-
Medical journals
- Career
Epidemiological factors influencing the development of relapsing and severe Clostridium difficile infection
Authors: L. Vojtilová 1; M. Freibergerová 1; J. Juránková 2; Z. Bortlíček 3; P. Husa 1
Authors‘ workplace: Clinic of Infectious Diseases, Faculty of Medicine, Masaryk University, The University Hospital Brno, Brno, Czech Republic 1; Department of Clinical Microbiology, The University Hospital Brno, Brno, Czech Republic 2; Institute of Biostatistics and Analyses Masaryk University, Brno, Czech Republic 3
Published in: Epidemiol. Mikrobiol. Imunol. 63, 2014, č. 1, s. 27-35
Category: Review articles, original papers, case report
Overview
Introduction:
Clostridium difficile infection (CDI) is currently the most frequent cause of nosocomial infectious diarrhea in adults in the developed countries. The goal of the study was to evaluate risk factors for relapsing and severe CDI in a set of patients hospitalized at the Clinic of Infectious Diseases at the University Hospital Brno.Materials and methods:
A retrospective analysis of epidemiological, clinical and laboratory data of 281 patients with proved CDI diagnosis hospitalized in the period from 1. 1. 2007 to 31. 12. 2010.Results:
Patient age over 65 is a risk for severe CDI (OR 2.95, p < 0.001) and extends hospitalization at the first episode of CDI by about 3.2 days on average. Patients with 2 or more comorbidities (p < 0.05) or with a history of recent hospitalization (p ≤ 0.001) are at risk for both relapsing CDI and severe CDI. The use of proton pump inhibitors may increase the number of relapses (OR 1.94, p < 0.05). If the CDI symptoms appear within 7 days of taking antibiotics, there is a greater risk of relapse (OR 2.32, p < 0.05). If the symptoms occur after a longer period, a mild or moderate course of the disease can be expected (OR 0.31, p < 0.05).Conclusions:
To determine the risk level for development of relapsing or severe CDI, focus on risk factors from the patients’ medical history and their clinical and laboratory status is appropriate at the outset of CDI patients treatment. An early intensive monitoring of vital functions and administration of aggressive treatment can reduce complications, mortality and relapses of CDI.Keywords:
Clostridium difficile infection – risk factors for relapsing CDI – risk factors for severe CDIINTRODUCTION
Considering the increasing number of reported cases, Clostridium difficile-caused infections represent a major health problem in the developed countries.
Increasing is not only incidence, but also severity of the disease, mortality, the number of treatment failures and relapses. The infection caused by C. difficile is now the leading cause of nosocomial infectious diarrhea in adults in the developed countries [1, 2]. Severity of the disease is alarming especially in geriatric patients, where it leads to increased morbidity and mortality [3]. The rising costs of prevention, diagnosis and treatment associated with this infection represent a significant financial burden to health care.
C. difficile, including its toxigenic strains, counts among the common commensals of colonic mucosa in humans and animals. Infection occurs only under certain circumstances. Infection risk factors include intestinal dysmicrobia after antibiotic treatment, patient age over 65, comorbidity, and disorders of the immune system – particularly mucosal immunity [4]. A previous hospitalization poses a significant risk of colonization with hospital C. difficile strains. The disease is associated with frequent and repeated relapses, which can lead to exhaustion of the patient and death.
The risk factors of the disease are well described. However, factors determining relapse or severe course of CDI associated with poor patient prognosis are far less known. The focus of this study is to identify the factors leading to relapsing or severe colitis caused by CDI.
MATERIALS AND METHODS
The retrospective analysis evaluated epidemiological, clinical and laboratory data of patients with proved diagnosis of C. difficile infection hospitalized at the Clinic of Infectious Diseases (CID) of The University Hospital (UH) Brno from 1. 1. 2007 to 31. 12. 2010. Data about the patiens’ age, gender, comorbidities, the number and length of hospitalizations at CID, other previous hospitalizations, previous antibiotic use, gastric acid suppressions, corticosteroids and chemotherapies were extracted from the patients’ medical records.
The study icluded inpatients with clinical signs of CDI, who had confirmed toxins A or B in the stool sample. If the sample was negative, and there was a persistent clinical suspicion of CDI, the proof of the toxin in the stool sample was repeated several times with samplings at least one day apart. Cultivated stool samples of all patients were also examined to exclude obligatory intestinal pathogens as alternative cause of infectious diarrhea. Patients diagnosed with CDI at another medical facility and admitted to our clinic only for treatment were excluded from the study, because their laboratory tests were not repeated. Microbiological examinations were conducted at the Department of Clinical Microbiology (DCM) of The University Hospital Brno. In 2007–2009, toxins A and B of C. difficile were analyzed by ELISA (Enzyme Linked Immunosorbent Assay) method using the mini-VIDAS set (Bio-Merieux). The immunochromatographic set C.diff Quick chek complete (Techlab) was used in 2010.
CDI relapse was defined as re-appearence of clinical symptoms after an asymptomatic period following an episode of CDI. Each relapse was confirmed by a repeated proof of the CDI toxin in the stool sample and an exclusion of another infectious cause of the diarrhea. The criteria for severe CDI were adopted from the document "Diagnosis and Therapy of Clostridium difficile infection: The Czech National Guidelines", published in 2012 [4]. The disease is regarded as severe, if any one of these symptoms presents: fever (> 38 ˚C), rigors and chills, haemodynamic instability including signs of septic shock, signs of peritonitis, signs of paralytic ileus, leukocytosis (> 15 × 109/L), marked left shift (band neutrophils > 20 % of leukocytes), elevated serum creatinine (> 50% above the baseline), elevated serum lactate, pseudomembranous colitis (by endoscopy) or distension of large intestine (by imaging).
Statistical evaluation
Statistical analysis of data from 281 patients was carried out in two parts. The first analysis evaluated risk factors according to the frequency of CDI relapse, the second one evaluated risk factors according to the severity of CDI. Patients who died during the first hospitalization (n = 42, n – number of patients) as well as patients who died within 30 days of discharge (n = 6) were excluded from the CDI relapse evaluation. Therefore, only 233 patients were analyzed. The second analysis – risk factors for severe CDI – included all 281 patients. According to the patiens’ clinical and laboratory status data during all CDI episodes, a subset of patients with severe course of CDI (n = 181) was identified.
Odds ratio (OR) for all parameters was calculated according to univariate logistic regression for relapse or for severe course of the disease. The estimates always included a 95% confidence interval (CI) and a significance level p (Wald test). The selected level of significance was α = 0.05. The reference category is always a result related to the complement of patients to the overall 233 patients (the first analysis) or 281 patients (the second analysis), unless stated otherwise. When evaluating the length of the first hospitalization relative to the patient age, statistical significance was assessed non-parametrically using the Mann-Whitney test (MW test).
RESULTS
In 2007–2010, 281 patients with CDI, confirmed by laboratory tests at the DCM UH Brno, were hospitalized at the Clinic of Infectious Diseases. 73 patients were hospitalized repeatedly, 106 (37.7%) of them were male and 175 (62.3%) were female. Average patient age was 73.2 with a minimum of 19 and a maximum of 99, the median age was 79. Figures 1 and 2 shows annual distribution of cases and patient age distribution, respectively.
Fig. 1 The number of hospitalized CDI patients in years 2007–2010 divided into age subsets Graf. 1 Počet hospitalizovaných pacientů s CDI v jednotlivých letech s věkovým rozdělením 
Fig. 2 Distribution of CDI patients by age Graf. 2 Věkové rozložení pacientů s CDI 
Hospitalization period was 1–55 days. The average length of hospitalization at the first episode of CDI was 16 days (median 14 days). Comparison of age groups ≤ 65 and over 65 has shown a statistically significant difference in the length of the first hospitalization (the result of M-W test = 0.003). The length of the first hospitalization in the group of younger patients was 13.5 days on average (median 12), but in the group of elderly patients, the average was 16.7 days (median 15). Patients older than 65 stayed in the hospital by 3.2 days longer on average.
1. The risk factors for relapsing CDI
In the first analysis of data from 233 patients, 87 (37.3%) patients had relapsing disease and 146 (62.7%) patients had only a single episode of CDI. Out of the 174 patients in the age group over 65, 70 patients (40.2%) relapsed, p = 0.119. Neither age over 65, nor gender was a risk for relapse according to our analysis. Data about the following comorbidities were collected from the patients’ medical history: ischemic heart disease, diabetes mellitus, cerebrovascular diseases, chronic renal failure, immobility, chronic obstructive pulmonary disease and oncological diseases. A coincidence of 2 or more comorbidities represented a significantly higher risk of relapse, p = 0.013 (Table 1).
1. Evaluation of CDI relapse relative to the patients’ medical history Tabulka 1. Vyhodnocení rekurence CDI vzhledem k anamnestickým údajům pacientů 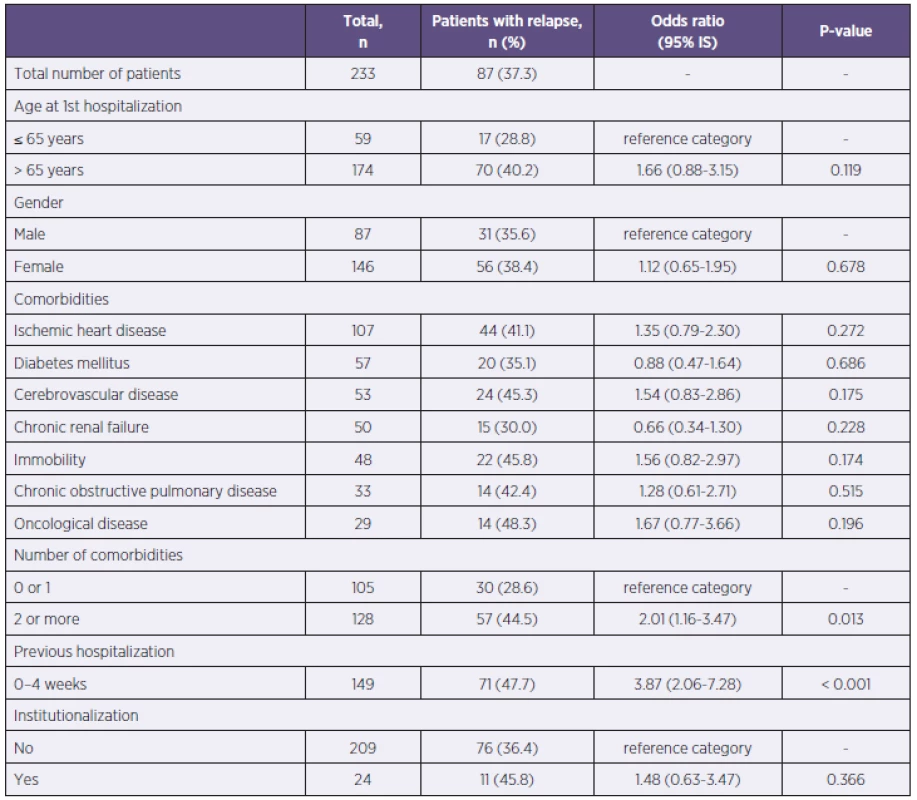
A hospitalization within 4 weeks before the onset of CDI was a significant risk factor. There were 149 previously hospitalized patients, of whom 71 had relapsed (47.7%), p < 0.001. Other previous institutionalizations (a stay in a retirement home or a rest home) did not represent a risk of relapsing CDI (see Table 1).
A previous antibiotic treatment within 60 days before the CDI diagnosis was documented in 207 patients, 82 of them received 2 or more antibiotics. Neither the type of antibiotics, nor their combination factored in the relapse. The interval between the onset of CDI symptoms and the antibiotic treatment was also evaluated. The patients were more prone to relapse if their symptoms of diarrhea occurred during the first week after the end of antibiotic treatment, p = 0.035 (Table 2).
2. Evaluation of CDI relapse relative to history of antibiotics administration, gastric acid suppression and the use of immunosupressive drugs Tabulka 2. Vyhodnocení rekurence CDI vzhledem k předchozímu užívání antibiotik, antiulcerózní a imunosupresivní terapie 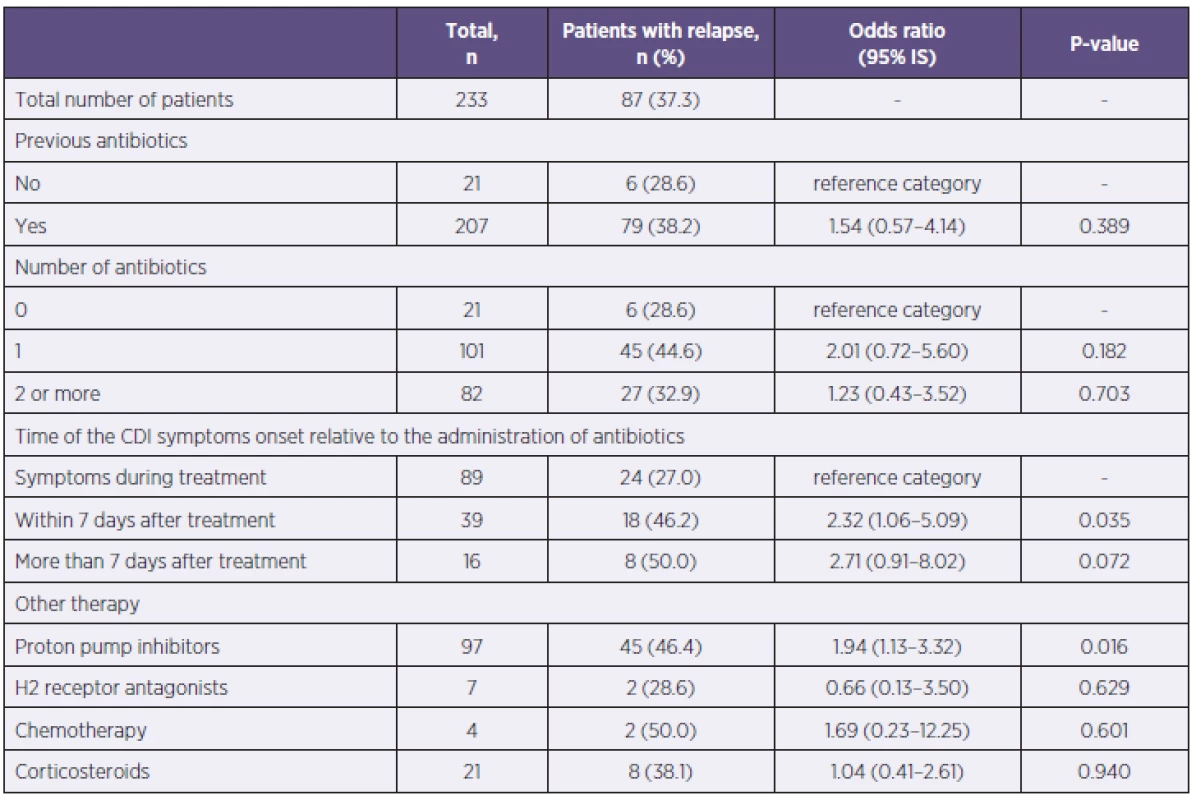
Previous use of antiulcer drugs and immunosupressive drugs was also monitored. Out of 97 patients of the entire set, who had a history of proton pump inhibitors use, 45 patients (46.4%) had relapsed, p = 0.016. Consequently, the use of proton pump inhibitors increased the risk of relapse. The previous use of histamine H2 receptor antagonists, treatments with systemic corticosteroids (dose ≥ 5 mg prednisone) at the time of CDI, or the use of chemotherapy in the last 60 days did not affect the emergence of the CDI relapse (see Table 2).
2. The risk factors for severe CDI
Severe CDI during any episode of the disease was observed in 181 (64.4%) of all 281 patients, while the remaining 100 (35.6%) patients had a mild or moderate course of CDI.
Patient age over 65 was definitely a risk factor for severe CDI. Out of 216 patients over 65 years old, 152 (70.4%) had severe CDI, p = 0.001. Gender was not in correlation with the severity of the disease. Among the observed comorbidities, a group of patients with a history of ischemic heart disease and chronic renal failure were at risk for severe CDI, as were also patients with 2 or more comorbidities (Table 3).
3. Evaluation of severe CDI relative to the patients’ medical history Tabulka 3. Vyhodnocení těžkého průběhu CDI vzhledem k anamnestickým údajům pacientů 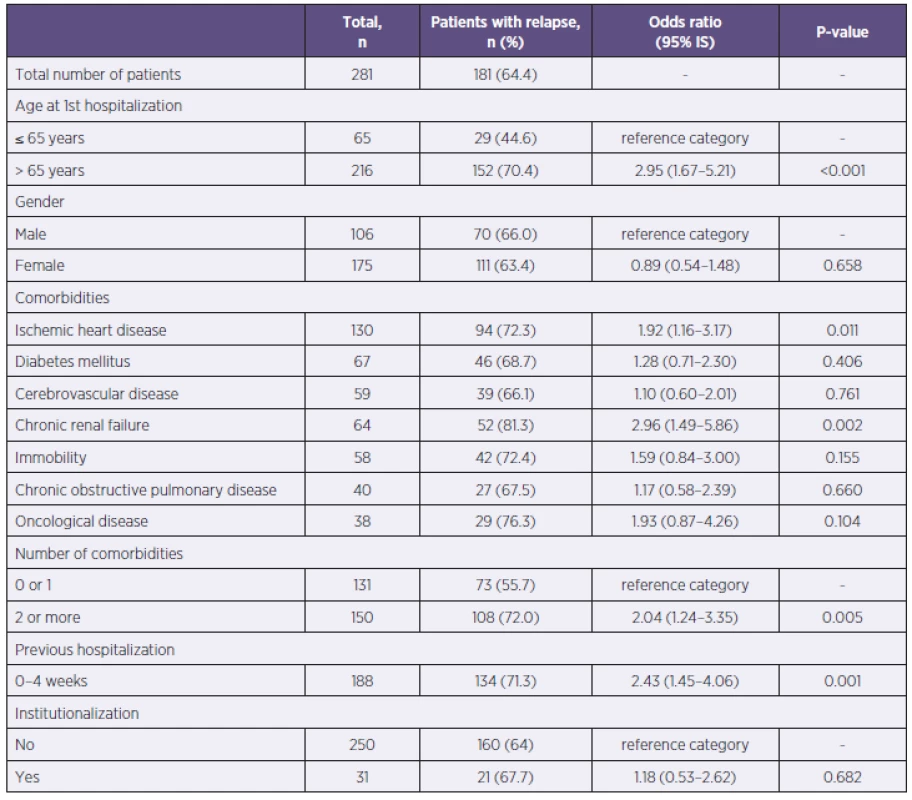
Patients, previously hospitalized 4 weeks before the onset of CDI, had more likely a severe course of the disease. Out of the 188 previously hospitalized patients, 134 patients had severe CDI (71.3%), p = 0.001. Previous institutionalization was not a risk of severe CDI (see Table 3).
Previous use of antibiotics singly or in a combination did not pose a risk of severe CDI development. The patients with CDI symptoms onset more than 7 days after antibiotic treatment were statistically less affected by severe CDI than patients who had symptoms during the antibiotic treatment or within one week since. The use of antiulcer drugs, treatment with systemic corticosteroids at the time of CDI, or the use of chemotherapy in the last 60 days did not affect the severity of CDI (Table 4).
4. Evaluation of severe CDI relative to history of antibiotics administration, gastric acid suppression and the use of immunosupressive drugs Tabulka 4. Vyhodnocení těžkého průběhu CDI vzhledem k předchozímu užívání antibiotik, antiulcerózní a imunosupresivní terapie 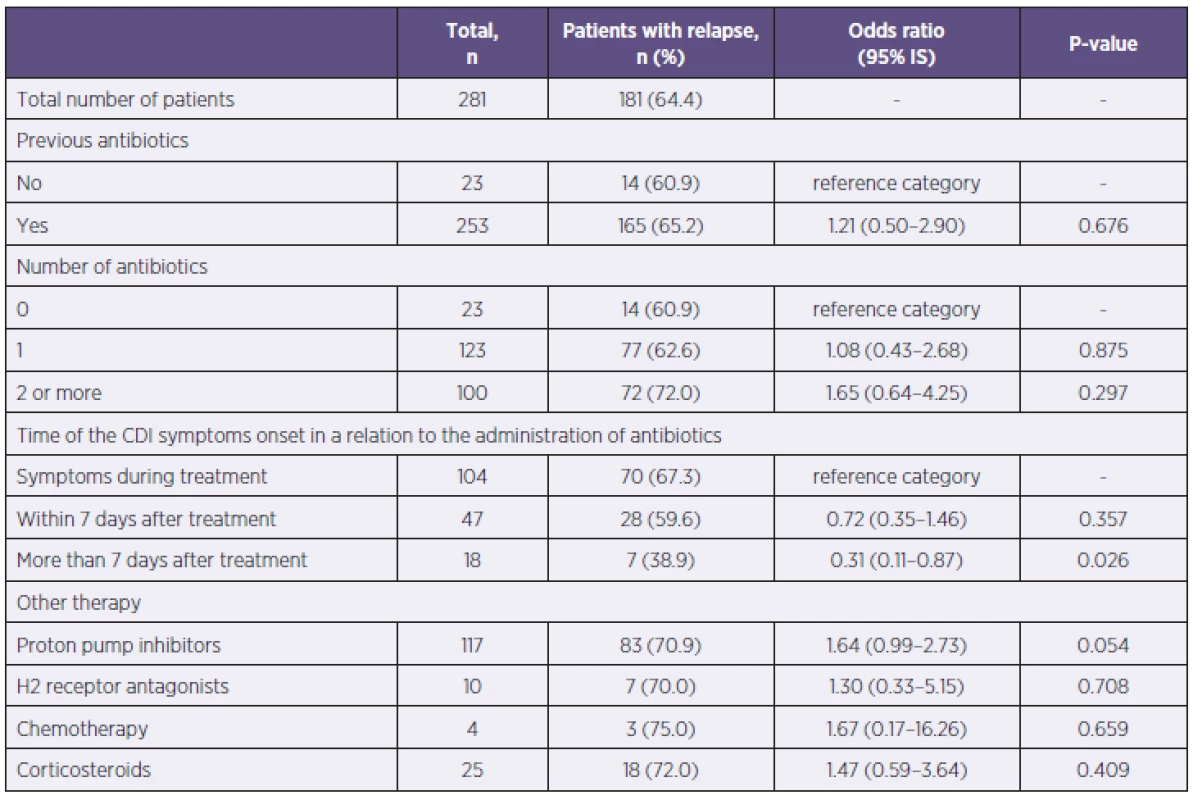
DISCUSSION
Since 2007, a significant increase in the number of CDI cases was noted at our clinic. Until then, it was only individual cases. This trend follows the incidence of CDI reported in the Czech Republic. According to the Epidat database of The National Institute of Public Health in the Czech Republic, 303 cases of CDI were reported in 2008, 1,552 cases in 2011, and a total of 2,241 cases in 2012 [5]. By comparison, the study of authors Polívková et al. included 82 patients with CDI for the period from 1. 1. 2008 to 30. 6. 2010 [6]. However, the increased number of reported CDI cases in the Czech Republic in recent years is partly influenced by the implementation of better diagnostic methods and improved quality of reporting.
The average length of the first hospitalization of CDI patients was 16 days, almost twice longer than the average length of stay of all patients admitted to CID in 2007–2010 (8.3 days). In addition, the average length of the first hospitalization for the treatment of CDI in patients older than 65 was by 3.2 days longer than in younger patients. Aside from severe course of CDI, it might be caused by a greater frequency and severity of comorbidities at the older age.
Any course of CDI in the patients over 65 is considered serious, even without clinical signs of severe colitis; this was confirmed in our evaluation, too. Age over 65 did not represent a significant risk for CDI relapse. There were 1.5 times more women than men in our patient set, but the patients’ gender was not correlated to the severity or relapse of CDI. Many studies did not find a relationship between gender and severity of CDI, although one prospective study suggests a link between the male gender and severe course of CDI. The study did not prove a correlation between ethnicity and severity of the disease [7]. Pépin’s retrospective study of 2007, including 1,616 CDI inpatients at a Canadian tertiary care hospital, described the following risk factors for severe CDI: age over 65, male gender, immunosuppression, nosocomial infections, enteral nutritions, short duration of diarrhea, fever, leukocytosis and increased creatinine [8].
The number of comorbidities is correlated to the patient’s age; therefore the combination of 2 or more monitored comorbidities presents a risk of both relapsing and severe CDI. Our study did not confirm the expected impact of patient’s long-term immobility on relapse or severity of CDI. Some studies have reported pre-existing renal insufficiency or chronic pulmonary disease as a risk factor for increased mortality. A correlation between severe CDI and a history of malignancy has also been suggested [9, 10]. Interestingly, despite the widely accepted fact about increased susceptibility of diabetes mellitus patients to various infections, none of the studies has demonstrated a link between diabetes mellitus and severity of CDI [9].
Publications classify CDI as healthcare facility onset CDI (begins 48 hours after admission to the hospital), healthcare facility-associated community onset CDI (up to 4 weeks after hospitalization) or community associated CDI (symptoms begin more than 12 weeks after previous hospitalization). The period between 4–12 weeks after hospitalization is referred to as indeterminate [11]. Previous hospitalization within 4 weeks before the onset of CDI represents a significant risk factor for relapse and severity of CDI in our patient set. According to our results, a stay in a retirement home without prior hospitalization is not a risk for development of CDI. Asymptomatic faecal carriage of C. difficile was reported in 10% of residents in an Irish continuing care institution for the elderly, 7% of them were toxin positive [12]. There is no information available about C. difficile carriage in the Czech Republic.
The use of antibiotics is considered the most important risk factor for intestinal colonization with strains of C. difficile. Lincosamides, aminopenicillins, cephalosporins and quinolones represent the highest risk levels. Aminoglycosides, co-trimoxazole, penicillin, carbapenems and tetracyclines are considered relatively safe [13–15]. According to our results, the use of any specific class of antibiotics cannot be correlated with the risk of relapse or severe CDI, although the use of quinolones was described as a risk factor for relapsing CDI in a smaller patient set in the USA [16].
Suppressed acidity of gastric secretion predisposes to a variety of intestinal infections. Some studies indicate the use of proton pump inhibitors as a risk factor for CDI, but others have not confirmed this [17–20]. Howell et al. documented an increased risk of nosocomial infection of C. difficile depending on the pharmacological suppression of gastric acid secretion [18]. A connection between pharmacological suppression of gastric acid secretion and severity of CDI has not been demonstrated yet [21, 22]. Another study comprising 125 patients identified the use of proton pump inhibitors as well as the age over 65 and a low serum albumin (< 25 g/L) as significant risk factors for CDI relapse [23]. The relationship between the use of proton pump inhibitors and the risk of relapse, demonstrated by our study, indicates a reinfection with C. difficile rather than a relapse of previous disease. CDI patients contaminate the hospital environment with spores contained in the stool, which can be a source of reinfection of predisposed persons. It is important to realize that a failure of antibiotic treatment due to the resistance of C. difficile is not the cause of these recurrences [4].
Treatment with systemic corticosteroids at the time of CDI or the use of chemotherapy in the last 60 days did not influence relapses or disease severity in our patient set. Our results do not correspond with the described effect of immunosuppression as a risk factor for severe CDI [8], nor with the results of another study, which demonstrated a significant 2.1-fold increase in 30-day mortality in CDI patients who received corticosteroids for at least 15 days before the CDI diagnosis [24].
CONCLUSIONS
Retrospective analysis of clinical and epidemiological data of patients hospitalized at CID with a proved diagnosis of C. difficile infection in 2007–2010 leads to the following key conclusions:
- Patient age over 65 is a risk factor of severe CDI (OR 2.95, p < 0.001) and leads to extension of hospitalization at the first episode of CDI by about 3.2 days on average.
- Patients with 2 or more comorbidities (p ≤ 0.05) or with a history of recent hospitalization (p ≤ 0.001) are at risk for relapsing CDI and also severe CDI.
- The use of proton pump inhibitors may increase the number of relapses (OR 1.94, p < 0.05), probably because of reinfection with spores of C. difficile.
- If CDI symptoms appear within 7 days of taking antibiotics, there is a greater risk of relapse (OR 2.32, p < 0.05). If the symptoms occur after a longer period such as one week, a mild or moderate course of the disease can be expected (OR 0.31, p < 0.05).
At the beginning of CDI patients’ treatment, it is appropriate to focus on risk factors known from patients’ medical history and their clinical and laboratory status to assess risk of relapsing or severe disease. Early intensive monitoring of vital functions and administration of aggressive treatment can reduce complications, mortality and relapses of CDI.
Do redakce došlo dne 29. 7. 2013.
Adresa pro korespondenci:
MUDr. Lenka Vojtilová, Ph.D.
Jánošíkova 36
643 00 Brno
e-mail: lvojtilova@fnbrno.cz
Sources
1. Archbald-Pannone L, Sevilleja JE, Buerrant R. Diarrhea, Clostridium difficile, and Intestinal Inflammation in Residents of a Long-Term Care Facility. J Am Med Dir Assoc, 2010;11 : 263–267.
2. Markis AT, Gelone S. Clostridium difficile in the long-term care setting. J Am Med Dir Assoc, 2007;8 : 290–299.
3. Bielaková K, Weber P, Matějovská-Kubešová H, et al. The disease caused by Clostridium difficile in geriatric patients. Čas Lék Čes, 2011;150 : 334–338.
4. Beneš J, Husa P, Nyč O. Doporučený postup diagnostiky a léčby kolitidy vyvolané Clostridium difficile. Klin Mikrobiol Inf Lek, 2012;18(5):160–167.
5. Státní zdravotní ústav, databáze Epidat. Dostupné na www: www.szu.cz.
6. Polívková S, Sýkorová B, Džupová O, Reisingerová M, Beneš J. Výskyt a charakter infekcí vyvolaných Clostridium difficile u pacientů s průjmovým onemocněním v pražské fakultní nemocnici. Klin Mikrobiol Inf Lek, 2010;16(6):206–210.
7. Arora V, Kachroo S, Ghantoji SS, DupontHL, Garey KW. High Horn's index score predicts poor outcomes in patients with Clostridium difficile infection. J hosp Infect, 2011;79(1):23–26.
8. Pépin J, Valiquette L, Gagnon S, Routier S, Brazeau I. Outcomes of Clostridium difficile-associated disease treated with metronidazole or vancomycin before and after the emergence of NAP1/027. Am J Gastroenterol, 2007;102 : 2781–2788.
9. Potter VA, Aravinthan A. Identifying patients at risk of severe Clostridium difficile-associated disease. British Journal of Hospital Medicine, 2012;73(5):265–270.
10. Lungulescu OA, Cao W, Gatskevich E, Tlhabano L, Stratidis JG. CSI: a severity index for Clostridium difficile infection at the time of admission. J Hosp Infect, 2011;79(2):151–154.
11. Cohen SH, Gerding DN, Johnson S et al. Clinical Practice Guidelines for Clostridium difficile Infection in Adults: 2010 Update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol, 2010;31(5):431–455.
12. Ryan C, Murphy C, Twomey C et al. Asymptomatic carriage of Clostridium difficile in an Irish continuing care institution for eldery: prevalence and characteristics. Ir J Med Sci, 2010;179(2):245–250.
13. Owens RC. Clostridium difficile – associated disease. Changing epidemiology and implications for management. Drugs, 2007;67(4):487–503.
14. Beneš J, Sýkorová B. Kolitida vyvolaná Clostridium difficile. Zpráva z kongresu ICAAC 2006. Klin Mikrobiol Inf Lek, 2006;12(6):247–251.
15. Hubert B, Loo VG, Bourgault AM, et al. A portrait of the geographic dissemination of the Clostridium difficile North American pulsed-field type 1 strain and the epidemiology of C. difficile-associated disease in Québec. CID, 2007;44 : 238–244.
16. Cadena J, Thompson GR, Patterson JE, et al. Clinical predictors and risk factors for relapsing Clostridium difficile infection. Am J Med Sci, 2010;339(4):350–355.
17. Cloud J, Kelly CP. Update on Clostridium difficile associated disease. Current Opinion in Gastroenterology, 2007;23(1):4–7.
18. Howell MD, Novack V, Grgurich P, et al. Iatrogenic gastric acid suppression and the risk of nosocomial Clostridium difficile infection. Arch Intern Med, 2010;170(9):784–790.
19. DuPont HL, Garey K, Caeiro JP, Jiang ZD. New advances in Clostridium difficile infection: changing epidemiology, diagnosis, treatment and control. Curr Opin Infect Dis, 2008;21 : 500–507.
20. Dubberke ER, Reske KA, Yan Y, et al. Clostridium difficile – associated disease in a setting of endemicity: Identification of novel risk factors. CID, 2007;45 : 1543–1549.
21. Moshkowitz M, Ben-Paruch E, Kline Z, et al. Risk factors for severity and relapse of pseudomembranous colitis in an eldery population. Colorectal Dis, 2007;9(2):173–177.
22. Bishara J, Peled N, Pitlik S, Samra Z. Mortality of patients with antibiotic-associated diarrhoea: the impact of Clostridium difficile. J Hosp Infect, 2008;68(4):308–314.
23. Kim JW, Lee KL, Jeong JB, et al. Proton pump inhibitors as a risk factor for recurrence of Clostridium-difficile-associated diarrhea. World J Gastroenterol, 2010;16(28):3573–3577.
24. Das R, Feuerstadt P, Brandt LJ. Glucocorticoids Are Associated With Increased Risk of Short-Term Mortality in Hospitalized Patients with Clostridium difficile-Associated Disease. Am J Gastroenterol, 2010;105 : 2040–2049.
Labels
Hygiene and epidemiology Medical virology Clinical microbiology
Article was published inEpidemiology, Microbiology, Immunology

2014 Issue 1-
All articles in this issue
- Clinical and epidemiological characteristics of patients hospitalized with severe influenza in the season 2012–2013
- Bacteria of the Burkholderia cepacia complex: epidemiology and diagnosis of infection in patients with cystic fibrosis
- Genotyping results, laboratory diagnosis, and epidemiology of the mumps virus circulating in the Czech Republic in 2012
- Legionella infection – a neglected problem
- Evaluation of prophylactic precautions after exposure to biological materials
- Epidemiological investigation in five dental offices of the Clinic of Dentistry, Faculty of Medicine, Palacký University, Olomouc and of the Olomouc University Hospital
- Sequencing analysis of the antigens included in the four-component vaccine against serogroup B meningococcus in Czech isolates of Neisseria meningitidis from 2007–2013
- Influenza in seasons 2009-2013 in the Faculty Hospital Hradec Kralove, East Bohemia
- Epidemiological factors influencing the development of relapsing and severe Clostridium difficile infection
- Epidemiology of Cronobacter spp. isolates from patients admitted to the Olomouc University Hospital (Czech Republic)
- Epidemiology, Microbiology, Immunology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Legionella infection – a neglected problem
- Bacteria of the Burkholderia cepacia complex: epidemiology and diagnosis of infection in patients with cystic fibrosis
- Epidemiological investigation in five dental offices of the Clinic of Dentistry, Faculty of Medicine, Palacký University, Olomouc and of the Olomouc University Hospital
- Clinical and epidemiological characteristics of patients hospitalized with severe influenza in the season 2012–2013
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

