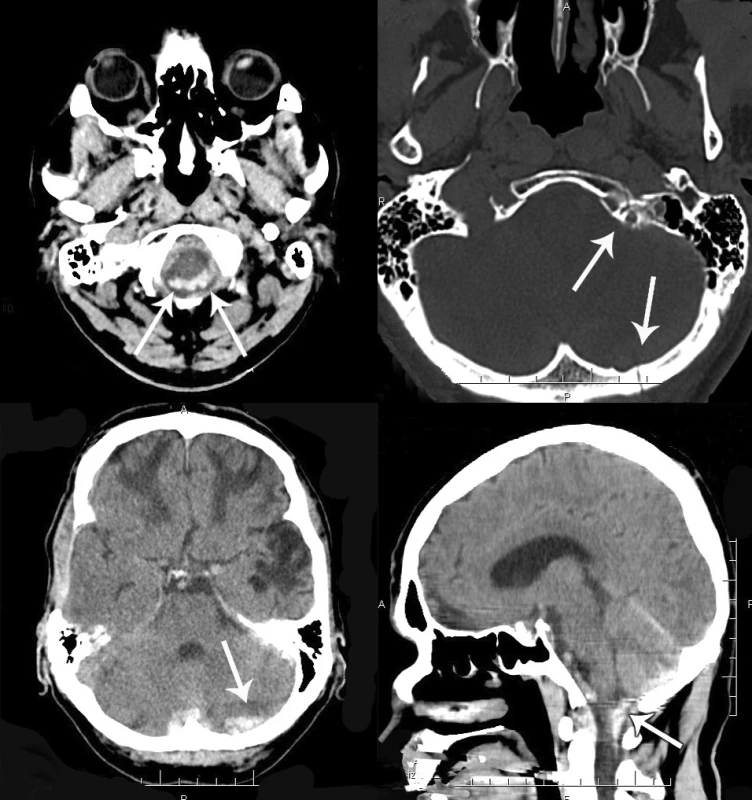
2. A – resolution of the previously observed contusion and hematoma of the left temporal lobe. B – CT scan (sagittal section), diffuse cerebral and cerebellar atrophy, complete resolution of the previously observed subdural hematoma in the posterior fossa. 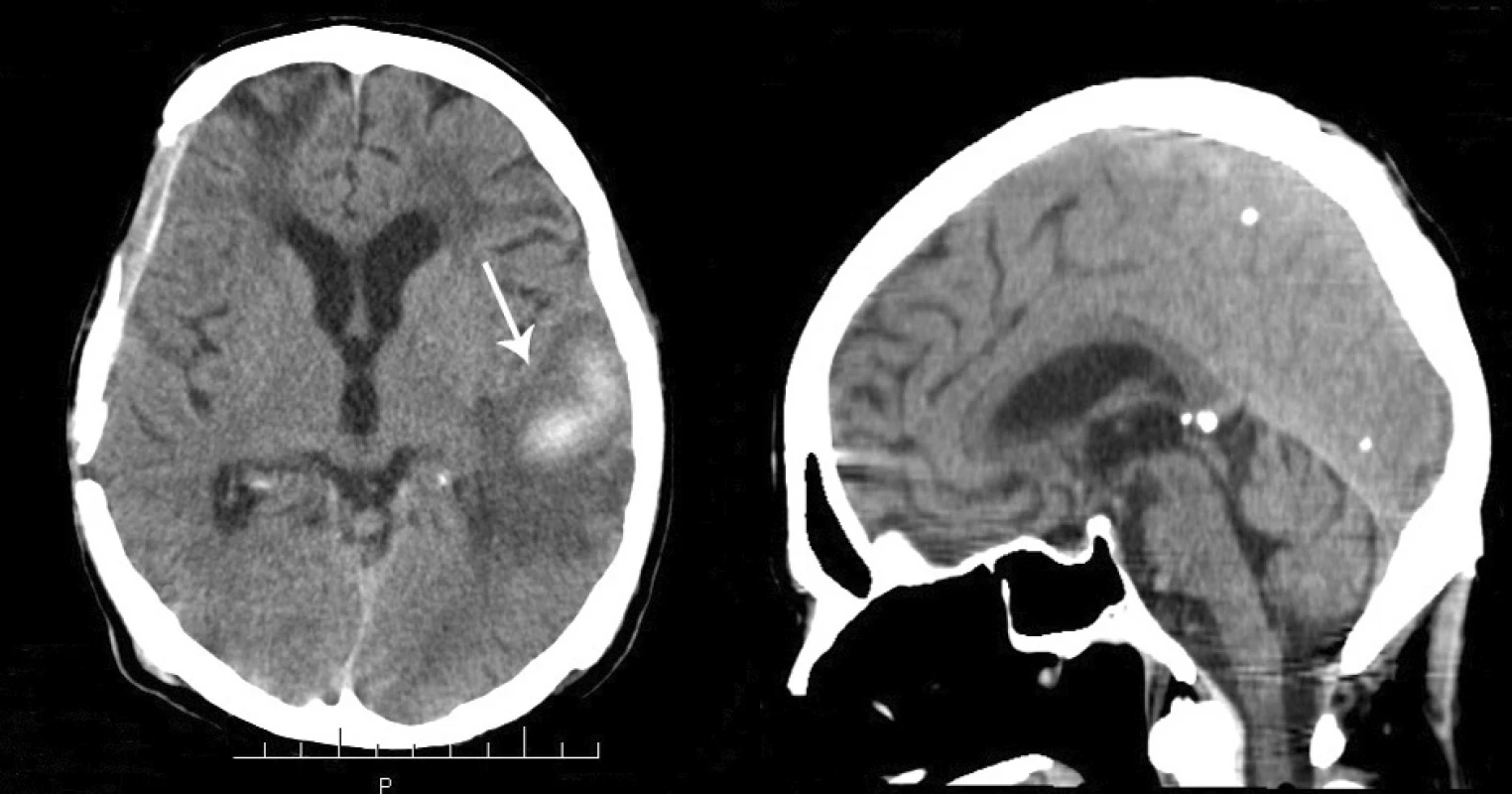
3. A – CT scan (coronal section), intraventricular hemorrhage in the right lateral ventricle and fourth ventricle, subdural collection overlying the left occipital lobe, bilateral subdural hematoma in the posterior fossa and in the cervical spinal canal. B – massive subdural collection surrounding the medulla and in the cervical spinal canal. C – CT scan (sagittal section), massive supratentiorial hemorrhage overlying the occipital lobes, third and fourth ventricular bleeding, subdural hematoma surrounding the medulla and pons and in the cervical region of the spinal cord. D – CT scan (transverse section), severe diffuse cerebellar edema and bilateral subdural and subarachnoid hematoma. 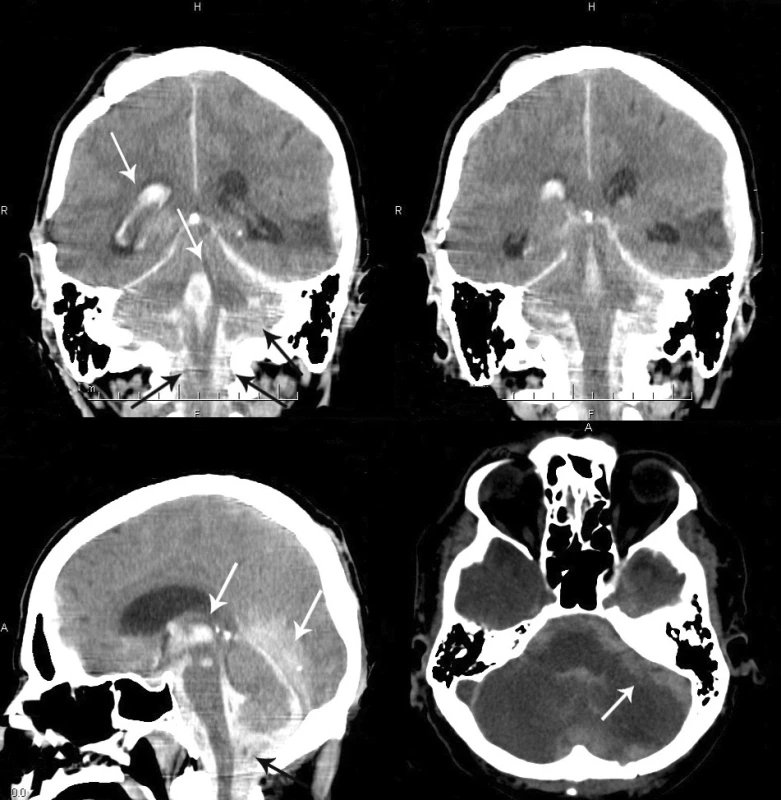
An autopsy was performed 24 hours after death. External examination revealed a surgically repaired laceration in the left parieto-occipital region and swelling of the skin and subcutaneous tissue in the left occipito-cervical region. There was evidence of the recent fronto-temporo-parietal decompressive craniectomy. Internal examination demonstrated acute bilateral subdural hematoma in the posterior fossa measuring 46 g and a thin film of subdural hematoma in the middle fossa. The cranio-cervical junction was intact. The dura mater was stripped showing fracture of the occipital and the left temporal bone extending into the parietal bones (Fig. 4). The brain showed extreme gyral flattening and compressed sulci with bilateral tonsillar herniation. Anatomical structures in the posterior fossa showed significant autolytic changes. An 8 mm thick subarachnoid hemorrhage overlying the cerebellar hemispheres, pons and the medulla was present. Coronal sections of the cerebral hemispheres revealed compressed ventricles with focal bleeding in the right lateral ventricle. Sections of the cerebellum showed secondary hemorrhages in the white matter. The spinal cord was uncovered with a dorsal laminectomy. The dura mater appeared dark and slightly distended in the cervical and mid-thoracic areas. On the opening, SSH spreading to the 6th thoracic vertebra was reavealed (Fig. 5). There was no sign of epidural hemorrhage. No fracture of the cervical and thoracic vertebrae was observed. Hematoxylin-eosin, Palmgren and anti-neuron specific enolase stained sections of the corpus callosum and dorsolateral pons showed diffuse axonal injury (Fig. 6). In addition, red neurons were present in the cerebral cortex and cervical spinal cord as a result of hypoxia. Cross-sections of the upper cervical cord also showed subarachnoidal hemorrhage. Other finding included mild atherosclerosis. Death was due to traumatic brain edema with bilateral tonsilar herniation complicating a severe diffuse traumatic brain injury.
4. Fracture of the occipital and the left temporal bone running across the sigmoid sinus (horizontal arrow) and spinal subdural hematoma (vertical arrow). 
5. Dorsal view of the cervical and mid-thoracic epidural space with subdural hematoma visible through the intact dura mater. The dura is incised to show the spinal subdural hematoma in situ (inset). 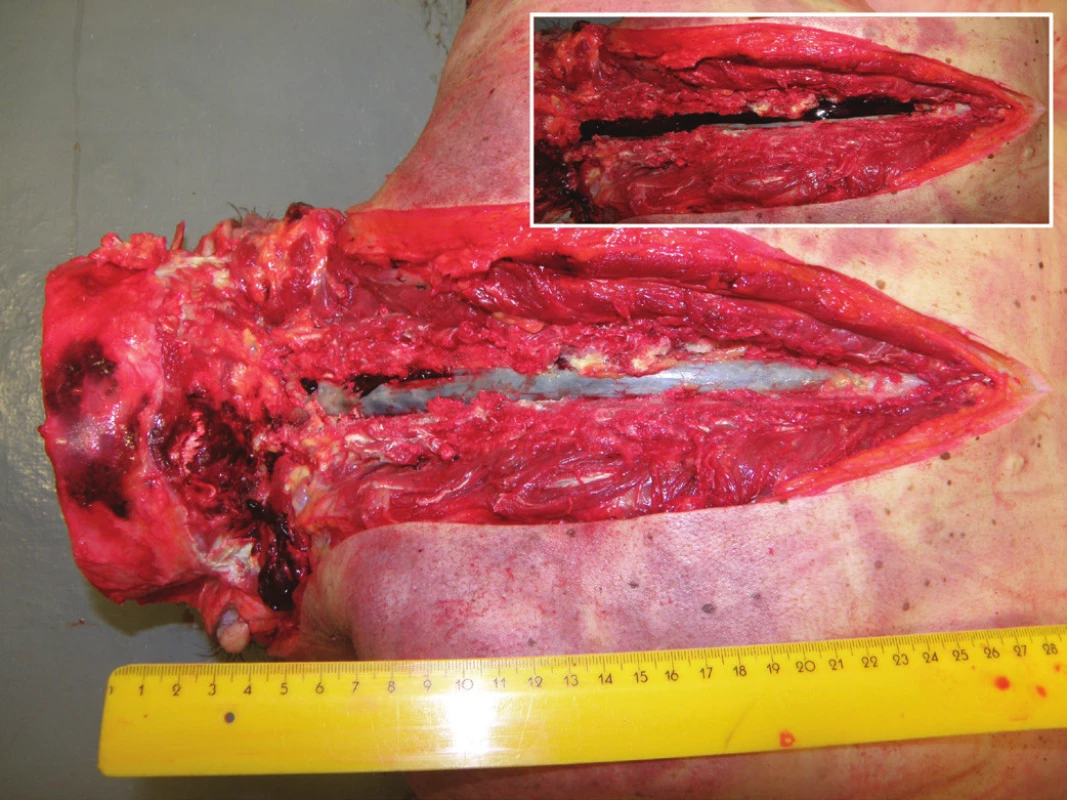
6. Anti-neuron specific enolase stained section of the dorsolateral pons showing diffuse axonal injury (anti-NSE x400). 
DISCUSSION
A subdural hemorrhage is a collection of blood between the dura and the meninges. In contrast to epidural hematomas, subdural hematomas are not usually well circumscribed and do not have distinct borders. Intracranial subdural hematoma is usually caused by tears in cortical bridging veins. It may also arise from tears in cortical surface veins or cortical arteries, or tears in a dural sinus (1). Subdural hematomas can be acute (symptoms present within 72 hours), subacute (symptoms present between 3 days and 2-3 weeks), or chronic (more than 3 weeks after injury) (14). Rare cases of traumatic subdural hematoma with concomitant epidural hematoma have been reported (15).
In this case after cranial computed tomography scan evaluation we assume that bilateral ISH in the middle fossa was caused by tears in cortical bridging veins, whereas bilateral ISH in the posterior fossa was rather caused by tears in a dural sinus (sigmoid or transverse sinus) due to occipital bone fracture on the left side after fall. The deceased did not have preexisting coagulopathy and was not on anticoagulant therapy. ISH is a well-known entity in neuropathology (1,2) and forensic pathology (3) which can be either trauma related or non-trauma related. SSH is a rare and potentionally life-threatening condition which is characterized by an accumulation of blood in the subdural space within the spinal canal that can mechanically compress the spinal cord causing irreversible damage. The aforementioned conditions (traumatic and non-traumatic) can be associated with blood accumulation in the spinal subdural space. SSH might be related to a progressive migration of subdural blood to the spinal subdural space (9). In this case follow-up CT angiography of the brain revealed no vascular anomaly that could be associated with SSH. Therefore, we concluded that SSH was related to a progressive migration of ISH in the posterior fossa to the spinal subdural space. This migration was accelerated due to diffuse cerebral atrophy causing the subdural space to enlarge and resulting in faster redistribution of ISH. We assume that the head trauma four months prior to his death may have contributed to the extent of cerebral atrophy and subdural space enlargement.
Balik et al. reported a case of spontaneous redistribution of an acute intracranial supratentorial subdural hematoma to the entire spinal subdural space (9). The most commonly affected areas are the lumbal and thoracolumbal regions of the spinal canal (4). Clinical manifestation of a traumatic SSH varies depending on the level and size and may occur in a few hours to 3 weeks after the primary insult. Clinical manifestation of SSH may include sudden back pain radiating into upper or lower extremities or to the trunk, sensory, motor and autonomic deficits (12), severe headache (13), and an extremely rare Pourfour du Petit syndrome (9). The reported case demonstrates traumatic brain injury including diffuse axonal injury in a comatose, non-responding patient comfirmed by histological examination and immunohistochemical investigation using antibodies against neuron specific enolase (16). Clinical evaluation of the patient’s symptoms was not possible due to diffuse axonal injury. The treatment of SSH (conservative or surgical intervention) depends on the patient’s initial neurological condition, the severity and the position of the hematoma (5). Rarely, ISH may be concomitant with SSH (17) as in the presented case. The demonstrated case is remarkable because it illustrates a patient with a recurrence of traumatic SSH related to its acute redistribution from the infratentorial region to the spinal subdural space, and a grade three diffuse axonal injury (18).
Discrepancy between clinical and autopsy diagnosis is not a rare phenomenon in the medico-legal practice. This case demonstrates the possibility of ISH redistribution to the spinal subdural space. In the clinical setting, SSH can be reliably diagnosed by magnetic resonance imaging (MRI). Currently, CT myelography is used only sporadically in routine clinical practice. The spinal subdural space can be visualized post-mortem by a number of modern imaging methods, such as computed tomography and MRI (19). If such techniques are not available, precise dissection of the spinal subdural space is of great importance.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest regarding the publication of this paper.
Correspondence address:
Alžbeta Ginelliová
Medico-Legal Department of the Health Care Surveillance Authority
Ipeľská 1, P.O.BOX G-44, 043 74 Košice, Slovak Republic
tel: +421915953365
fax: +421552852655
e-mail: e.ginelli@gmail.com
Sources
-
Dolinak D. Trauma. In: Prayson AR, Goldblum JR, eds. Neuropathology (a volume in the foundations in diagnostic pathology). Philadelphia: Elsevier Churchill Livingstone; 2005 : 76-78.
-
Fuller GN, Goodman JC. The central nervous system. In: Strayer DS, Rubin E, eds. Rubin’s Pathology. Clinicopathologic foundations of medicine. Philadelphia: Lippincott Wiliams & Wilkins; 2015 : 1428-1429.
-
Leestma JE. Forensic neuropathology. Boca Raton: CRC Press; 2009.
-
Domenicucci M, Ramieri A, Ciappetta P, et al. Nontraumatic acute spinal subdural hematoma: report of five cases and review of the literature. J Neurosurg 1999; 91 : 65-73.
-
Lee JK, Chae KW, Ju CI, et al. Acute cervical subdural hematoma with quadriparesis after cervical transforaminal epidural block. J Korean Neurosurg Soc 2015; 58 : 483-486.
-
Cha YH, Chi JH, Barbaro NM. Spontaneous spinal subdural hematoma associated with low-molecular-weight heparin: a case report. J Neurosurg Spine 2005; 2 : 612-613.
-
Subbiah M, Avadhani A, Shetty AP, et al. Acute spontaneous cervical epidural hematoma with neurological deficit after low-molecular-weight heparin therapy: role of conservative management. Spine J 2010; 10: e11-e15.
-
Post MJ, Becerra JL, Madsen PW, et al. Acute spinal subdural hematoma: MR and CT findings with pathologic correlates. AJNR Am J Neuroradiol 1994; 15 : 1895-1905.
-
Balik V, Kolembus P, Švajdler M, et al. A case report of rapid spontaneous redistribution of acute supratentorial subdural hematoma to the entire spinal subdural space presenting as a Pourfour du Petit syndrome and review of the literature. Clin Neurol Neurosurg 2013; 115 : 849-852.
-
Hamaguchi H, Osawa T, Koyama K, et al. Idiopathic lumbar spinal subdural hematoma. Orthopedics 2008; 31 : 715.
-
Metzger G, Singbartl G. Spinal epidural hematoma following epidural anesthesia versus spontaneous spinal subdural hematoma. Two case reports. Acta Anaesthesiol Scand 1991; 35 : 105-107.
-
Al B, Yildirim C, Zengin S, et al. Acute spontaneous spinal subdural haematoma presenting as paraplegia and complete recovery with non-operative treatment. BMJ Case Rep 2009; doi:10.1136/bcr.02.2009.1599.
-
Oh JH, Jwa SJ, Yang TK, et al. Intracranial vasospasm without intracranial hemorrhage due to acute spontaneous spinal subdural hematoma. Exp Neurobiol 2015; 24 : 366-370.
-
Dettmeyer RB. Forensic histopathology. Fundamentals and perspetives. Berlin Heidelberg: Springer; 2011.
-
Balik V, Lehto H, Hoza D, et al. Posterior fossa extradural haematomas. Cent Eur Neurosurg 2010; 71 : 167-172.
-
Toupalík P, Bouska I. Diagnosis of traumatical and hypoxial changes of the CNS - immunohistochemical study. Soud Lek 2005; 50 : 42-44.
-
Kim MS, Sim SY. Spinal subdural hematoma associated with intracranial subdural hematoma. J Korean Neurosurg Soc 2015; 58 : 397-400.
-
Graham DI, Gennarelli TA. Trauma. In: Graham DI, Lantos PL, eds. Greenfield’s neuropathology. London: Arnold; 1997 : 229.
-
Kučerová S, Safr M, Ublová M, et al. The application of X-ray imaging in forensic medicine. Soud Lek 2014; 59 : 34-38.
Labels
Anatomical pathology Forensic medical examiner Toxicology
Article was published inForensic Medicine

2018 Issue 3
Most read in this issue
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

