-
Medical journals
- Career
Pathological evaluation of colorectal cancer specimens: advanced and early lesions
Authors: Annika Resch; Nora I. Schneider; Cord Langner
Authors‘ workplace: Institute of Pathology, Medical University of Graz, Graz, Austria
Published in: Čes.-slov. Patol., 51, 2015, No. 1, p. 12-22
Category: Reviews Article
Overview
Surgical resection is the treatment of choice for patients with locally confined disease, but early cancers may be adequately treated by endoscopic resection alone. In advanced colorectal cancers, accurate staging including pathological lymph node assessment is crucial for patient counselling and decision making. In addition to the extent of surgical lymph node removal and the thoroughness of the pathologist in dissecting the cancer specimen lymph node recovery is related to the actual number of regional lymph nodes that is related to patient demographics, tumor location and biology. Current guidelines recommend a minimum of twelve nodes harvested as the standard of care. In patients with node-negative tumors a variety of histological features may be used for adjusted risk assessment, including histological subtyping, lymphatic and venous invasion, tumor budding and tumor necrosis as well as the anti-tumor host inflammatory response which has been identified as favorable feature in several studies. In rectal cancer, involvement of the circumferential resection margin and the plane of surgery are important prognostic factors. Early or superficial colorectal cancer is defined as invasive adenocarcinoma invading into, but not beyond the submucosa. A number of features require special attention because they are used to determine the necessity for radical surgery. In addition to the assessment of completeness of excision, these include the recording of parameters that predict the presence of lymph node metastasis, namely the depth of invasion into the submucosa, tumor grade, and the presence of additional risk factors, such as angioinvasion and tumor budding. The combination of these parameters allows the stratification of affected individuals into low-risk and high-risk categories.
Keywords:
colorectal cancer - early colorectal cancer - lymph node metastasis - pathological features - risk factors - prognosis
Colorectal cancer (CRC) is the third most common cancer worldwide in men and the second in females. In the United States, approximately 96,830 new cases of colon cancer and 40,000 new cases of rectal cancer have been estimated for 2014. For the same time period, 50,310 deaths from colorectal cancer have been calculated, accounting for about 9 % of all cancer deaths (1).
The pathological work-up of cancer specimens plays a central role in patient counselling and decision making, concerning both advanced and early lesions. In advanced CRC, accurate staging including thorough lymph node assessment is inevitable. Although tumor staging according to the AJCC/UICC TNM system is currently regarded as the standard for staging of patients with CRC, this system seems to be of limited value in predicting the outcome of patients with intermediate levels of disease (2). A variety of histological features may be used for risk assessment in these cases. In early CRC, the decision for additional surgery after primary endoscopic treatment is almost exclusively based upon the histological evaluation of the resection specimen. Only cases that lack features indicating increased risk of lymph node metastasis can be cured by local therapy alone (3,4).
In this review, we will summarize the histological features of CRC which are pivotal for clinical management and hence need to be addressed in the pathology report, with special focus on advanced and early lesions. Data for this review were compiled using MEDLINE/PubMed and Thomson Reuters Web of Science®, assessing articles published before April 2014. Only articles published in English were considered.
ADVANCED LESIONS
A. Lymph node evaluation
For patients with CRC, the indication for adjuvant therapy is mainly guided by the presence of positive lymph nodes (5,6). It is a matter of fact that both the extent of surgical lymph node removal and the thoroughness of the pathologist in dissecting the resection specimen may have severe impact on the clinical significance of lymph node staging. Several parameters that are related to the pathological work-up of the dissected nodes have to be considered. These include changing definitions of lymph nodes, involved lymph nodes, and tumor deposits in different editions of the AJCC/UICC TNM system as well as the minimum number of nodes that need to be dissected (7). Outcome prediction based on the lymph node ratio, defined as the number of positive lymph nodes divided by the total number of retrieved nodes, may be superior to the absolute numbers of involved nodes. Extracapsular invasion has been identified as additional prognostic factor. The clinical value of more recent technical advances, such as sentinel lymph node biopsy and molecular analysis of lymph nodes tissue still remains to be defined (8).
Minimum number of lymph nodes
Manual dissection with subsequent histological assessment based on routinely hematoxylin and eosin (H&E) stained slides is the standard approach in the examination of regional lymph nodes in cancer specimens (8). The conventional hypothesis is that by examining more lymph nodes, the accuracy of staging will be improved. An established body of evidence exists, demonstrating an association between a higher total lymph node count and improved survival, particularly in stage II disease, whereas in node positive cancers the relation between survival and lymph node harvest is less clear (9).
There is, however, ongoing debate regarding the optimal number of lymph nodes required for adequate staging. The evaluation of at least 12 lymph nodes is currently regarded as standard of care and widely cited in clinical guidelines (10-14). This number was first proposed 1990 by the Working Party Report to the World Congress of Gastroenterology in Sydney (15). Arguing against the minimum of 12 lymph nodes required for adequate staging several authors have suggested other cut-off values ranging from 6 to 21 (9).
The variability in the number of retrieved lymph nodes remains to be a major problem in patient management since often the recommended minimum number of 12 lymph nodes is not achieved (16). This may be due to differences in the extent of surgical lymph node removal, the thoroughness of the pathologist in dissecting the cancer specimen, and/or the actual number of regional lymph nodes that is found to be related to patient demographics, tumor location and biology (9,17). In rectal cancer, the increasing use of neoadjuvant therapy represents another important factor since under combined chemo - and radiotherapy regional lymph nodes undergo a process of regression and remain undetectable during routine pathological work-up (18). Despite these limitations lymph node harvest is widely used as a marker of quality control in colorectal cancer treatment, since it is easily measured and comparably between centers (19).
Fat clearing methods, methylene blue-assisted lymph node dissection, as well as acetone elution with subsequent compression of adipose tissue (“acetone compression”) have been introduced as techniques to increase the lymph node harvest, resulting in dramatically increased lymph node counts (21,22). However, according to recent data, the application of these techniques may not be associated with increased detection of positive nodes (18,22).
Lymph node ratio
The presence of positive lymph nodes defines stage III disease. However, due to the recognized variability of lymph nodes across individuals and inadequacy of lymph node harvest in a considerable amount of patients it may be more appropriate to investigate the percentage of positive nodes rather than just the absolute number (8,9). In this regard, the lymph node ratio, which is defined as the number of positive lymph nodes divided by the total number of retrieved nodes, has gained increasing attraction in recent years (23-25).
The lymph node ratio was identified as an independent predictor of disease-free survival, overall survival, and cancer-specific survival, irrespective of the number of nodes examined, and it remained independent even after neoadjuvant therapy, despite reduction of the absolute number of retrieved nodes (26,27). Although data suggest that a higher lymph node ratio equates to worsening survival, debate remains concerning the ideal cut-off value for accurate outcome prediction. Pathologists need to be aware of the fact that the numerical values of the lymph node ratio will be disproportionally high if the total lymph node harvest is under-representative. Therefore, although the lymph node ratio was introduced to overcome dependence on absolute lymph node numbers, the calculation of an accurate lymph node ratio still relies on an adequate total lymph node harvest (9).
Technical aspects
The appropriate number of sections that should be made during the histological lymph node evaluation has not yet been determined, and recommendations in this regard are currently lacking in international guidelines (12-14). Increasing the number of sections and/or levels may, however, increase the detection of metastatic deposits, thereby increasing the diagnostic accuracy.
Indirect evidence can be derived from studies evaluating the value of sentinel node biopsy procedures in patients with CRC. From these studies we know that the extent of pathological work-up significantly affects the clinical performance of the concept, since several studies showed that lymph nodes initially classified as negative by routine H&E staining were in fact positive after extensive evaluation (28-30). According to a recent meta-analysis, adding step sectioning and immunohistochemistry (using antibodies directed against pankeratin) to the work-up of sentinel nodes results in a mean upstaging of 18.9% (range 0 - 50%). True upstaging defined as micrometastases (pN1mi+) rather than isolated tumor cells (pN0(i+)) is 7.7% (31).
B. Histological Subtypes
Approximately 90% of CRCs are adenocarcinomas. For the residual cases, the World Health Organization (WHO) classification of carcinomas of the colon and rectum lists several distinct histological variants or subtypes which often carry distinct prognostic significance (Table 1) (32).
Mucinous adenocarcinoma
Mucinous adenocarcinomas constitute 4 - 19 % of CRC worldwide (33). The designation is used when more than 50 % of the lesion is composed of pools of extracellular mucin that contain malignant epithelium as acinar structures, layer of tumor cells, or individual tumor cells including signet ring cells (Fig. 1A). Carcinomas with extracellular mucin covering less than 50 % of the tumor are categorized as having a mucinous component (32).
Fig. 1. Histological variants of colorectal cancer. 
A, mucinous adenocarcinoma characterized by abundant extracellular mucin production. B, signet ring cell carcinoma with prominent intracytoplasmic mucin deposition, causing displacement and molding of tumor cell nuclei. C, medullary carcinoma characterized by sheets of malignant cells with vesicular nuclei with prominent nucleoli and prominent lymphocytic infiltration of the tumor tissue. D, micropapillary adenocarcinoma with characteristic small papillary and trabecular tumor cell clusters within stromal spaces mimicking vascular channels. The prognostic value of mucinous differentiation in CRC remains controversial. According to a recent meta-analysis, mucinous differentiation leads to a 2 - 8 % increased hazard of death, which persists after correction for stage (34). It is of interest that the prognostic value may vary depending on tumor location. Thus, in rectal cancer mucinous histology has been shown to indicate poor outcome, whereas it was a protective survival indicator in right-sided colon cancer (35). This may be due to the fact, that right-sided mucinous adenocarcinomas are often microsatellite instable (36). Hence, grading of mucinous cancers is, if at all done mainly by molecular analysis: mucinous cancers that are high-level microsatellite instable (MSI-H) are considered low-grade, while those that are microsatellite stable (MSS) or show low-level MSI (MSI-L) are considered high-grade (32,34). The outcome of adenocarcinomas with a mucinous component does not seem to differ from that of classical adenocarcinomas (33).
Signet ring cell carcinoma
About 1 % of CRC are signet ring cell carcinomas (37,38).This subtype is defined by the presence of more than 50 % of tumor cells with prominent intracytoplasmic mucin, typically with displacement and molding of the nucleus (Fig. 1B). Carcinomas with signet ring cells covering less than 50 % of the tumor are categorized as adenocarcinoma with a signet ring cell component (32).
Signet ring cell carcinomas mainly occur as right-sided tumors. Patients present at higher tumor stage, and the prognosis is usually poor (37,38). Already a minor signet ring cell carcinoma may cause adverse outcome. Sung et al. (39) demonstrated that mucinous adenocarcinomas with signet ring cells have a significantly poorer disease-specific survival compared to tumors without signet ring cells, indicating the need to report a signet ring cell component, i.e. the percentage of signet ring cells when present.
Finally, tumors with signet ring cell differentiation (and to a lesser extent also cancers with mucinous differentiation) have a propensity to cavitary metastatic spread with metastases to the peritoneum and ovaries. This is in contrast to conventional colorectal adenocarcinomas that metastasize predominantly to liver and lungs (40).
Medullary carcinoma
This rare mainly right-sided subtype is characterized by sheets of malignant cells with vesicular nuclei with prominent nucleoli, and abundant eosinophilic cytoplasm exhibiting prominent infiltration by intraepithelial lymphocytes (Fig. 1C) (32). Medullary carcinomas are almost invariably MSI-H and usually have a favorable prognosis: Follow-up data showed 1 - and 2 - year survival rates of 92.7 % and 73.8 %, respectively (41,42).
Micropapillary adenocarcinoma
This subtype is defined by small papillary tumor cell clusters within stromal spaces mimicking vascular channels (Fig. 1D). The pattern is mainly seen as a minor component of conventional adenocarcinoma (32). Upon immunohistochemistry, micropapillary adenocarcinoma shows a characteristic “inside-out” staining-pattern, i.e. reversed polarity for MUC1 (EMA) and villin (43).
Adenocarcinomas with a micropapillary component bear a high malignant potential with higher frequency of infiltrative pattern, lymphovascular and perineural invasion, as well as increased likelihood of positive lymph nodes compared to conventional adenocarcinomas (43,44). It is of note that already a small micropapillary component, such as 5 - 10% of the tumor area may significantly increase the risk of local (40 - 74 %) and distant (8 - 16 %) metastatic spread (43).
C. Prognostic variables
CRC is known to show different patterns of growth and invasion, which have been linked with prognosis in several studies. Within the last decade, the anti-tumor host response, characterized by intra - and/or peritumoral inflammation has similarly attracted attention and has been characterized as additional prognostic feature (Table 1).
1. Established and novel histological markers for the prognostication of patients with colorectal cancer. 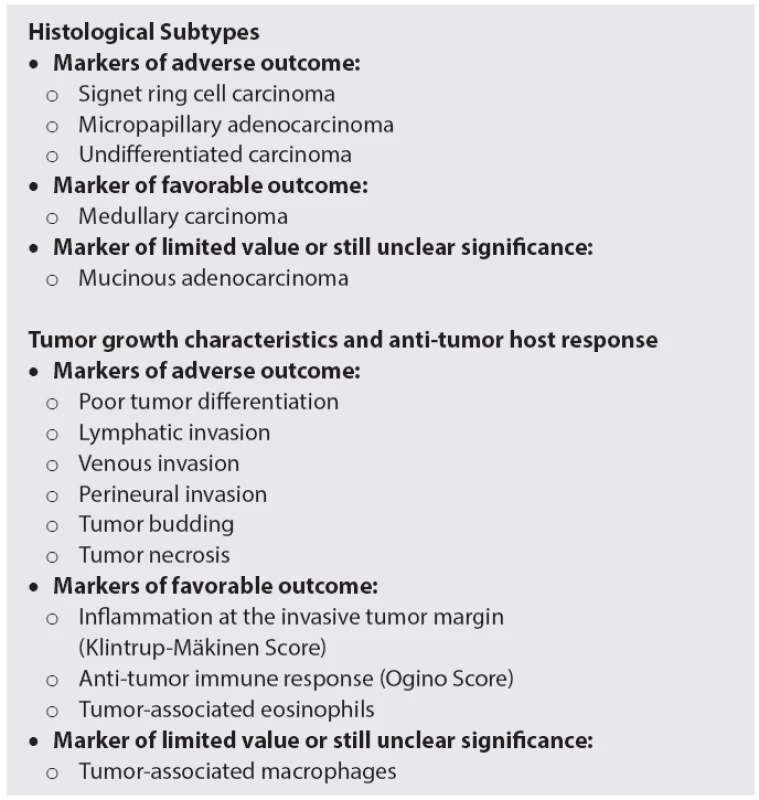
Lymph and blood vessel invasion
The invasion of tumor cells into lymph or blood vessels plays a crucial role in the metastatic process. Lymphatic invasion is diagnosed, when tumor cells are present in vessels with an unequivocal endothelial lining, yet lacking a thick (muscular) wall (Fig. 2A). Blood vessel invasion refers to the involvement of veins and is characterized histologically by the presence of tumor cells in vessels with a thick (muscular) wall or in vessels containing red blood cells (Fig. 2B) (45). In some studies, both types of vascular invasion have been lumped together and referred to as “lymphovascular invasion” or simply as “vascular invasion”, which is problematic, since the term lymphovascular invasion in other studies refers only to lymphatic invasion and the term vascular invasion only to venous invasion (45).
Fig. 2. Histological prognostic variables in colorectal cancer. 
A, lymphatic invasion is diagnosed when tumor cells are present in vessels with an unequivocal endothelial lining, yet lacking a thick (muscular) wall. B, venous invasion is characterized histologically by the presence of tumor cells in vessels with a thick (muscular) wall or in vessels containing red blood cells. C, perineural invasion is defined by tumor cell invasion of nerves and/or spread along nerve sheaths. D, tumor budding is characterized by the presence of isolated single cells or small clusters of cells composed of less than five cells scattered in the stroma at the invasive tumor margin. E, coagulative tumor necrosis reflects chronic ischemic injury due to rapid tumor growth. Assessing the anti-tumor inflammatory response is a new promising prognostic tool which commonly indicates favorable outcome: F, marked overall inflammation at the tumor margin, characterized by a mixed inflammatory infiltrate with destruction of cancer cell islets; G, anti-tumor immune response, characterized by dense peritumoral lymphocytic infiltration; H, eosinophilic infiltration of the tumor area (“tumor-associated tissue eosinophilia”). Despite these limitations, both lymph and blood vessel invasion have emerged as major prognostic variables in patients with CRC, and, consequently, both the Association of Directors of Anatomic and Surgical Pathology (46) and the College of American Pathologists (12) emphasize the recording of vascular invasion during the routine pathological work-up of cancer specimens.
We recently evaluated the prognostic significance of lymphatic and venous invasion in a large cohort of patients with CRC (47). The prognostic value of venous invasion was superior to lymphatic invasion, the prognostic impact of extramural invasion superior to intramural invasion, respectively. In multivariate analysis, the prognostic impact of venous invasion was comparable to that of T classification, stronger than that of tumor grade and lymphatic invasion, yet inferior to that of lymph node metastasis. In AJCC/UICC stage II tumors, venous invasion proved to be an independent predictor of both disease progression and cancer-related death.
Venous invasion is widely believed to be an underreported finding with significant variability in its reported incidence (48). The Royal College of Pathologists recommends that pathology departments audit their reports at regular intervals to ensure that their overall results are not significantly different from what might be expected (10). In particular, these guidelines state that the frequency of extramural venous invasion should be at least 25%.
In our study the prognostic value of angioinvasion assessed by review pathology was superior to routine pathology (47). Although the rate of vascular invasion, especially lymphatic invasion, was comparable, false positive reports, mostly resulting from overestimation of retraction artifacts, and false negative reports were noted in comparable frequencies. These observations illustrate the need for high quality pathology reporting and quality control, but they also clearly demonstrate that quality standards merely relying on the fulfillment of target values, e.g. the frequency of vascular invasion can be misleading (47). Ancillary techniques, such as Elastica van Gieson and immunostaining for CD31 and D2-40 for the detection of endothelial cells as well as alpha smooth muscle actin for the detection of vessel walls may increase the diagnostic accuracy in difficult cases (49,50).
Perineural invasion
Perineural invasion is defined by tumor cell invasion of nervous structures, as illustrated by neoplastic invasion of nerves and/or spread along nerve sheaths (Fig. 2C). Its presence constitutes a process for neoplastic invasion and cancer spread, independent of blood and lymphatic vessels (51).
Perineural invasion is more common in tumors with an aggressive phenotype, as illustrated by significant associations with lymph and blood vessel invasion, tumor growth pattern and budding, as well as poor tumor differentiation (52). The prognostic significance of perineural invasion has been investigated by several groups, proving this marker to be an independent predictor of adverse outcome, with respect to both disease progression and survival (52,53). In rectal cancer, node-negative tumors with perineural invasion were shown to have a 2.5-fold higher 5-year local recurrence rate than tumors without perineural invasion (22.7 % versus 7.9 %) (54). Likewise, in rectal cancers with clear resection margin (R0 resection), perineural invasion proved to be the only independent predictor of local tumor progression (adjusted hazard ratio = 5.62, 95 % confidence interval = 1.97 - 15.99, p = 0.001) (52).
Tumor budding
Histological growth characteristics at the invasive front reflect tumor aggressiveness and have thus been considered as prognostic markers in CRC (55). Tumor budding has been defined as the presence of isolated single cells or small clusters of cells composed of less than five cells, scattered in the stroma at the invasive tumor margin (Fig. 2D) (56,57). Biologically, tumor budding is closely related to the process of epithelial-mesenchymal transition (EMT). During EMT, epithelial cells lose intercellular and cell-matrix contacts mediated by E-cadherin, and the tumor cell complexes dissociate, thereby promoting invasion and ultimately metastatic cancer spread (58).
Several studies proved tumor budding to be a major prognostic marker, independently predicting high risk of recurrence and poor survival (56,57,59,60). In patients with AJCC/UICC stage II disease the extent of tumor budding could be used to select patients at high risk for recurrence for adjuvant therapy (61-64). Currently budding appears to be the most interesting prognostic variable in CRC. However, it has often been criticized because of non-standardized criteria for evaluation and unclear reproducibility of the numerous methods for tumor budding measurement (65).
To overcome these problems, Karamitopoulou et al. (66) recently presented a 10-high-power-fields method for the assessment of tumor budding. According to this proposal, high-grade budding can be defined as an average of ≥ 10 buds across 10-high-power-fields and was significantly associated with higher tumor grade, vascular invasion, infiltrating tumor border configuration, higher TNM stage, and poor outcome. Multivariate analysis confirmed the independent prognostic effect of tumor budding when adjusted for TNM stage and adjuvant therapy (66). In a second publication, the same group of authors applied this system selectively to AJCC/UICC stage II tumors. In this study only tumor budding had significant prognostic effects on patient survival, with high prognostic accuracy over 10-year follow-up (67).
In 2012, Ueno et al. (68) proposed a new grading system for CRC based upon the quantification of “poorly differentiated clusters”. These are defined as clusters of ≥ 5 cancer cells infiltrating the stroma at the invasive tumor margin and lacking gland-like structures. These poorly differentiated clusters affected outcome, independent of T and N classification. Morphologically, poorly differentiated clusters are closely related to tumor budding (the nests are slightly larger) and also to the micropapillary variant of CRC (compare above). Future studies are needed to prove the originality of poorly differentiated clusters, as histological feature and as prognostic variable.
Tumor necrosis
Tumor necrosis has been related to rapid tumor growth and consequent chronic ischemic injury (Fig. 2E). Increased cellular hypoxia correlates with increased metastatic potential and worse prognosis as well as resistance to radiotherapy and chemotherapy in several types of cancer (69-72).
Studies assessing the prognostic value of tumor necrosis in CRC are rare. In the study by Pollheimer et al. (73), the extent of necrosis was significantly associated with T classification, N classification, TNM stage, poor tumor differentiation, large tumor size, and angioinvasion. Tumor necrosis was identified as independent predictor of progression free and cancer specific survival in multivariate analysis. These findings were validated by other groups. (74-76). Richards et al. (74,75) noted an association between tumor necrosis and the host systemic as well as the local inflammatory response (intra/peritumoral inflammatory infiltrate). The authors provided supportive evidence for the hypothesis that tumor necrosis is associated with elevated circulating IL-6 and VEGF concentrations, thereby modulating both local and systemic inflammatory responses as well as angiogenesis which, in turn, may promote tumor progression and metastasis (77).
Inflammatory response
The anti-tumor inflammatory response is a distinct histological feature and a new promising prognostic factor in CRC pathology. In contrast to the histological features presented above, which all reflect tumor aggressiveness, the inflammatory cells act as “defenders”, their presence generally indicating favorable outcome. Several aspects need to be considered: the overall inflammatory response at the tumor margin, the anti-tumor immune response, characterized by lymphocytic intra-/peritumoral infiltration and the infiltration by eosinophils and macrophages.
The Klintrup-Mäkinen criteria (78) are widely used to score the intensity of inflammation at the invasive tumor margin (Fig. 2F). This is done using a four-degree scale which takes into consideration the numbers of neutrophilic and eosinophilic granulocytes, lymphoid cells and macrophages and their relation to the invading tumor (with or without destruction of cancer cell islets). The favorable effect of high-grade inflammation was first shown by Klintrup et al. (78), and later confirmed by others (74,79).
Ogino et al. (80) developed a scoring system for the anti-tumor immune response, based upon the evaluation of four distinct features: tumor-infiltrating lymphocytes, intra-/peritumoral lymphocytic stroma infiltration, and Crohn’s-like lymphoid reaction (Fig. 2G). The lymphocytic anti-tumor immune response has been associated with MSI-H status (81) and favorable prognosis in several studies (82,83). Recent data suggest that specific immunotyping of the infiltrate (“immunoscore”) may be of additional prognostic value (84), in particular if assessed together with tumor budding (85).
The prognostic value of tumor-associated eosinophils and macrophages is less well established. Eosinophilis are easy-assessable in routine pathology and therefore of major interest. Increased numbers have been favorably associated with disease recurrence and survival in several studies (Fig. 2H) (82,86,87). A high number of CD68-positive macrophages have been identified as favorable histological feature (88,89).
D. Circumferential margin
Total mesorectal excision (TME) is considered the standard of care for rectal cancer treatment. Failure to remove the entire mesorectum may explain local and distant recurrences. Several studies suggest that the quality of the mesorectum after TME surgery (as determined by pathological evaluation) may have influence on prognosis. A muscularis propria resection plane (“incomplete” excision) was found to increase the risk of local recurrence (hazard ratio (HR) 2.72; 95% confidence interval (CI) 1.36 to 5.44) and overall recurrence (HR 2.00; 95% CI 1.17 to 3.42) compared to an (intra)mesorectal plane (“complete” or “nearly complete” excision). Hence, the plane of surgery is an important prognostic factor, and the documentation by pathologists is essential for the improvement of TME quality and patient outcome (90).
There is strong relationship between the plane of surgery and involvement of the circumferential resection margin. Since the initial description of its clinical importance in 1986, the involvement of this margin (also called lateral or radial resection margin) has been associated with poor prognosis. Nagtegaal and Quirke demonstrated that, after neoadjuvant therapy (both radiotherapy and radiochemotherapy), the predictive value of the circumferential resection margin for local recurrence is significantly higher than when no preoperative therapy has been applied (HR 6.3 vs. 2.0, respectively). Furthermore, involvement of the circumferential resection margin is a powerful predictor of both development of distant metastases (HR 2.8; 95% CI 1.9 to 4.3) and survival (HR 1.7; 95% CI 1.3 to 2.3) (91).
Very recently, the EURECCA consensus conference on multidisciplinary management of colorectal cancer stressed the importance of the circumferential resection margin (92). Minimal requirements for margin assessment in rectal cancer include the following (93): Reporting margins of excisions is mandatory in all pathology reports, and the R classification should only be reported in conjunction with clinical information (e.g. R1 resection = positive microscopic margin without gross residual disease); the distance to the circumferential resection margin should be recorded in cross-sectional slices, and 1 mm or less is considered involved; not only the primary tumor is relevant, but any tumor involvement, including discontinuous spread (“tumor satellites”), positive nodes as well as lymphatic, venous, or perineural invasion.
EARLY LESIONS
Early or superficial colorectal cancer is defined as invasive adenocarcinoma invading into, but not beyond the submucosa. While the principles of pathological reporting are the same as in major resections, a number of features require special attention in local excision of early cancers with curative intent because they are used to determine the necessity for radical surgery (10). In addition to the assessment of completeness of excision, these include the recording of parameters that predict the presence of lymph node metastasis, namely the depth of invasion into the submucosa, tumor grade, and the presence of additional risk factors, such as angioinvasion and tumor budding. Ultimately, the pathology report should sum up these features and designate early cancer lesions as low-risk or high-risk regarding regional lymph node spread (based upon the combination of these factors).
A. Specimen handling
Specimen handling is an important issue, as poor handling can impair diagnostic accuracy. The handling starts with the local excision of the tumor and ends with the histopathological diagnosis and report. The European guidelines for quality assurance in colorectal cancer screening and diagnosis stress the need for a close relationship between clinicians and histopathologists (65).
Polyps must be sliced and totally embedded. While smaller polyps may be bisected through the stalk (or resection margin), larger polyps should be trimmed to leave a central section containing the intact stalk (or resection margin). The resection margin deserves special attention: It should be either dissected tangentially (into an extra cassette) or sliced in a way that allows complete assessment. For mucosal excisions, performed endoscopically or, in the case of early rectal tumors, under direct vision it is helpful to pin out specimens on a cork board or another suitable type of material, with mucosal surface upwards. The needles should be inserted through the periphery of the specimen, and should not be placed directly through a lesion. The specimens should be transversely sectioned into 3 mm slices and submitted for histology in sequentially labeled cassettes, so that involvement of the deep and lateral surgical margins can be evaluated. Inking margins is recommended. In general, three or more levels should be cut through each block (10,65).
B. Depth of invasion
The depth of invasion into the submucosal layer can be assessed using the Haggitt (for pedunculated lesions) or Kikuchi (for non-polypoid lesions) classification systems or by direct measurement (65).
The Haggitt classification system (94) is defined as follows: level 1, carcinoma invading into the submucosa, limited to the head of the polyp; level 2, carcinoma invading to the level of the neck (the junction of the head and stalk) of the polyp; level 3, carcinoma invading any part of the stalk; level 4, carcinoma invading into the submucosa of the bowel wall below the level of the stalk but above the muscularis propria.
Kudo (95) developed a classification system for flat and depressed types of early colorectal cancer, classifying the depth of penetration into three segments termed sm1, sm2 or sm3. Kikuchi et al. (96) modified the Kudo classification by dividing the submucosa into three parts: sm1, tumor invasion of the upper third of the submucosa; sm2, tumor invasion of the middle third of the submucosa; sm3, tumor invasion of the lower third of the submucosa. There is a significantly higher risk of nodal metastasis for sm3 (12-25 %) compared with sm1 and sm2 lesions (0-8 %), which has been confirmed in two recent meta-analyses (3,4).
The Kikuchi classification system cannot be accurately applied in the absence of muscularis propria, i.e. for the majority of local excisions. For these specimens direct measurement of the absolute thickness of invasion, from the muscularis mucosae to the leading edge of invasion, is recommended. Different cut-off values have been introduced, and meta-analyses proved invasion >1000 µm and >2000 µm into the submucosa to be significantly related with increased risk of lymph node metastasis (3,4).
C. Lymph and blood vessel invasion
As in advanced lesions the presence of angioinvasion is an important prognostic variable and is significantly associated with an increased risk of regional lymph node spread (97-99). In the seminal paper by Hassan et al. (100) lymphovascular invasion was present in 18 % of malignant polyps. Lymph node metastasis occurred in 35 % of polyps when lymphovascular invasion was present and in 7 % when it was absent. In fact, two recent meta-analyses identified lymphatic invasion as the most powerful predictor of lymph node metastasis in early lesions (3,4). Regarding lymphatic invasion, the sensitivity and specificity of nodal involvement were calculated as 69.5 % and 73.8 %, respectively (4). The prognostic impact of venous invasion is less well established, and in many studies no (independent) prognostic significance was identified. For venous invasion, the sensitivity and specificity of nodal involvement were calculated as 33.9 % and 82.3 %, respectively (4).
D. Tumor budding
Tumor budding has been presented as promising new prognostic factor for advanced lesions. In early CRCs, studies assessing the prognostic value of tumor budding are still comparably rare. In the study by Tateishi et al. (99) budding was observed in one third of cases. When budding was positive 20.6 % of patients had lymph node metastases, compared to 8 % when budding was negative. In the study by Ueno et al. (101) lymph node metastasis was present in 42.1 % of tumors classified as positive for tumor budding. Although tumor budding proved to be a powerful predictor of regional lymph node metastasis in two recent meta-analyses (3,4), it is currently not recommended for routine use in the European Guidelines for Quality Assurance in Colorectal Cancer Screening and Diagnosis (65).
E. Tumor grade
Assessing tumor differentiation, i.e. tumor grade represents the most traditional prognostic tool in cancer pathology. Poorly differentiated carcinomas are identified by predominantly solid tumor growth with less than 50 % gland formation (32). In the absence of good evidence, the European Guidelines for Quality Assurance in Colorectal Cancer Screening and Diagnosis recommend to diagnose early cancers as poorly differentiated when ANY area of the lesion shows poor differentiation (65).
Poor differentiation is an unusual finding in malignant polyps of the colon and rectum, occuring more frequently in sessile compared to pedunculated polyps. In the study by Hassan et al. (100), poor differentiation was associated with a statistically significant increase in positive lymph nodes (23 % vs. 7 %), distant metastasis (10 % vs. 3 %), and cancer related death (15 % vs. 2 %).
F. Resection margin status
The European Guidelines for Quality Assurance in Colorectal Cancer Screening and Diagnosis (65) stress the importance to record whether the deep (basal, vertical) resection margin is involved by invasive tumor (that may be a reason for further surgery) and/or whether the lateral mucosal resection margin is involved by cancer or the preexisting adenoma (in which case a further local excision may be attempted). A positive resection margin is significantly more frequent in sessile compared to pedunculated polyps. In the study by Hassan et al. (100), residual and recurrent disease occurred in 30 % and 17 % of patients with positive resection margin compared to 3 % and 1 % of patients with negative resection margin. Butte et al. (102) reported residual invasive disease in the colon wall in 8 of 50 (16 %) with <1 mm (positive) polypectomy margin and 0 of 44 with ≥1 mm (negative) polypectomy margin. The authors of the European Guidelines recommend that clearance of 1 mm or less should indicate margin involvement (65).
G. Prognostic stratification: low - or high-risk lesion?
In many cases, early CRCs will have more than one risk factor for residual disease and/or lymph node metastasis. By combination of these factors, in particular depth of invasion, angioinvasion, tumor grade, and resection margin status, risk stratification of affected individuals into low-risk and high-risk groups is possible. Ueno et al. (101) reported lymph node metastasis to occur in 0.7 % of tumors with no risk factor, compared to 20.7 % of those with a single risk factor, and 36.4 % of those with multiple risk factors.
A low-risk early colorectal cancer is defined as a completely excised Haggitt level 1-3 or Kikuchi sm1 adenocarcinoma with no evidence of poor differentiation, high grade tumor budding, lymphatic or venous invasion. For these tumors local excision is generally regarded adequate treatment, as the risk of failure is less or equal to the risk of death from surgery. In contrast, surgical resection is indicated for high-risk lesions, which show at least one of the following features: Haggitt level 4 or Kikuchi sm3 invasion, presence of lymphatic (or venous) invasion, poor differentiation, high grade tumor budding, or positive resection margin (Fig. 3) (65,103,104). In the study by Hassan et al. (100), 58 % of lesions were classified as low-risk, the remaining 42 % as high-risk. In low-risk lesions, reported frequencies of residual disease, recurrent disease, and lymph node metastasis were 1.2 %, 0 %, and 5.1 %. In high-risk lesions, residual disease, recurrent disease, and lymph node metastasis occurred in 21.4 %, 9.2 %, and 11.2 % of lesions, respectively.
Fig. 3. Risk of regional lymph node metastasis in patients with early colorectal cancer: The subdivision into low- and high-risk groups is based upon the combination of different histological parameters. 
CONCLUSION
In advanced CRC, accurate staging including thorough lymph node assessment is crucial for patient counselling and decision making since the indication for adjuvant therapy is mainly guided by the presence of regional lymph node metastasis. Current guidelines recommend a minimum of 12 nodes harvested as the standard of care. In node-negative patients a variety of histological features may be used for adjusted risk assessment, including histological subtyping, lymphatic and venous invasion, tumor budding and necrosis as well as the anti-tumor host inflammatory response which has been identified as favorable feature. In rectal cancer, involvement of the circumferential resection margin and the plane of surgery are important prognostic factors. Standard risk assessment of early CRCs following local excision is based upon the evaluation of different histological risk parameters, such as depth of invasion, angioinvasion, budding, tumor grade, and resection margin status. The combination of these parameters allows the stratification of affected individuals into low-risk and high-risk categories.
Correspondence address:
Cord Langner, MD
Institute of Pathology
Medical University of Graz
Auenbruggerplatz 25, A-8036 Graz, Austria
tel.: +43 (0)316 385 13665, fax +43 (0)316 385 13432
e-mail: cord.langner@medunigraz.at
Sources
1. Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin 2014; 64(1): 9-29.
2. Compton CC. Optimal pathologic staging: defining stage II disease. Clin Cancer Re. 2007; 13(22 Pt 2): 6862-6870.
3. Beaton C, Twine CP, Williams GL, Radcliffe AG. Systematic review and meta-analysis of histopathological factors influencing the risk of lymph node metastasis in early colorectal cancer. Colorectal Dis 2013; 15(7): 788-797.
4. Bosch SL, Teerenstra S, de Wilt JH, Cunningham C, Nagtegaal ID. Predicting lymph node metastasis in pT1 colorectal cancer: a systematic review of risk factors providing rationale for therapy decisions. Endoscopy 2013; 45(10): 827-834.
5. Washington MK. Colorectal carcinoma: selected issues in pathologic examination and staging and determination of prognostic factors. Arch Pathol Lab Med 2008; 132(10): 1600-1607.
6. Benson AB 3rd, Bekaii-Saab T, Chan E, Chen YJ, Choti MA, Cooper HS, Engstrom PF, et al. Localized colon cancer, version 3.2013: featured updates to the NCCN Guidelines. J Natl Compr Canc Net. 2013; 11(5): 519-528.
7. Nagtegaal ID, Tot T, Jayne DG, McShane P, Nihlberg A, Marshall HC, et al. Lymph nodes, tumor deposits, and TNM: are we getting better? J Clin Oncol 2011; 29(18): 2487-2492.
8. Resch A, Langner C. Lymph node staging in colorectal cancer: old controversies and recent advances. World J Gastroenterol 2013; 19(46): 8515-8526.
9. McDonald JR, Renehan AG, O’Dwyer ST, Haboubi NY. Lymph node harvest in colon and rectal cancer: Current considerations. World J Gastrointest Surg 2012; 4(1): 9-19.
10. Williams GT, Quirke P, Shepherd NA. The royal college of pathologists. Standards and datasets for reporting cancers. Dataset for colorectal cancer, 2nd edition, 2007 (http://www.rcpath.org/publications-media/publications/datasets/colorectal-cancer.htm).
11. Jass JR, O’Brien J, Riddell RH, Snover DC. Association of Directors of Anatomic and Surgical Pathology. Recommendations for the reporting of surgically resected specimens of colorectal carcinoma: Association of Directors of Anatomic and Surgical Pathology. Am J Clin Pathol 2008; 129(1): 13-23.
12. Washington MK, Berlin J, Branton P, Burgart LJ, Carter DK, Fitzgibbons PL, et al. Members of the Cancer Committee, College of American Pathologists. Protocol for the examination of specimens from patients with primary carcinoma of the colon and rectum. Arch Pathol Lab Med 2009; 133(10): 1539-1551.
13. Benson AB 3rd, Arnoletti JP, Bekaii-Saab T, Chan E, Chen YJ, Choti MA, et al. Colon Cancer. J Natl Compr Canc Net. 2011; 9(11): 1238-1290.
14. Benson AB 3rd, Bekaii-Saab T, Chan E, Chen YJ, Choti MA, Cooper HS, Rectal cancer. J Natl Compr Canc Netw 2012; 10(12): 1528-1564.
15. Fielding LP, Arsenault PA, Chapuis PH, Dent O, Gathright B, Hardcastle JD, et al. Clinicopathological staging for colorectal cancer: an International Documentation System (IDS) and an International Comprehensive Anatomical Terminology (ICAT). J Gastroenterol Hepatol 1991; 6(4): 325-344.
16. Baxter NN, Virnig DJ, Rothenberger DA, Morris AM, Jessurun J, Virnig BA. Lymph node evaluation in colorectal cancer patients: a population-based study. J Natl Cancer Inst 2005; 97(3): 219-225.
17. Bilimoria KY, Palis B, Stewart AK, Bentrem DJ, Freel AC, Sigurdson ER, et al. Impact of tumor location on nodal evaluation for colon cancer. Dis Colon Rectum 2008; 51(2): 154-161.
18. Gehoff A, Basten O, Sprenger T, Conradi LC, Bismarck C, Bandorski D, et al. Optimal lymph node harvest in rectal cancer (UICC stages II and III) after preoperative 5-FU-based radiochemotherapy. Acetone compression is a new and highly efficient method. Am J Surg Pathol 2012; 36(2): 202-213.
19. Baxter NN. Is lymph node count an ideal quality indicator for cancer care? J Surg Oncol 2009; 99(4): 265-268.
20. Märkl B, Rössle J, Arnholdt HM, et al. The clinical significance of lymph node size in colon cancer. Mod Pathol 2012; 25(10): 1413-1422.
21. Märkl B, Kerwel TG, Wagner T, Anthuber M, Arnholdt HM. Methylene blue injection into the rectal artery as a simple method to improve lymph node harvest in rectal cancer. Mod Pathol 2007; 20(7): 797-801.
22. Märkl B, Schaller T, Krammer I, Cacchi C, Arnholdt HM, Schenkirsch G, et al. Methylene blue-assisted lymph node dissection technique is not associated with an increased detection of lymph node metastases in colorectal cancer. Mod Pathol 2013; 26(9): 1246-1254.
23. Berger AC, Sigurdson ER, LeVoyer T, Hanlon A, Mayer RJ, Macdonald JS, et al. Colon cancer survival is associated with decreasing ratio of metastatic to examined lymph nodes. J Clin Oncol 2005; 23(34): 8706-8712.
24. Sjo OH, Merok MA, Svindland A, Nesbakken A. Prognostic impact of lymph node harvest and lymph node ratio in patients with colon cancer. Dis Colon Rectum 2012; 55(3): 307-315.
25. Medani M, Kelly N, Samaha G, Duff G, Healy V, Mulcahy E, et al. An appraisal of lymph node ratio in colon and rectal cancer: not one size fits all. Int J Colorectal Dis 2013; 28(10): 1377-1384.
26. Chen SL, Steele SR, Eberhardt J, Zhu K, Bilchik A, Stojadinovic A. Lymph node ratio as a quality and prognostic indicator in stage III colon cancer. Ann Surg 2011; 253(1): 82-87.
27. Kang J, Hur H, Min BS, Lee KY, Kim NK. Prognostic impact of the lymph node ratio in rectal cancer patients who underwent preoperative chemoradiation. J Surg Oncol 2011; 104(1): 53-58.
28. Bembenek AE, Rosenberg R, Wagler E, Gretschel S, Sendler A, Siewert JR, et al. Sentinel lymph node biopsy in colon cancer: a prospective multicenter trial. Ann Surg 2007; 245(6): 858-863.
29. van der Zaag ES, Kooij N, van de Vijver MJ, Bemelman WA, Peters HM, Buskens CJ. Diagnosing occult tumour cells and their predictive value in sentinel nodes of histologically negative patients with colorectal cancer. Eur J Surg Oncol 2010; 36(4): 350-357.
30. Märkl B, Arnholdt HM, Jähnig H, Spatz H, Anthuber M, Oruzio DV, et al. A new concept for the role of ex vivo sentinel lymph nodes in node-negative colorectal cancer. Ann Surg Oncol 2010; 17(10): 2647-2655.
31. van der Zaag ES, Bouma WH, Tanis PJ, Ubbink DT, Bemelman WA, Buskens CJ. Systematic review of sentinel lymph node mapping procedure in colorectal cancer. Ann Surg Oncol 2012; 19(11): 3449-3459.
32. Hamilton SR, Bosman FT, Boffetta P, Ilyas M, Morreau, Nakamura SI, et al. Carcinoma of the colon and rectum. In: Bosman FT, Carneiro F, Hruban RH, Theise ND, eds. WHO classification of tumours of the digestive system. 4th edition. Lyon France: IARC Press; 2010 : 134–146.
33. Langner C, Harbaum L, Pollheimer MJ, Kornprat P, Lindtner RA, Schlemmer A, et al. Mucinous differentiation in colorectal cancer--indicator of poor prognosis? Histopathology 2012; 60(7): 1060-1072.
34. Verhulst J, Ferdinande L, Demetter P, Ceelen W. Mucinous subtype as prognostic factor in colorectal cancer: a systematic review and meta-analysis. J Clin Pathol 2012; 65(5): 381-388.
35. Gao P, Song YX, Xu YY, Sun Z, Sun JX, Xu HM, et al. Does the prognosis of colorectal mucinous carcinoma depend upon the primary tumour site? Results from two independent databases. Histopathology 2013; 63(5): 603-615.
36. Minoo P, Zlobec I, Peterson M, Terracciano L, Lugli A. Characterization of rectal, proximal and distal colon cancers based on clinicopathological, molecular and protein profiles. Int J Oncol 2010; 37(3): 707-718.
37. Nitsche U, Zimmermann A, Späth C, Müller T, Maak M, Schuster T, et al. Mucinous and signet-ring cell colorectal cancers differ from classical adenocarcinomas in tumor biology and prognosis. Ann Surg 2013; 258(5): 775-782. Discussion pp. 782-783.
38. Thota R, Fang X, Subbiah S. Clinicopathological features and survival outcomes of primary signet ring cell and mucinous adenocarcinoma of colon: retrospective analysis of VACCR database. J Gastrointest Oncol 2014; 5(1): 18-24.
39. Sung CO, Seo JW, Kim KM, Do IG, Kim SW, Park CK. Clinical significance of signet-ring cells in colorectal mucinous adenocarcinoma. Mod Pathol 2008; 21(12): 1533-1541.
40. Pande R, Sunga A, Levea C, Wilding GE, Bshara W, Reid M, et al. Significance of signet-ring cells in patients with colorectal cancer. Dis Colon Rectum 2008; 51(1): 50-55.
41. Thirunavukarasu P, Sathaiah M, Singla S, Sukumar S, Karunamurthy A, Pragatheeshwar KD, et al. Medullary carcinoma of the large intestine: a population based analysis. Int J Oncol 2010; 37(4): 901-907.
42. Chetty R. Gastrointestinal cancers accompanied by a dense lymphoid component: an overview with special reference to gastric and colonic medullary and lymphoepithelioma-like carcinomas. J Clin Pathol 2012; 65(12): 1062-1065.
43. Verdú M, Román R, Calvo M, Rodón N, García B, González M, et al. Clinicopathological and molecular characterization of colorectal micropapillary carcinoma. Mod Pathol 2011; 24(5): 729-738.
44. Lee HJ, Eom DW, Kang GH, Han SH, Cheon GJ, Oh HS, et al. Colorectal micropapillary carcinomas are associated with poor prognosis and enriched in markers of stem cells. Mod Pathol 2013; 26(8): 1123-1131.
45. Betge J, Langner C. Vascular invasion, perineural invasion, and tumour budding: predictors of outcome in colorectal cancer. Acta Gastroenterol Belg 2011; 74(4): 516-529.
46. Jass JR, O’Brien J, Riddell RH, Snover DC. Association of Directors of Anatomic and Surgical Pathology. Recommendations for the reporting of surgically resected specimens of colorectal carcinoma: Association of Directors of Anatomic and Surgical Pathology. Am J Clin Pathol 2008; 129(1): 13-23.
47. Betge J, Pollheimer MJ, Lindtner RA, Kornprat P, Schlemmer A, Rehak P, et al. Intramural and extramural vascular invasion in colorectal cancer: prognostic significance and quality of pathology reporting. Cancer 2012; 118(3): 628-638.
48. Messenger DE, Driman DK, Kirsch R. Developments in the assessment of venous invasion in colorectal cancer: implications for future practice and patient outcome. Hum Pathol 2012; 43(7): 965-973.
49. Harris EI, Lewin DN, Wang HL, Lauwers GY, Srivastava A, Shyr Y et al. Lymphovascular invasion in colorectal cancer: an interobserver variability study. Am J Surg Pathol 2008; 32(12): 1816-1821.
50. van Wyk HC, Roxburgh CS, Horgan PG, Foulis AF3, McMillan DC2. The detection and role of lymphatic and blood vessel invasion in predicting survival in patients with node negative operable primary colorectal cancer. Crit Rev Oncol Hematol 2014; 90(1): 77-90.
51. Liebig C, Ayala G, Wilks JA, Berger DH, Albo D. Perineural invasion in cancer: a review of the literature. Cancer 2009; 115(15): 3379-3391.
52. Poeschl EM, Pollheimer MJ, Kornprat P, Lindtner RA, Schlemmer A, Rehak P, et al. Perineural invasion: correlation with aggressive phenotype and independent prognostic variable in both colon and rectum cancer. J Clin Oncol 2010; 28(21): e358-360.
53. Liebig C, Ayala G, Wilks J, Verstovsek G, Liu H, Agarwal N, et al. Perineural invasion is an independent predictor of outcome in colorectal cancer. J Clin Oncol 2009; 27(31): 5131-5137.
54. Peng J, Sheng W, Huang D, Venook AP, Xu Y, Guan Z, et al. Perineural invasion in pT3N0 rectal cancer: the incidence and its prognostic effect. Cancer 2011; 117(7): 1415-1421.
55. Zlobec I, Lugli A. Invasive front of colorectal cancer. dynamic interface of pro-/anti-tumor factors. World J Gastroenterol 2009 15(47): 5898-5906.
56. Hase K, Shatney C, Johnson D, Trollope M, Vierra M. Prognostic value of tumor “budding” in patients with colorectal cancer. Dis Colon Rectum 1993; 36(7): 627-635.
57. Ueno H, Murphy J, Jass JR, Mochizuki H, Talbot IC. Tumour ‘budding’ as an index to estimate the potential of aggressiveness in rectal cancer. Histopathology 2002; 40(2): 127-132.
58. Lugli A, Karamitopoulou E, Zlobec I. Tumour budding: a promising parameter in colorectal cancer. Br J Cancer 2012; 106(11): 1713-1717.
59. Kanazawa H, Mitomi H, Nishiyama Y, Kishimoto I, Fukui N, Nakamura T, et al. Tumour budding at invasive margins and outcome in colorectal cancer. Colorectal Dis 2008;10(1):41-47.
60. Park KJ, Choi HJ, Roh MS, Kwon HC, Kim C. Intensity of tumor budding and its prognostic implications in invasive colon carcinoma. Dis Colon Rectum 2005; 48(8): 1597-1602.
61. Okuyama T, Nakamura T, Yamaguchi M. Budding is useful to select high-risk patients in stage II well-differentiated or moderately differentiated colon adenocarcinoma. Dis Colon Rectum 2003; 46(10): 1400-1406.
62. Nakamura T, Mitomi H, Kanazawa H, Ohkura Y, Watanabe M. Tumor budding as an index to identify high-risk patients with stage II colon cancer. Dis Colon Rectum 2008; 51(5): 568–572.
63. Betge J, Kornprat P, Pollheimer MJ, Lindtner RA, Schlemmer A, Rehak P, et al. Tumor budding is an independent predictor of outcome in AJCC/UICC stage II colorectal cancer. Ann Surg Oncol 2012; 19(12): 3706-3712.
64. Mitrovic B, Schaeffer DF, Riddell RH, Kirsch R. Tumor budding in colorectal carcinoma: time to take notice. Mod Pathol 2012; 25(10): 1315-1325.
65. Quirke P, Risio M, Lambert R, von Karsa L, Vieth M. Quality assurance in pathology in colorectal cancer screening and diagnosis—European recommendations. Virchows Arch 2011; 458(1): 1-19.
66. Karamitopoulou E, Zlobec I, Kölzer V, Kondi-Pafiti A, Patsouris ES, Gennatas K, et al. Proposal for a 10-high-power-fields scoring method for the assessment of tumor budding in colorectal cancer. Mod Pathol 2013; 26(2): 295-301.
67. Horcic M, Koelzer VH, Karamitopoulou E, Terracciano L, Puppa G, Zlobec I, et al. Tumor budding score based on 10 high-power fields is a promising basis for a standardized prognostic scoring system in stage II colorectal cancer. Hum Pathol 2013; 44(5): 697-705.
68. Ueno H, Kajiwara Y, Shimazaki H, Shinto E, Hashiguchi Y, Nakanishi K, et al. New criteria for histologic grading of colorectal cancer. Am J Surg Pathol 2012; 36(2): 193-201.
69. Swinson DE, Jones JL, Richardson D, Cox G, Edwards JG, O’Byrne KJ. Tumour necrosis is an independent prognostic marker in non-small cell lung cancer: correlation with biological variables. Lung Cancer 2002; 37(3): 235-240.
70. Frank I, Blute ML, Cheville JC, Lohse CM, Weaver AL, Zincke H. An outcome prediction model for patients with clear cell renal cell carcinoma treated with radical nephrectomy based on tumor stage, size, grade and necrosis: the SSIGN score. J Urol 2002; 168(6): 2395-2400.
71. Langner C, Hutterer G, Chromecki T, Leibl S, Rehak P, Zigeuner R. Tumor necrosis as prognostic indicator in transitional cell carcinoma of the upper urinary tract. J Urol 2006; 176(3): 910-913;
72. Zigeuner R, Shariat SF, Margulis V, Karakiewicz PI, Roscigno M, Weizer A, et al. Tumour necrosis is an indicator of aggressive biology in patients with urothelial carcinoma of the upper urinary tract. Eur Urol 2010; 57(4): 575-581.
73. Pollheimer MJ, Kornprat P, Lindtner RA, Harbaum L, Schlemmer A, Rehak P, et al. Tumor necrosis is a new promising prognostic factor in colorectal cancer. Hum Pathol 2010; 41(12): 1749-1757.
74. Richards CH, Flegg KM, Roxburgh CS, Going JJ, Mohammed Z, Horgan PG, et al. The relationships between cellular components of the peritumoural inflammatory response, clinicopathological characteristics and survival in patients with primary operable colorectal cancer. Br J Cancer 2012; 106(12): 2010-2015.
75. Richards CH, Roxburgh CS, Anderson JH, McKee RF, Foulis AK, Horgan PG, et al. Prognostic value of tumour necrosis and host inflammatory responses in colorectal cancer. Br J Surg 2012; 99(2): 287-294.
76. Komori K, Kanemitsu Y, Kimura K, Hattori N, Sano T, Ito S, et al. Tumor necrosis in patients with TNM stage IV colorectal cancer without residual disease (R0 Status) is associated with a poor prognosis. Anticancer Res 2013; 33(3): 1099-1105.
77. Guthrie GJ, Roxburgh CS, Richards CH, Horgan PG, McMillan DC. Circulating IL-6 concentrations link tumour necrosis and systemic and local inflammatory responses in patients undergoing resection for colorectal cancer. Br J Cancer 2013; 109(1): 131-137.
78. Klintrup K, Mäkinen JM, Kauppila S, Väre PO, Melkko J, Tuominen H, et al. Inflammation and prognosis in colorectal cancer. Eur J Cancer 2005; 41(17): 2645-2654.
79. Roxburgh CS, Salmond JM, Horgan PG, Oien KA, McMillan DC. Tumour inflammatory infiltrate predicts survival following curative resection for node-negative colorectal cancer. Eur J Cancer 2009; 45(12): 2138-2145.
80. Ogino S, Nosho K, Irahara N, Meyerhardt JA, Baba Y, Shima K, et al. Lymphocytic reaction to colorectal cancer is associated with longer survival, independent of lymph node count, microsatellite instability, and CpG island methylator phenotype. Clin Cancer Res 2009; 15(20): 6412-6420.
81. Greenson JK, Huang SC, Herron C, Moreno V, Bonner JD, Tomsho LP, et al. Pathologic predictors of microsatellite instability in colorectal cancer. Am J Surg Pathol 2009; 33(1): 126-33.
82. Nagtegaal ID, Marijnen CA, Kranenbarg EK, Mulder-Stapel A, Hermans J, van de Velde CJ, et al. Local and distant recurrences in rectal cancer patients are predicted by the nonspecific immune response; specific immune response has only a systemic effect--a histopathological and immunohistochemical study. BMC Cancer 2001; 1 : 7.
83. Richards CH, Roxburgh CS, Powell AG, Foulis AK, Horgan PG, McMillan DC. The clinical utility of the local inflammatory response in colorectal cancer. Eur J Cancer 2014; 50(2): 309-319.
84. Galon J, Mlecnik B, Bindea G, Angell HK, Berger A, Lagorce C, et al. Towards the introduction of the ‘Immunoscore’ in the classification of malignant tumours. J Pathol 2014; 232(2): 199-209.
85. Lugli A, Karamitopoulou E, Panayiotides I, Karakitsos P, Rallis G, Peros G, et al. CD8+ lymphocytes/ tumour-budding index: an independent prognostic factor representing a ‘pro-/anti-tumour’ approach to tumour host interaction in colorectal cancer. Br J Cancer 2009; 101(8): 1382-1392.
86. Nielsen HJ, Hansen U, Christensen IJ, Reimert CM, Brünner N, Moesgaard F. Independent prognostic value of eosinophil and mast cell infiltration in colorectal cancer tissue. J Pathol 1999; 189(4): 487-95.
87. Fernandez-Acenero MJ, Galindo-Gallego M, Sanz J, Aljama A. Prognostic influence of tumor-associated eosinophilic infiltrate in colorectal carcinoma. Cancer 2000; 88(7): 1544-1548.
88. Forssell J, Oberg A, Henriksson ML, Stenling R, Jung A, Palmqvist R. High macrophage infiltration along the tumor front correlates with improved survival in colon cancer. Clin Cancer Res 2007; 13(5): 1472-1479.
89. Chaput N, Svrcek M, Aupérin A, Locher C, Drusch F, Malka D, et al. Tumour-infiltrating CD68+ and CD57+ cells predict patient outcome in stage II-III colorectal cancer. Br J Cancer 2013; 109(4): 1013-1022.
90. Bosch SL, Nagtegaal ID. The importance of the pathologist’s role in assessment of the quality of the mesorectum. Curr Colorectal Cancer Rep 2012; 8(2): 90-98.
91. Nagtegaal ID, Quirke P. What is the role for the circumferential margin in the modern treatment of rectal cancer? J Clin Oncol 2008; 26(2): 303-312.
92. van de Velde CJ, Boelens PG, Borras JM, Coebergh JW, Cervantes A, Blomqvist L, et al. EURECCA colorectal: multidisciplinary management: European consensus conference colon & rectum. Eur J Cancer 2014; 50(1): 1.e1-1.e34.
93. Quirke P, West NP, Nagtegaal ID. EURECCA consensus conference highlights about colorectal cancer clinical management: the pathologists expert review. Virchows Arch 2014; 464(2): 129-134.
94. Haggitt RC, Glotzbach RE, Soffer EE, Wruble LD. Prognostic factors in colorectal carcinomas arising in adenomas: implications for lesions removed by endoscopic polypectomy. Gastroenterology 1985; 89(2): 328-336.
95. Kudo S. Endoscopic mucosal resection of flat and depressed types of early colorectal cancer. Endoscopy 1993; 25(7): 455-461.
96. Kikuchi R, Takano M, Takagi K, Fujimoto N, Nozaki R, Fujiyoshi T, Uchida Y. Management of early invasive colorectal cancer. Risk of recurrence and clinical guidelines. Dis Colon Rectum 1995; 38(12): 1286-1295.
97. Sakuragi M, Togashi K, Konishi F, Koinuma K, Kawamura Y, Okada M, et al. Predictive factors for lymph node metastasis in T1 stage colorectal carcinomas. Dis Colon Rectum 2003; 46(12): 1626-1632.
98. Egashira Y, Yoshida T, Hirata I, Hamamoto N, Akutagawa H, Takeshita A, et al. Analysis of pathological risk factors for lymph node metastasis of submucosal invasive colon cancer. Mod Pathol 2004; 17(5): 503-511.
99. Tateishi Y, Nakanishi Y, Taniguchi H, Shimoda T, Umemura S. Pathological prognostic factors predicting lymph node metastasis in submucosal invasive (T1) colorectal carcinoma. Mod Pathol 2010 23(8): 1068-1072.
100. Hassan C, Zullo A, Risio M, Rossini FP, Morini S. Histologic risk factors and clinical outcome in colorectal malignant polyp: a pooled-data analysis. Dis Colon Rectum 2005; 48(8): 1588-1596.
101. Ueno H, Mochizuki H, Hashiguchi Y, Shimazaki H, Aida S, Hase K, et al. Risk factors for an adverse outcome in early invasive colorectal carcinoma. Gastroenterology 2004; 127(2): 385-394.
102. Butte JM, Tang P, Gonen M, Shia J, Schattner M, Nash GM, et al. Rate of residual disease after complete endoscopic resection of malignant colonic polyp. Dis Colon Rectum 2012; 55(2): 122-127.
103. Williams JG, Pullan RD, Hill J, Horgan PG, Salmo E, Buchanan GN, et al. Management of the malignant colorectal polyp: ACPGBI position statement. Colorectal Dis 2013; 15 Suppl 2 : 1-38.
104. Ramirez M, Schierling S, Papaconstantinou HT, Thomas JS. Management of the malignant polyp. Clin Colon Rectal Surg 2008; 21(4): 286-290.
Labels
Anatomical pathology Forensic medical examiner Toxicology
Article was published inCzecho-Slovak Pathology

2015 Issue 1-
All articles in this issue
-
A revolution postponed indefinitely.
WHO classification of tumors of the breast 2012: the main changes compared to the 3rd edition (2003) - Hopes and pitfalls of the molecular classification of breast cancer
- Neurofibromatosis von Recklinghausen type 1 (NF1) – clinical picture and molecular-genetics diagnostic
- Small cell type (Ewing-like) clear cell sarcoma of soft parts: a case report
- Pathological evaluation of colorectal cancer specimens: advanced and early lesions
- Mammary fibroadenoma with pleomorphic stromal cells
- Four bilateral synchronous benign and malignant kidney tumours: A case report
-
A revolution postponed indefinitely.
- Czecho-Slovak Pathology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Neurofibromatosis von Recklinghausen type 1 (NF1) – clinical picture and molecular-genetics diagnostic
- Pathological evaluation of colorectal cancer specimens: advanced and early lesions
- Mammary fibroadenoma with pleomorphic stromal cells
- Small cell type (Ewing-like) clear cell sarcoma of soft parts: a case report
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

