-
Medical journals
- Career
Primary vaginal squamous cell carcinoma arising in a squamous inclusion cyst: Case report
Authors: K. Němejcová 1; P. Dundr 1; C. Povýšil 1; J. Sláma 2
Authors‘ workplace: Department of Pathology, First Faculty of Medicine and General University Hospital, Charles University in Prague Czech Republic 1; Oncogynecological Centre, Department of Obstetrics and Gynecology, First Faculty of Medicine and General University Hospital, Charles University in Prague, Czech Republic 2
Published in: Čes.-slov. Patol., 48, 2012, No. 3, p. 153-155
Category: Original Articles
Overview
We report the case of a 39-year-old female with primary vaginal squamous cell carcinoma arising from a squamous inclusion cyst of the posterior wall. The tumor was located in the vaginal wall and extended into the rectovaginal septum. The overlying mucosa was intact. Histologically, there was invagination of the surface squamous vaginal epithelium forming a cystic lesion. In some areas of this invagination, the squamous epithelium showed dysplastic changes (VAIN3) transitioning into invasive squamous cell carcinoma. To the best of our knowledge, we have documented the first case of primary squamous cell carcinoma arising in a vaginal cyst in a patient without having undergone a previous hysterectomy.
Keywords:
squamous cell carcinoma – vagina – squamous inclusion cyst – embryonal remnants – rectovaginal septumVaginal squamous cell carcinomas (SCCs) are rare tumors, accounting for approximately 1–2 % of all malignant neoplasms of the female genital tract (1,2). The risk factor of vaginal carcinoma is similar to that of cervical cancer, which includes a strong association with persistent human papillomavirus infection. Painless vaginal bleeding is the most common symptom of vaginal cancer. Other symptoms frequently reported include increased vaginal discharge, dyspareunia and postcoital bleeding. Most tumors occur in the upper third of the vagina and are located on the posterior wall. We report a patient with primary vaginal SCC arising from a squamous inclusion cyst of the posterior wall.
Case Report
A 39-year-old woman, 2-para with a short history of dyspareunia and postcoital bleeding was referred with a palpable vaginal tumor to the Oncogynecological center. The patient had not yet been treated for any malignancy, and had had no history of pelvic irradiation. Her last Papanicolaou smear screening, which had been done six month prior to her referral, was normal. The initial vaginal colposcopy showed the intact vaginal mucosa without any abnormality. Colonoscopic and rectoscopic examination was normal too. However, the transrectal and endovaginal ultrasound, as well as the MRI and PET, revealed a tumor, suspected to be malignant, localised in the rectovaginal septum. The spread of disease was not apparent, and both tumor markers (CA-125 and SCCA) were negative. An ultrasound-guided tru-cut biopsy from the rectovaginal septum showed a moderately differentiated nonkeratinizing invasive SCC. Subsequently, the patient underwent a radical hysterectomy type C2, partial colpectomy and the resection of the rectosigmoid bowel with terminal colostomy. No adjuvant treatment was indicated. The patient has been under regular follow-up controls every three months for 9 months without any sign of disease recurrence.
Materials and Methods
Sections from formalin-fixed, paraffin-embedded tissue blocks were stained with hematoxylin-eosin. Selected sections were analysed immunohistochemically using the avidin-biotin complex method with the antibody directed against p16 (ready to use, Dako).
Results
Grossly, the vaginal, cervical and rectal mucosa was intact. Under the mucosa of the posterior wall of the vagina there was a firm, poorly circumscribed tumor, measuring 30 x 30 x 25 mm, extending into the rectovaginal septum (Fig. 1).
1. Tumor of the posterior wall of vagina. 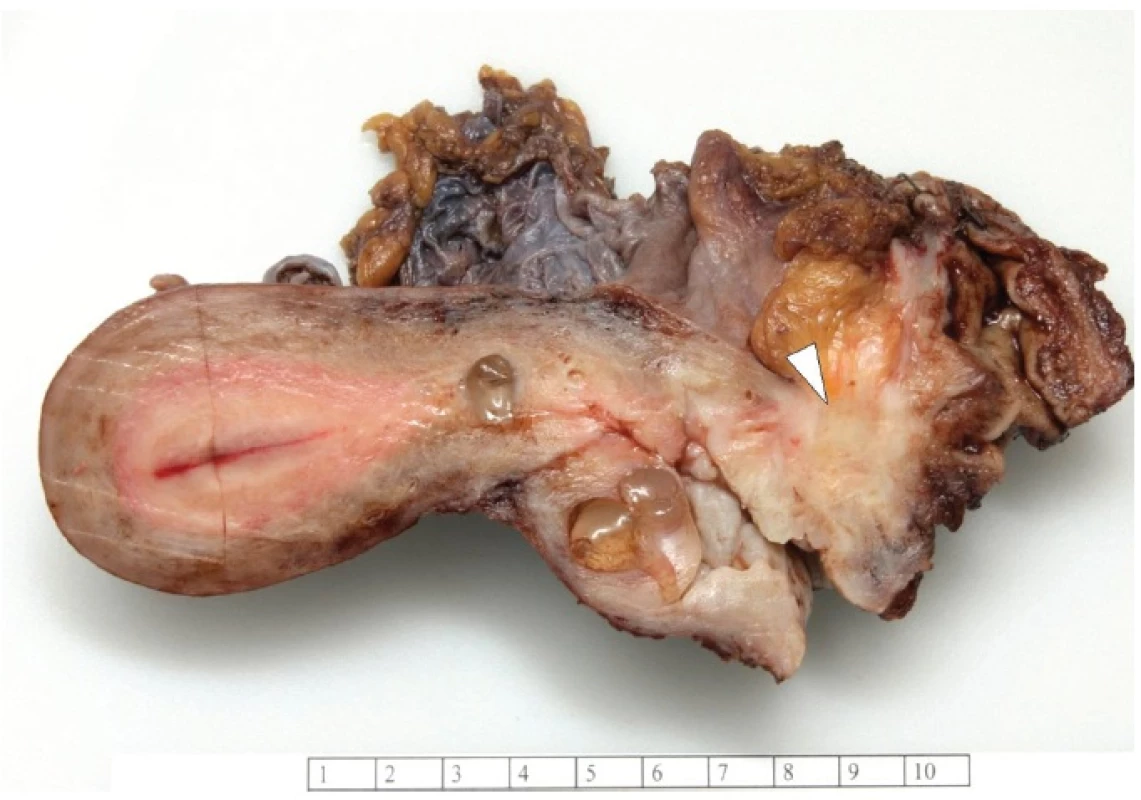
Microscopically, the uterine cervix was covered by stratified squamous epithelium. There was squamous cell metaplasia of the transformation zone. Dysplastic changes in the cervical epithelium were not found. The vagina was covered with nonkeratinizing squamous epithelium without atypia. In one area of the posterior wall, we found the invagination of the surface epithelium forming a squamous inclusion cyst (Fig. 2). In some areas of the squamous cyst, the epithelium showed vaginal intraepithelial neoplasia (VAIN3) transitioning into invasive SCC with focal pseudoglandular arrangement (Fig. 3, 4). The carcinoma spread through the vaginal wall to the rectovaginal septum and the external layers of muscularis mucosae of the rectum. The rectal mucosa was intact. Immunohistochemically, the tumor cells showed a diffuse strong expression of p63 and p16.
2. Invagination of the surface epithelium forming a squamous inclusion cyst (H&E, original magnification 20x). Arrow indicates transition between normal and dysplastic squamous epithelium. 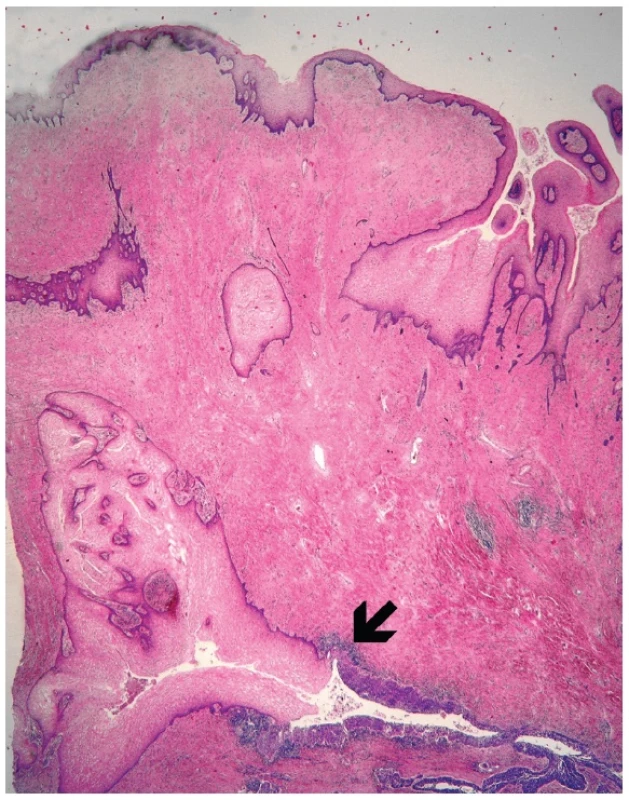
3. Normal squamous epithelium (upper left) and dysplastic epithelium lining invagination with apparent transition into invasive squamous cell carcinoma (H&E, original magnification 100x). 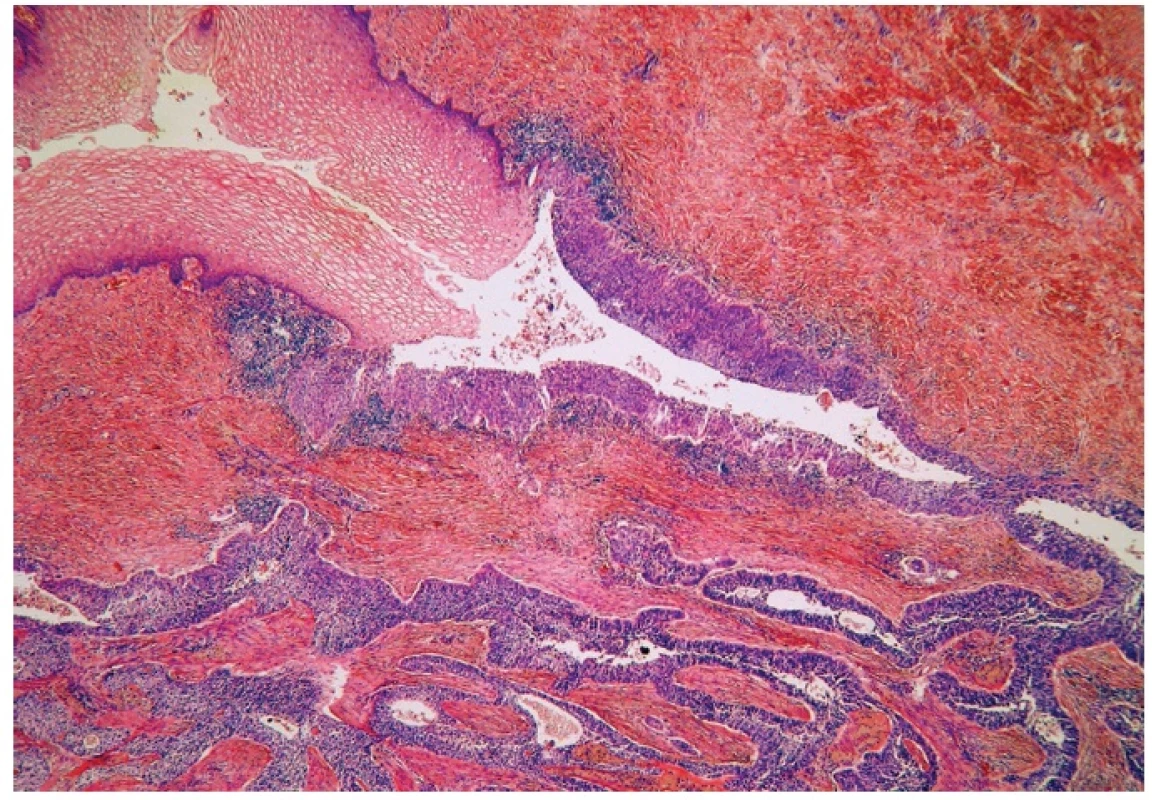
4. Invasive squamous cell carcinoma (H&E, original magnification 200x). 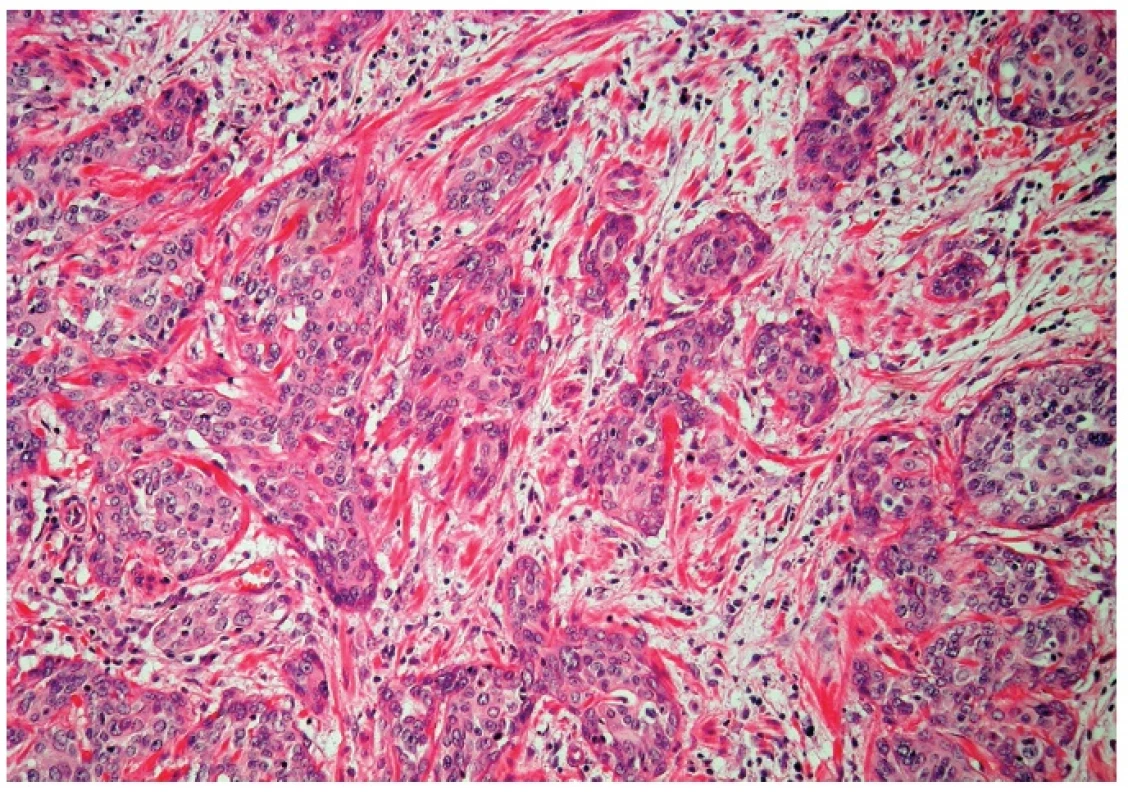
DISCUSSION
Primary SCC of the vagina is a rare malignancy approximately accounting for 1–2 % of all malignant tumours of the female genital tract (1,2). Most vaginal SCCs represent a direct extension of either primary cervical or vulvar carcinoma. Only tumors located in the vagina not having clinical or histological evidence of involvement of the cervix or vulva can be classified as primary vaginal (1). According to the International Federation of Gynaecology and Obstetrics (FIGO), a vaginal tumor involving the uterine cervix or vulva should be classified as a primary cervical or vulvar cancer, respectively (2). Most primary tumors occur in the upper third of the vagina and are located on the posterior wall.
Cysts of the vagina are relatively uncommon. These cysts include squamous inclusion cysts, mesonephric cysts, and müllerian cysts (1,3,4). Squamous inclusion cysts are probably the most common vaginal cysts, resulting primarily from the entrapment of fragments of mucosa during the repair of a vaginal laceration, episiotomy, or appear in vaginal cuffs after a hysterectomy. They are often asymptomatic and vary from a few millimeters to several centimeters in diameter. In rare cases, VAIN or invasive SCC can arise in vaginal squamous inclusion cysts. These instances are rare, and only three cases of VAIN and 2 cases of SCCs arising within vaginal cuff inclusion cysts or sinuses have been described in patients who had previously undergone a hysterectomy (5). In one of these patients, the hysterectomy was performed because of cervical cancer, in two for cervical intraepitelial neoplasia, and in the other two the cause for hysterectomy was not reported. In our case, however, the tumor arose from the invagination of squamous epithelium located in the posterior vaginal wall, which most likely represented a squamous inclusion cyst in a patient without a previous hysterectomy. The patient had neither an anamnestic nor current clinical or histological evidence of the involvement of the cervix or vulva.
Differential diagnosis in our case includes the origin of SCC from embryonal remnants in the rectovaginal septum or in the longitudinal vaginal septum, or from endometriosis. However, the tumor origin from the squamous inclusion cyst of the posterior wall is fare more likely, because we had found the particular site of the invagination of the surface vaginal epithelium. Tumors arising from endometriosis of the rectovaginal septum are rare neoplasms, which can mimic either primary vaginal or rectal tumors. However, these tumors mostly represent carcinomas of müllerian origin such as endometrioid and serous adenocarcinoma. Moreover, the foci of endometriosis are usually found close to the tumor (6).
In conclusion, we described a case of primary SCC probably arising from a squamous inclusion cyst of the vaginal wall. To the best of our knowledge our case represents the first case of SCC arising in a vaginal cyst in a patient without a prior hysterectomy. Only two other cases of SCCs arising in vaginal squamous cysts have been described to date, however, these tumors were found in patients who had previously undergone a hysterectomy (5). In addition, one report described a case of vaginal SCC which was cystic and grew endophytically into the rectovaginal septum (7). The possibility of carcinomas originating in a vaginal wall cyst should be borne in mind in the differential diagnosis of tumors located in the vaginal wall and the rectovaginal septum.
Correspondence address:
Kristýna Němejcová, MD
First Faculty of Medicine and General University Hospital, Charles
University in Prague, Studničkova 2, Prague 2, 12800, Czech Republic
tel: +420224968632
fax: +420224911715
email: kristyna.nemejcova@vfn.cz
Sources
1. Kurman RJ (ed.) Blaustein’s pathology of the female genital tract. 5th ed. New York, NY: Springer-Verlag, 2002 : 172–182.
2. Tavassoli FA, Devilee P (eds) World Health Organization Classification of Tumours. Pathology and Genetics Tumours of the Breast and Female Genital Organs: IARC Press: Lyon 2003 : 291–293.
3. Brown KL, Segal AJ, Hurd GB. An unusual cyst of the vaginal wall mucous cyst of mid posterior vaginal wall, Gartner duct-type, complicated by rectovaginal septum abscess. Ann Surg 1957; 145(3): 423–427.
4. Klein FA, Vick CW 3rd, Broecker BH. Neonatal vaginal cysts: diagnosis and management. J Urol 1986; 135(2): 371–372.
5. Hoffman MS, Roberts WS, LaPolla JP, Sterghos S, Cavanagh D. Neoplasia in vaginal cuff epithelial inclusion cysts after hysterectomy. J Reprod Med 1989; 34(6): 412–414.
6. Yang Q, Wang H, Cho HY, et al. Carcinoma of müllerian origin presenting as colorectal cancer: a clinicopathologic study of 13 cases. Ann Diagn Pathol 2011; 15 : 12–18.
7. Eltabbakh GH, Field JM, Trask CE, McDay JB, Swift PD. Primary vaginal squamous cell carcinoma presenting as a cystic pelvic mass. Gynecol Oncol 2000; 76(2): 213–217.
8. Georg C Jr. Cysts of Gaertner’s Duct. J Michigan M Soc 1911; 10 : 515.
Labels
Anatomical pathology Forensic medical examiner Toxicology
Article was published inCzecho-Slovak Pathology

2012 Issue 3-
All articles in this issue
- Melanocytic pseudotumors
- Differential diagnosis of the chronic pancreatitis and the pancreatic ductal adenocarcinoma
- Giant-cell lesions of bone and their differential diagnosis
- Pseudotumors of the testis and testicular adnexa
- Sarcomatoid (metaplastic) spindle cell carcinoma arising in a phylloid tumor with massive squamous metaplasia – a case report and review of the literature
- Primary vaginal squamous cell carcinoma arising in a squamous inclusion cyst: Case report
- Histopathological autoptic findings in 8 patients with pandemic influenza A (H1N1) pneumonia
- Immunoexpression of type-1 adiponectin receptor in the human intestine
- Czecho-Slovak Pathology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Giant-cell lesions of bone and their differential diagnosis
- Differential diagnosis of the chronic pancreatitis and the pancreatic ductal adenocarcinoma
- Sarcomatoid (metaplastic) spindle cell carcinoma arising in a phylloid tumor with massive squamous metaplasia – a case report and review of the literature
- Melanocytic pseudotumors
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

