-
Medical journals
- Career
Digoxin at sub-cardiotonic dose modulates the anticonvulsive potential of valproate, levetiracetam and topiramate in experimental primary generalized seizures
Authors: Vadim Tsyvunin; Sergiy Shtrygol; Mariia Mishchenko; Diana Shtrygol
Published in: Čes. slov. Farm., 2022; 71, 78-88
Category:
doi: https://doi.org/https://doi.org/10.5817/CSF2022-2-76Overview
The prevalence of epilepsy in the world population together with a high percentage of patients resistant to existing antiepileptic drugs (AEDs) stimulates the constant search for new approaches to the treatment of the disease. Previously a significant anticonvulsant potential of cardiac glycoside digoxin has been verified by enhancing a weak activity of AEDs in low doses under screening models of seizures induced by pentylenetetrazole and maximal electroshock. The aim of the present study is to investigate the influence of digoxin at a sub-cardiotonic dose on the anticonvulsant activity of valproate, levetiracetam, and topiramate in models of primary generalized seizures with different neurochemical mechanisms. A total of 264 random-bred male albino mice have been used. AEDs were administered 30 min before seizure induction once intragastrically at conditionally effective (ED50) and sub-effective (½ ED50) doses: sodium valproate and topiramate – at doses of 300 and 150 mg/kg; levetiracetam – at doses of 100 and 50 mg/kg. Digoxin was administered once subcutaneously at a dose of 0.8 mg/kg body weight (1/10 LD50) 10–15 min before seizure induction. Picrotoxin (aqueous solution 2.5 mg/kg, subcutaneously), thiosemicarbazide (aqueous solution 25 mg/kg, intraperitoneally), strychnine (aqueous solution 1.2 mg/kg, subcutaneously), camphor (oil solution 1000 mg/kg, intraperitoneally) have been used as convulsive agents for seizure induction. It was found that under the conditions of primary generalized seizures induced by picrotoxin, thiosemicarbazide, strychnine, and camphor, digoxin not only shows its own strong anticonvulsant activity but also significantly enhances the anticonvulsant potential of classical AEDs sodium valproate, levetiracetam, and topiramate. The obtained results substantiate the expediency of further in-depth study of digoxin as an anticonvulsant drug, in particular, the in-depth study of neurochemical mechanisms of its action.
Keywords:
seizures – digoxin – topiramate – adjuvant – anticonvulsant – valproate – levetiracetam
Introduction
The prevalence of epilepsy in the world population together with the a percentage of patients resistant to existing antiepileptic drugs (AEDs)1–3) stimulates the constant search for new approaches to the treatment of the disease. One of the promising ways to improve the traditional pharmacotherapy of epilepsy is the use of adjuvant drugs with new targets that have an original basic mechanism of action not inherent in the known AEDs4). The anticonvulsant potential of drugs from various pharmacological groups has been investigated in particular for antiarrhythmic and antihypertensive drugs5–7), nonsteroidal antiinflammatory drugs8, 9), synthetic antidiabetic medicines10, 11), statins12, 13), etc. The well-known cardiotonic glycoside digoxin draws special attention as a drug for adjuvant therapy of epilepsy (including its refractory forms)14). In previous studies, we verified significant potentiation of the effect of seven widespread AEDs in sub-effective doses by a low dose of the cardiac glycoside digoxin on baseline screening models of seizures induced by pentylenetetrazole and maximal electroshock15–17). However, neurochemical mechanisms of anticonvulsant action – both digoxin per se and its combinations with classical commonly used AEDs – remain unexplored.
The aim of the study is to investigate the influence of digoxin at sub-cardiotonic dose on the anticonvulsant activity of valproate, levetiracetam, and topiramate in mice models of primary generalized seizures with different neurochemical mechanisms.
The present work is a part of the scientific project “Rationale for improving the treatment of multidrugresistant epilepsy through the combined use of classical anticonvulsant medicines with other drugs” (No. 0120U102460, 2020/2022) supported by the Ministry of Health of Ukraine and carried out at the expense of the State Budget of Ukraine.
Experimental part
Materials
Classical AEDs, as well as a cardiac glycoside, have been used in the form of officinal drugs: sodium valproate (Depakine® 57.64 mg/ml syrup, Sanofi Aventis, France), topiramate (Topamax® 50 mg filmcoated tablets, Janssen-Cilag SpA, Italy), levetiracetam (Keppra® 500 mg film-coated tablets, UCB Pharma, Belgium) and digoxin (Digoxin-Zdorovie® 0.25mg/ml solution for injection, DNCLZ / Zdorovie, Ukraine).
Convulsive agents (convulsants) picrotoxin, thiosemicarbazide, strychnine and camphor have been used in the form of substances (Sigma, USA).
Methods
Study design
Classical AEDs were administered once intragastrically in conditionally effective (ED50) and sub-effective (½ ED50) doses 30 min before seizure simulation: sodium valproate and topiramate – at doses of 300 and 150 mg/kg; levetiracetam – at doses of 100 and 50 mg/kg15, 18). Digoxin was administered once subcutaneously at a dose of 0.8 mg/kg (1/10 LD50)19) 10–15 minutes before seizure induction. Control animals received intragastrically purified water in an appropriate volume (0.1 ml per 10 g of weight).
The anticonvulsant effect of digoxin and its combinations with AEDs was studied in chemoinduced models of seizures caused by picrotoxin (aqueous solution 2.5 mg/kg, subcutaneously), thiosemicarbazide (aqueous solution 25 mg/kg, intraperitoneally), strychnine (aqueous solution 1.2 mg/kg, subcutaneously) and camphor (oil solution 1000 mg/kg, intraperitoneally)20, 21).
After convulsant administration, the animals were immediately placed into separate transparent plastic cylindrical containers (diameter – 15 cm; height – 30 cm) and continuously monitored for 60 min (240 min on the model of thiosemicarbazideinduced seizures). The effectiveness of anticonvulsant drugs and their combinations were evaluated by the following indicators: latency period of first convulsions (latency), the number of clonic-tonic seizures in 1 mouse, percentage of animals in the group separately with clonic and tonic convulsions, the severity of seizures – in points (1 point – single tremors, 2 points – “manege” running or “kangaroo” position, 3 points – clonic convulsions without lateral position, 4 points – clonic-tonic convulsions with lateral position, 5 points – tonic extension of hind limbs, and 6 points – tonic extension, which led to the death of the animal), duration of the convulsive period (period of seizures), life expectancy of animals to death (time to death), and lethality. If seizures were not observed for 1 h, the latency was considered to be 60 min (240 min on the model of thiosemicarbazideinduced seizures)15–17).
Experimental animals and grouping
The experiments were conducted on 264 random-bred male albino mice weighing 22–25 g which were kept on a standard vivarium diet with free access to water under controlled conditions of the Central Scientific and Research Laboratory of the Educational and Scientific Institute of Applied Pharmacy of the National University of Pharmacy (Kharkiv, Ukraine) with constant humidity (60%) and temperature (+18–20 °С).
The experiments were carried out in accordance with the U.K. Animals (Scientific Procedures) Act 1986 and the principles and requirements of the E.U. Directive 2010/63/E.U. (2010) on the protection of animals used for scientific purposes. All the experimental protocols have been approved by the Bioethics Commission of the National University of Pharmacy (Protocol No. 3, September 10, 2020).
Mice were randomly assigned to groups of 6 animals each: 1st group – control (untreated seizures), the remaining groups – animals with modeling seizures, which were administered sodium valproate/levetiracetam/topiramate, as well as their combinations with digoxin.
Statistical analysis
The results are expressed as mean ± standard error of mean. The level of statistical significance was considered as p < 0.05. Statistical differences between groups were analyzed using a non-parametric Mann-Whitney U-test. For the results in the alternative form (lethality, percentage of mice with clonic and tonic convulsions) the Fisher’s angular transformation was used.
Results
Picrotoxin-induced seizures
The picrotoxin-induced model of seizures stimulates primary generalized clonic-tonic seizures associated with chlorine-ionophore blockade of GABA-receptor and reduction of GABA-ergic inhibitory transmission22). Clinically, picrotoxin-induced seizures are initially detected by alternating clonic and clonictonic convulsions. In two-thirds (67%) of animals, there is a further transition to the tonic extension of the hind limbs with a lateral position, which ends with the animal’s death (Table 1) due to respiratory arrest.
1. Anticonvulsant effect of digoxin, AEDs and their combinations in the picrotoxin-induced seizures in mice (M ± m) 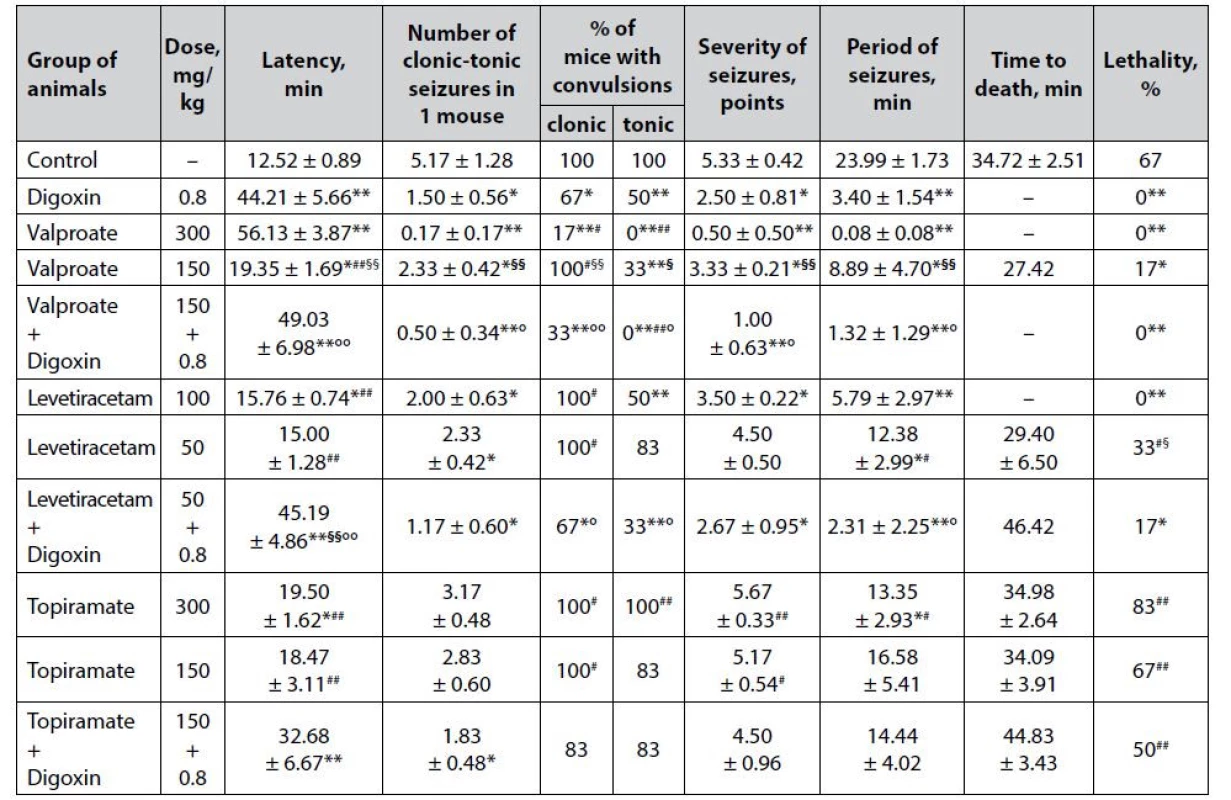
*p < 0.05 when compared with control, **p < 0.01 when compared with control
#p < 0.05 when compared with digoxin, ##p < 0.01 when compared with digoxin
§p < 0.05 when compared with appropriate antiepileptic drug at an ED50, §§p < 0.01 when compared with appropriate antiepileptic drug at an ED50
°p < 0.05 when compared with appropriate antiepileptic drug at a ½ ED50, °°p < 0.01 when compared with appropriate antiepileptic drug at a ½ ED50Digoxin per se in the model of picrotoxin-induced seizures has a pronounced anticonvulsant effect (Table 1). The protective action of cardiac glycoside was verified not only by complete prevention of animal death (lethality – 0%) but also by a statistically significant prolongation of the latency period of the first convulsions (p < 0.01), as well as a reduction in the number of mice with clonic and tonic convulsions (p < 0.05), reducing the severity of seizures (p < 0.05) and the duration of the convulsive period (p < 0.01) compared with similar indicators of the control group. The pronounced anticonvulsant properties of digoxin on the picrotoxin-induced seizure model correlate with a strong protective effect on the model of paroxysms induced by the known GABA-blocker pentylenetetrazole, which we studied earlier15) and additionally indicates its stable activity. It can be assumed that the influence on GABA-mediated neurotransmission is indirect, as there are still no relevant scientific data on the affinity of cardiac glycosides for the GABA-barbiturate-benzodiazepine receptor complex. The possibility of reciprocal interaction cannot be ruled out either – in particular, it is known about the enhancement of GABA release in brain synaptosomes under the influence of endogenous digitalis-like factor (ouabain)23).
Sodium valproate shows a predicted expressive and dose-dependent anticonvulsant effect on the picrotoxin-induced seizure model (Table 1). At ED50, the known AED not only completely prevents the death of animals in the experimental group but also prevents the development of severe tonic seizures (p < 0.01). In addition, against the background of sodium valproate at this dose, there was detected a high (p < 0.01) prolongation of the latency period of the first attacks, as well as reducing the number of seizures in 1 mouse % of animals with clonic paroxysms, the severity of convulsions and duration of seizure period compared with the control group indicators. Meanwhile, the anticonvulsant effect of sodium valproate at ½ ED50 under conditions of picrotoxininduced seizures is less pronounced – starting from a statistically significant (p < 0.05), but incomplete prevention of animal death (lethality – 17%) compared with control. In addition, the sub-effective dose of sodium valproate with a lower significance (p < 0.05) affects other indicators of experimental seizures – in particular, it prolongs the latency period of seizures, reduces the number of paroxysms in 1 mouse (solely due to the tonic component – % of animals with clonic seizures does not differ from the same indicator of the control group), reduces the severity of seizures and the duration of the convulsive period. The dosedependence of the anticonvulsant effect of sodium valproate is confirmed statistically – off almost all analyzed indicators of seizures (except lethality) in the group of animals receiving AED at ½ ED50 in high significance (p < 0.01) differ from similar markers on the background of the drug at ED50.
Digoxin potentiates the anticonvulsant effect of sodium valproate at ½ ED50. The combined use of drugs in terms of the effect on the course of seizures is statistically significantly higher than the values of similar markers on the background of AED at ½ ED50, closely approaching the level of anticonvulsant effect of sodium valproate at ED50. This, in particular, was established for the complete prevention of animal lethality, as well as a totally reduction of the tonic component of seizures (p < 0.01) compared with control.
The anticonvulsant effect of levetiracetam on the picrotoxin-induced seizure model is also dosedependent (Table 1). Thus, the drug at ED50 completely prevents picrotoxin-induced death of animals (lethality – 0%), and statistically significant compared with control, prolongs the latency of the first seizures, reduces the number of attacks in 1 mouse (solely due to the tonic component, halves decreasing the number of animals with tonic convulsions, p < 0.01), and also reduces the severity of paroxysms as well as the duration of the convulsive period. At the same time, half the effective dose of levetiracetam showed quite weak anticonvulsant properties, causing only a significant (p < 0.05) reduction in the number of clonic-tonic attacks in 1 mouse as well as the duration of the convulsive period.
Co-administration of levetiracetam at ½ ED50 with digoxin significantly enhances the anticonvulsant potential of low-dose classical AED, which is established not only by a statistically significant (p < 0.05) reduction in animal lethality but also by a positive influence on other indicators of experimental seizures. Thus, the combination of levetiracetam + digoxin significantly (p < 0.01), both with control, and the groups of animals treated with the corresponding AED at ED50 and ½ ED50, prolongs the latency period of the first attacks. In addition, the combination of drugs statistically significantly reduces the number of seizures in 1 mouse (not only due to the tonic, as levetiracetam at ED50, but also the clonic component) as well as the severity of convulsions (p < 0.05) and statistically decreases the duration of the convulsive period in animals – both in terms of control and in relation to a similar indicator on the background of levetiracetam in ½ ED50.
Topiramate in the model of picrotoxin-induced seizures has almost no anticonvulsant effect (Table 1). At ED50, the drug only significantly (p < 0.05) prolongs the latency of the first seizures and reduces the duration of the convulsive period, while at ½ ED50, topiramate is generally devoid of statistically significant influence on all studied indicators of experimental convulsions.
The combined use of topiramate at ½ ED50 with digoxin although it does not show a significant influence on the main integral indicator of efficiency – % of lethality in the group, still contributes to a significant prolongation of the latency period of the first seizures (p < 0.05) and significantly reduces the number of clonic tonic paroxysms in 1 mouse (p < 0.01).
Thiosemicarbazide-induced seizures
The model of thiosemicarbazide-induced seizures has specific mechanisms of development and individual features of the course17, 24). The convulsive effect of thiosemicarbazide has a double mechanism: due to the blockade of the cerebral enzyme glutamate decarboxylase, the pool of the main inhibitory neurotransmitter GABA is depleted with the simultaneous accumulation in neurons of its precursor glutamate, which has a powerful stimulating effect on the CNS. Clinically, thiosemicarbazideinduced seizures are characterized by a rather slow development with a prolonged “shaking” period, which is manifested by increased motor activity, agitation, jumping, skeletal muscle hypertonia (“kangaroo” position, Straub’s symptom, etc.). Further, the animals have alternating clonic, clonic-tonic, and tonic convulsions with lateral position and subsequent extension of the hind limbs. The rigidity of the model is confirmed by the high lethality – 100% of mice did not come out of long-term tonic extension (Table 2).
2. Anticonvulsant effect of digoxin, AEDs and their combinations in the tiosemicarbaside-induced seizures in mice (M ± m) 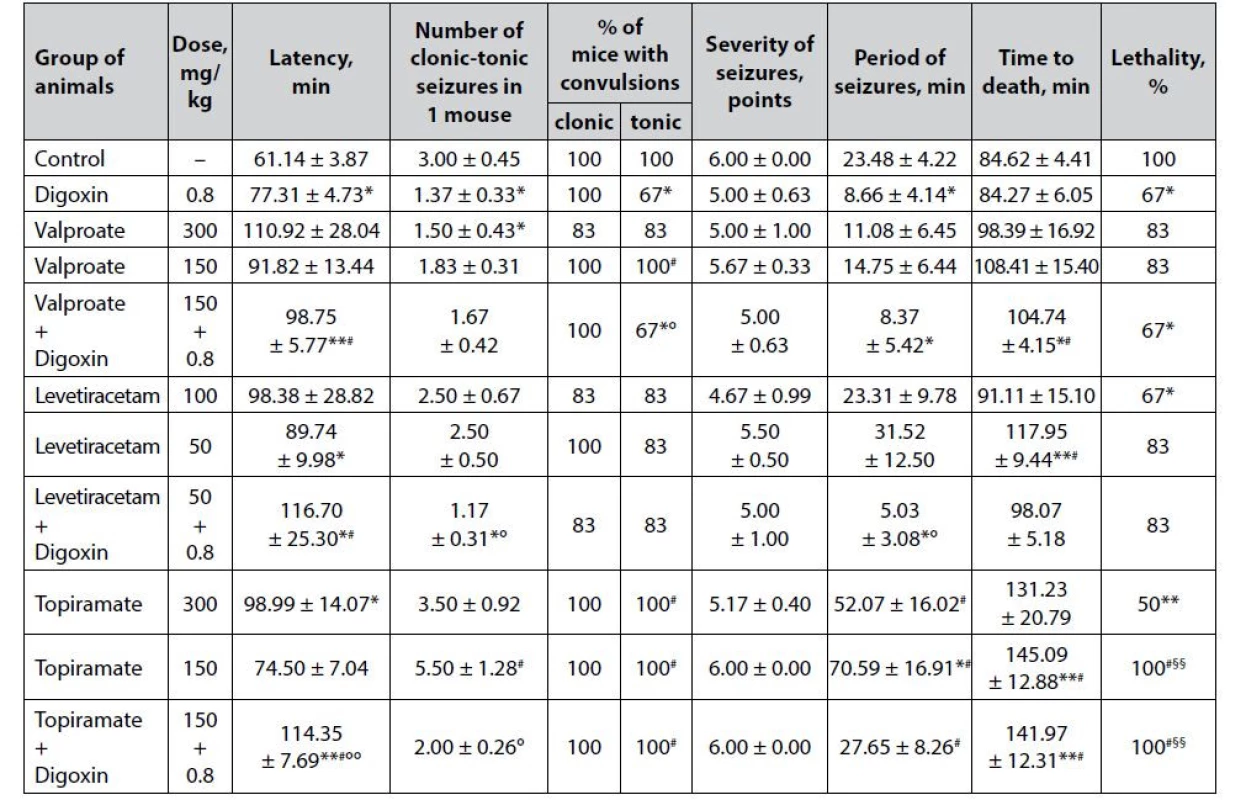
*p < 0.05 when compared with control, **p < 0.01 when compared with control
#p < 0.05 when compared with digoxin, ##p < 0.01 when compared with digoxin
§p < 0.05 when compared with appropriate antiepileptic drug at an ED50, §§p < 0.01 when compared with appropriate antiepileptic drug at an ED50
°p < 0.05 when compared with appropriate antiepileptic drug at a ½ ED50, °°p < 0.01 when compared with appropriate antiepileptic drug at a ½ ED50Prophylactic administration of digoxin contributes to a significant reduction of the thiosemicarbazideinduced convulsive syndrome (Table 2). Thus, cardiac glycoside not only significantly (p < 0.05) prolongs the latency period of the first attacks but also statistically reduces the number of paroxysms in 1 mouse (primarily due to the tonic component) and decreases the duration of the convulsive period. Moreover, digoxin reduces the lethality of animals in the group (67% compared to 100% in control, p < 0.05).
Sodium valproate is a commonly used AED with multiple mechanisms of anticonvulsant action, a firstline drug in the vast majority of clinical forms of epilepsy – in both ½ ED50 and ED50 has almost no significant effect on the course of thiosemicarbazide-induced seizures, which further indicates the severity of experimental model. The only exception is a significant reduction in the number of clonic-tonic seizures in 1 mouse in the valproate group at ED50 (p < 0.05). Although against the background of AED, there is a prolongation of the latency period of the first attacks and the lifespan of animals to death, as well as reducing the severity of paroxysms and the duration of the convulsive period, these differences are due to high dispersion and do not reach statistical significance.
Co-administration of sodium valproate at ½ ED50 with digoxin gives the classic low-dose AED a distinct anticonvulsant potential. Against the background of the combination, there is a statistically significant reduction in animal lethality – at the level of digoxin per se (p < 0.05), as well as significant prolongation of the latency period of the first seizures and life expectancy of animals – compared not only with control but also digoxin. In addition, the combination of valproate + digoxin statistically significantly relative control reduces the % of mice with tonic paroxysms and the duration of the convulsive period.
The anticonvulsant effect of levetiracetam under conditions of thiosemicarbazide-induced seizures is quite moderate (Table 2). Against the background of the drug at ED50, statistically significant differences compared with control are observed only for the reduction of animal lethality (67% vs. 100%), while at ½ ED50, levetiracetam causes a significant prolongation of the latency period of the first attacks (p < 0.05) and life expectancy of mice (p < 0.01).
The combination of levetiracetam at ½ ED50 and digoxin, although not likely to affect the main integral efficacy indicator – % of animal lethality, but statistically significant (p < 0.05) compared with control increases the latency of the first attacks, while reducing the number of clonic-tonic convulsions by 1 mouse and the duration of the convulsive period.
Topiramate in the model of thiosemicarbazideinduced seizures has a dose-dependent anticonvulsant effect (Table 2). Thus, at ED50, the drug halves the lethality of animals in the group (p < 0.05) and significantly increases the latency period of seizures. At ½ ED50, topiramate only statistically significantly prolongs the duration of the convulsive period and increases the lifespan of mice to death, which, however, due to the 100% lethality of animals can not indicate in favor of anticonvulsant properties of the drug at a sub-effective dose.
The combined use of topiramate at ½ ED50 with digoxin has a significant (p < 0.01) influence on the latency period of the first seizures and life expectancy of mice again due to the death of all animals in the experimental group (lethality – 100%) but does not allow to speak about of expressive anticonvulsant potential in such a combination.
Strychnine-induced seizures
Strychnine-induced seizures have an original mechanism of development associated with the blockade of the effects of the inhibitory neurotransmitter, the amino acid glycine25). Clinically, this model is characterized by the rapid development of tonic seizures, which are rarely preceded by phases of clonic and clonic-tonic paroxysms. Then, 100% of experimental animals go into a tonic extension of the hind limbs with opisthotonos, accompanied by cerebral edema. A short convulsive period ends with the death of mice (Table 3).
3. Anticonvulsant effect of digoxin, AEDs and their combinations in the strychnine-induced seizures in mice (M ± m) 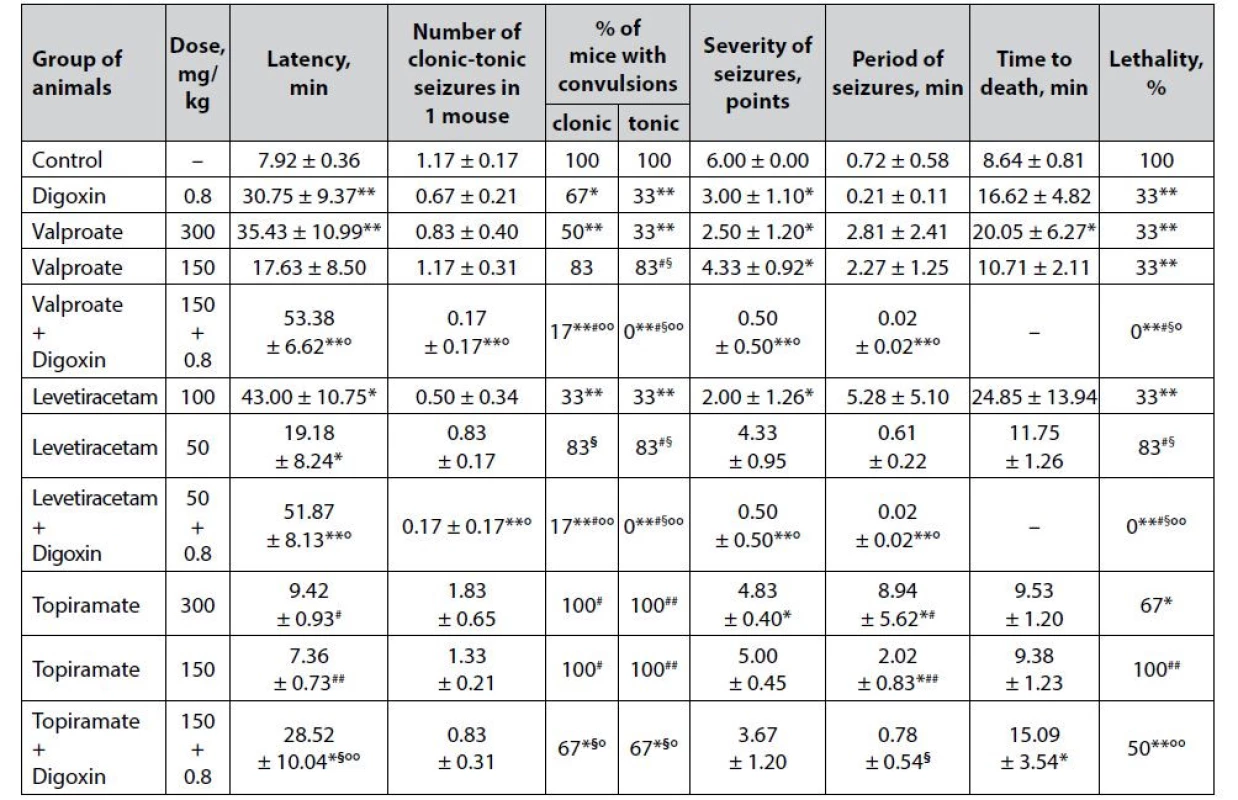
*p < 0.05 when compared with control, **p < 0.01 when compared with control
#p < 0.05 when compared with digoxin, ##p < 0.01 when compared with digoxin
§p < 0.05 when compared with appropriate antiepileptic drug at an ED50, §§p < 0.01 when compared with appropriate antiepileptic drug at an ED50
°p < 0.05 when compared with appropriate antiepileptic drug at a ½ ED50, °°p < 0.01 when compared with appropriate antiepileptic drug at a ½ ED50The pronounced anticonvulsant effect of digoxin in the model of strychnine-induced seizures (Table 3) was verified not only by a statistically significant decrease in lethality (33% vs. 100% in the group of animals with untreated seizures) but also by a significant increase in the latency period of the first convulsions (p < 0.01), % of mice with both clonic and tonic seizures, as well as a twofold reduction in the severity of seizures (p < 0.05) relative to control.
Sodium valproate in the strychnine-induced seizure model has a pronounced dose-dependent effect (Table 3). Although in both ED50 and ½ ED50, the drug significantly reduces the death of animals in the experimental groups (33% vs. 100% in control, p < 0.01) as well as the severity of seizures (p < 0.05), only valproate at a higher dose can prolong latency period of the first convulsions and increase the life expectancy of mice, as well as reduce the % of animals with clonic and tonic seizures.
The addition of digoxin to sodium valproate at ½ ED50 significantly enhances the anticonvulsant potential of low-dose classical AED. Thus, the combination of valproate + digoxin completely protects animals from death (lethality – 0%), which is statistically significant not only compared with control (p < 0.01) but also significantly different from similar indicators on the background of sodium valproate at both doses (½ ED50 and ED50) and digoxin per se (p < 0.05). In addition, the combined use of sodium valproate with digoxin is significantly outweigh the effect of low-dose AED monotherapy in terms of prolonging the latency period of the first seizures, as well as reducing the number of clonic-tonic seizures (due to both clonic and tonic components), the severity and duration of convulsions. According to the criterion of tonic paroxysms prevention (% of mice with tonic convulsions), the combination is statistically significant (p < 0.05) and exceeds even the effect of monotherapy with sodium valproate at ED50.
Clear dose-dependent anticonvulsant properties in the model of strychnine-induced convulsions, like for sodium valproate, have been established for levetiracetam (Table 3). At ED50, the drug significantly (p < 0.05) increases the latency of the first attacks and reduces the severity of seizures, as well as statistically significant compared with control (p < 0.01) reduces the % of mice with both clonic and tonic seizures, reduces animal lethality, while at ½ ED50 levetiracetam has only a significant (p < 0.05) influence on the latency period of seizure development.
The combined use of digoxin with levetiracetam at ½ ED50 has a pronounced anticonvulsant effect under conditions of strychnine-induced seizures. The combination of levetiracetam + digoxin completely prevents the death of animals, which reaches the level of statistical significance not only compared to control and levetiracetam at ½ ED50 (p < 0.01), but also significantly exceeds the effect of both the corresponding AED at ED50 and digoxin (p < 0.05). Moreover, the combined use of levetiracetam and digoxin is significantly outweighing the effect of AED per se at ½ ED50 by increasing the latency period of the first convulsions, as well as reducing the number of clonic-tonic seizures in 1 mouse, the severity of paroxysms, and the duration of the convulsive period. The combination of classical AED at a sub-effective dose with digoxin significantly exceeds the anticonvulsant effect of levetiracetam at a conditionally effective dose – in particular, by complete reduction of the tonic component of experimental paroxysms.
Topiramate at ED50 (but not at ½ ED50) is moderately effective in counteracting strychnine-induced seizures (Table 3). AED at a relatively effective dose causes a significant reduction in animal lethality (67% vs. 100% in control) and the severity of seizures. In addition, against the background of topiramate at ED50, there is a statistically significant increase in the duration of the convulsive period (p < 0.05), which can be seen as an increase in resistance to paroxysms and prevention of death of animals from the first seizure. At ½ ED50 topiramate does not have a significant positive influence on any of the indicators of the course of experimental seizures.
A pronounced anticonvulsant effect is observed against the background of the combined use of topiramate at ½ ED50 with digoxin. Although the combination of topiramate + digoxin is significantly superior to topiramate at both ½ ED50 and EED50 in terms of individual effects, it should be noted that there are no statistically significant differences with the group of animals that received digoxin per se . This indicates that the anticonvulsant potential of the combination is due solely to cardiac glycoside alone.
Camphor-induced seizures
The results of the study of the pharmacodynamic interaction of valproate, levetiracetam, and topiramate with digoxin in the course of primary generalized seizures induced by camphor, are shown in Table 4. Unlike other chemo-induced seizures, the exact mechanism of convulsive action of camphor is not known. It has been suggested that camphor causes an imbalance of monoamines in the CNS26). According to other data, camphor affects certain components of the cytochrome oxidase system of brain neurons, which leads to rapid oxidation and depletion of the pool of phosphate macroergs27). The clinical course of camphor-induced seizures is similar to the classic picture of the development of GABA-negative paroxysms induced by pentylenetetrazole or picrotoxin: alternating clonic and clonic-tonic seizures. In half (50%) of the experimental animals, there is a further transition to the tonic extension of the hind limbs with a lateral position and subsequent animal death due to anoxia.
4. Anticonvulsant effect of digoxin, AEDs and their combinations in the camphor-induced seizures in mice (M ± m) 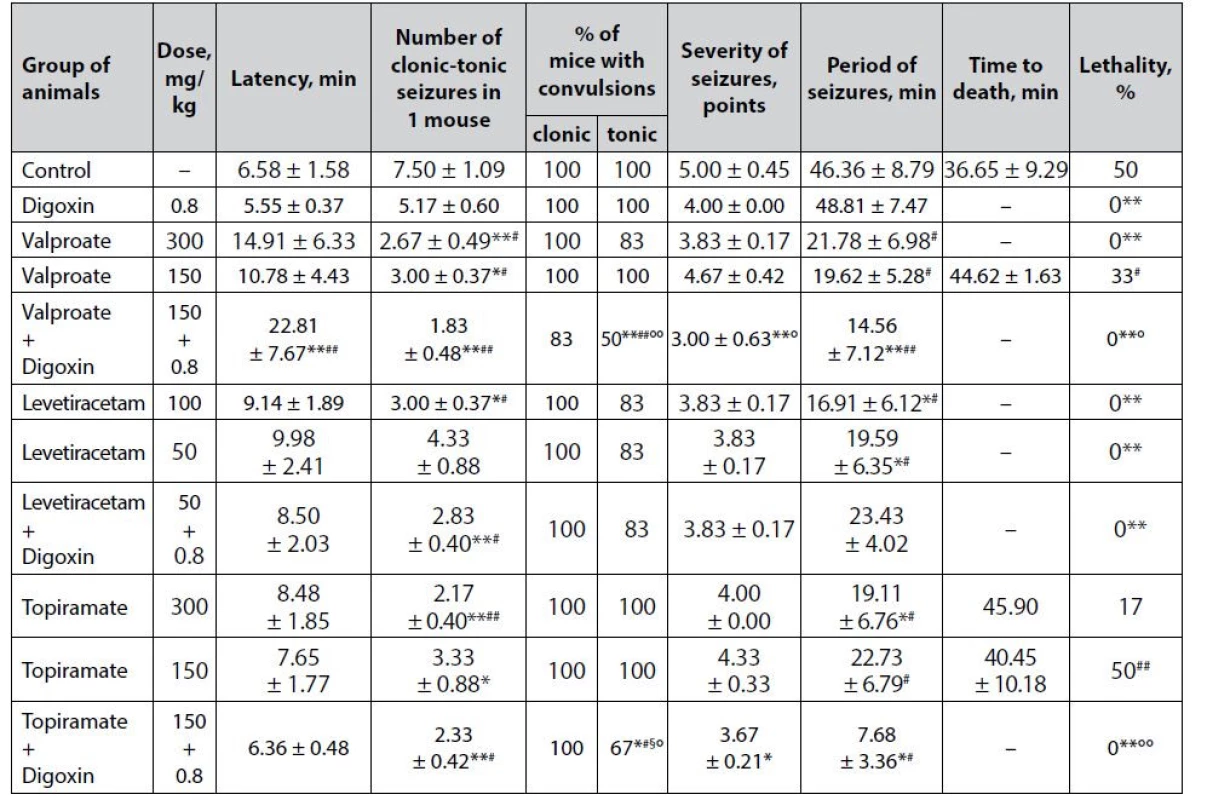
*p < 0.05 when compared with control, **p < 0.01 when compared with control
#p < 0.05 when compared with digoxin, ##p < 0.01 when compared with digoxin
§p < 0.05 when compared with appropriate antiepileptic drug at an ED50, §§p < 0.01 when compared with appropriate antiepileptic drug at an ED50
°p < 0.05 when compared with appropriate antiepileptic drug at a ½ ED50, °°p < 0.01 when compared with appropriate antiepileptic drug at a ½ ED50It is noteworthy that although digoxin does not protect tonic extension in the model of camphorinduced seizures (Table 4), the drug is statistically significant compared with control prevents the death of animals (lethality – 0%, p < 0.01). This may be in favor of the antihypoxic effect of digoxin, as tonic extension limits the ability to ventilate the lungs and therefore causes hypoxia. The anti-edematous effect of digoxin in the brain, which is known to respond rapidly to hypoxia by edema, cannot be ruled out28). These assumptions require special verification. However, there are no relevant data on the presence of antihypoxic effects in cardiac glycosides. It has been established. However, that digoxin inhibits hypoxia-inducible factor 1–3α (HIF1-3α), a protein that regulates the cellular response to hypoxia29).
Sodium valproate under the conditions of camphor-induced seizures has a dose-dependent anticonvulsant effect (Table 4). At both doses, the drug statistically significantly reduced the number of clonic-tonic paroxysms in 1 mouse relative to control, but only at ED50 sodium valproate completely prevents animal lethality (p < 0.01).
Co-administration of sodium valproate at ½ ED50 with digoxin demonstrates a pronounced anticonvulsant potential, which has been verified by a statistically significant influence on almost all indicators of camphor-induced seizures. Thus, the combination of valproate + digoxin not only completely prevents the death of animals in the experimental group (lethality 0% vs. 50% in control, p < 0.01), but also, in contrast to classical AED at both doses and digoxin per se , significantly prolongs the latency period of convulsions, decreases the number of paroxysms in 1 mouse, reducing the % of animals with tonic convulsions, the severity of seizures as well as the duration of the convulsive period. In terms of the effect on individual indicators, the combination statistically significantly outweighs the effectiveness of monotherapy with both digoxin and sodium valproate at ½ ED50.
The effectiveness of levetiracetam at both doses in the model of camphor-induced seizures was almost the same (Table 4). At both ED50 and ½ ED50, the drug is significantly preventing animal lethality (p < 0.01) and also statistically significantly reduces the duration of the convulsive period (p < 0.05) compared with control. The only difference is the significant additional reduction in the number of clonic-tonic paroxysms in 1 mouse when using levetiracetam at a high dose.
The addition of digoxin to levetiracetam at ½ ED50 does not affect the severity of the anticonvulsant effect of classical AED – the advantage of the combination at zero animal lethality is only statistically significant (both for the control group and for digoxin per se ) reduction in the number of clonic-tonic seizures in 1 mouse, similar to that in the levetiracetam at ED50 group.
The anticonvulsant effect of topiramate at both studied doses under conditions of paroxysms induced by camphor has been weak (Table 4) in the absence of a significant influence on lethality is manifested only in a statistically significant reduction in the number of clonic-tonic attacks in 1 mouse. In addition, at ED50 the drug significantly reduces the duration of the convulsive period relative to control (p < 0.05).
The combined use of topiramate at ½ ED50 with digoxin shows a clear protective influence against the convulsive effect of camphor. This has been verified by the complete prevention of animal deaths – significant (p < 0.01) not only compared with control but also relative to low-dose topiramate monotherapy. In addition, the background of the combination is also determined by a statistically significant decrease in the number of clonic-tonic seizures in 1 mouse, the % of animals with tonic convulsions, the severity of paroxysms, and the duration of the convulsive period. The combination is significantly superior to digoxin monotherapy in terms of its impact on individual parameters and even the effect of topiramate at ED50 (p < 0.05) in terms of the reduction in the % of mice with tonic convulsions.
Discussion
The difference in the severity of the anticonvulsant action of classical AEDs at conditionally effective doses in the corresponding experimental models of paroxysms is obviously due to different mechanisms of the anticonvulsant effect of drugs30–32). Thus, the pronounced anticonvulsant effect of sodium valproate and levetiracetam in the model of picrotoxininduced seizures confirms the presence of GABAergic properties in drugs33). The anti-glutamatergic effect was confirmed under thiosemicarbazideinduced seizures for levetiracetam and topiramate34). Significant effects of sodium valproate, levetiracetam, and topiramate on glycinergic neurotransmission in the strychnine antagonism test were verified. In addition, sodium valproate and levetiracetam (to a lesser extent topiramate) have been shown to have modulating properties for camphor-induced paroxysms, which may be due to the effect of drugs on the energy metabolism of neurons as well as cerebral monoamine balance.
The pronounced anticonvulsant effect of digoxin per se, as well as the ability of the drug to potentiate the weak anticonvulsant potential of sub-effective doses of classic AEDs sodium valproate, lamotrigine, and topiramate in all studied experimental models of seizures may indicate multiple mechanisms of action of cardiac glycoside: blockade of the effects of glutamate, normalization of energy metabolism of neurons, etc. On the other hand, given the known tropism of cardiac glycosides to a key enzyme supporting the membrane potential of excitable tissues (including cardiomyocytes and neurons) – Na+-, K+-ATPase35–38) – it is reasonable to assume that the anticonvulsant action of digoxin is likely to be due to resting potential due to the normalization of transmembrane sodium currents.
The obtained results indicate the expediency of further in-depth study of the mechanisms of realization of the anticonvulsant potential of cardiac glycosides (primarily digoxin), in particular, verification of the role of neuronal Na+-, K+-ATPase, endogenous digitalis factor, neuroactive amino acids, cytokines, neurotrophins, etc.
Thus, experimental models of primary generalized paroxysms with different neurochemical mechanisms have shown that digoxin has a stable anticonvulsant effect and enhances the anticonvulsant potential of sub-effective doses of classical antiepileptic drugs sodium valproate, levetiracetam and topiramate.
Conclusions
The impact of the cardiac glycoside digoxin in a sub-cardiotonic dose on the spectrum of anticonvulsant action of the classic commonly used antiepileptic drugs – sodium valproate, levetiracetam and topiramate has been studied. It was found that under the conditions of primary generalized seizures induced by picrotoxin, thiosemicarbazide, strychnine and camphor, digoxin causes a strong anticonvulsant effect per se at the same time significantly enhancing the anticonvulsant potential of classical antiepileptic drugs with various mechanisms of action: sodium valproate, levetiracetam and topiramate. The obtained results substantiate the expediency of further research of digoxin as an anticonvulsant drug, particularly the in-depth study of the neurochemical mechanisms of its action.
Conflicts of interest: none.
Received January 25, 2022 / Accepted March 30, 2022
Vadim Tsyvunin, PhD • S. Shtrygol’ • M. Mishchenko
Department of Pharmacology and Pharmacotherapy
National University of Pharmacy
Pushkinska str. 53, 61002 Kharkiv, Ukraine
e-mail: tsyvunin-vad@ukr.net
D. Shtrygol’
Department of Neurology, Psychiatry, Narcology and Medical Psychology
School of Medicine, V. N. Karazin Kharkiv National University, Kharkiv, Ukraine
Sources
1. Abramovici S., Bagić A. Epidemiology of epilepsy. Handb. Clin. Neurol. 2016; 138, 159–171.
2. Kalilani L., Sun X., Pelgrims B., Noack-Rink M., Villanueva V. The epidemiology of drug-resistant epilepsy: A systematic review and meta-analysis. Epilepsia 2018; 59(12), 2179–2193.
3. Pérez-Pérez D., Frías-Soria C. L., Rocha L. Drug-resistant epilepsy: From multiple hypotheses to an integral explanation using preclinical resources. Epilepsy & behavior 2021; 121(Pt B), 106430.
4. Łukawski K., Czuczwar S.J. Understanding mechanisms of drug resistance in epilepsy and strategies for overcoming it. Expert opinion on drug metabolism & toxicology 2021; 17(9), 1075–1090.
5. Borowicz K. K., Banach M. Antiarrhythmic drugs and epilepsy. Pharmacol. Reports 2014; 66(4), 545–551.
6. Zeiler F. A., Zeiler K. J., Kazina C. J., Teitelbaum J., Gillman L. M., West M. Lidocaine for status epilepticus in adults. Seizure 2015; 31, 41–48.
7. Łukawski K., Jakubus T., Janowska A., Raszewski G., Czuczwar S. J. Enalapril enhances the anticonvulsant activity of lamotrigine in the test of maximal electroshock. Pharmacol. Reports 2013; 65(4), 1012–1017.
8. Elgarhi R., Shehata M. M., Abdelsameea A. A., Salem A. E. Effects of Diclofenac Versus Meloxicam in Pentylenetetrazol - Kindled Mice. Neurochem. Res. 2020; 45(8), 1913–1919.
9. Suemaru K., Yoshikawa M., Tanaka A., Araki H., Aso H., Watanabe M. Anticonvulsant effects of acetaminophen in mice: Comparison with the effects of nonsteroidal anti-inflammatory drugs. Epilepsy Res. 2018; 140, 22–28.
10. Erdogan M. A., Yusuf D., Christy J., Solmaz V., Erdogan A., Taskiran E., et al. Highly selective SGLT2 inhibitor dapagliflozin reduces seizure activity in pentylenetetrazol - induced murine model of epilepsy. BMC Neurol. 2018; 18(1), 81.
11. Yimer E. M., Surur A., Wondafrash D. Z., Gebre A. K. The Effect of Metformin in Experimentally Induced Animal Models of Epileptic Seizure. Behav. Neurol. 2019; 2019, 6234758.
12. Quintana-Pájaro L. J., Ramos-Villegas Y., Cortecero - Sabalza E., Joaquim A. F., Agrawal A., Narvaez - Rojas A. R., et al. The Effect of Statins in Epilepsy: A Systematic Review. J. Neurosci. Rural. Pract. 2018; 9(4), 478–486.
13. Scicchitano F., Constanti A., Citraro R., de Sarro G., Russo E. Statins and epilepsy: preclinical studies, clinical trials and statin-anticonvulsant drug interactions. Curr. Drug Targets 2015; 16(7), 747–756.
14. Shtrygol’ S., Shtrygol’ D. Digoxin as an antiepileptic in children (clinical and experimental study). Ukrainian Medical Almanac 2010; 13, 164.
15. Tsyvunin V., Shtrygol’ S., Shtrygol’ D. Digoxin enhances the effect of antiepileptic drugs with different mechanism of action in the pentylenetetrazole-induced seizures in mice. Epilepsy Res. 2020; 167, 106465.
16. Tsyvunin V., Shtrygol’ S., Shtrygol’ D., Mishchenko M., Kapelka I., Taran A. Digoxin potentiates the anticonvulsant effect of carbamazepine and lamotrigine against experimental seizures in mice. Thai J. Pharm. Sciences 2021; 45(3), 165–171.
17. Tsyvunin V., Shtrygol’ S., Havrylov I., Shtrygol’ D. Low-dose digoxin enhances the anticonvulsive potential of carbamazepine and lamotrigine in chemo-induced seizures with different neurochemical mechanisms. ScienceRise: Pharm. Science 2021; 6(34), 58–65.
18. Duveau V., Pouyatos B., Bressand K., Bouyssières C., Chabrol T., Roche Y., et al. Differential Effects of Antiepileptic Drugs on Focal Seizures in the Intrahippocampal Kainate Mouse Model of Mesial Temporal Lobe Epilepsy. CNS neuroscience & therapeutics 2016; 22(6), 497–406.
19. Markova I. V., Mikhaĭlov I. B., Guzeva V. I. Digoksin - aktivnoe protivoépilepticheskoe sredstvo [Digoxin – an active antiepileptic agent]. Farmakologiia i toksikologiia 1991; 54(5), 52–54.
20. Hock F. J. Drug Discovery and Evaluation: Pharmacological Assays. Switzerland: Springer International Publishing 2016.
21. Mironov A. N., Bunyatyan N. D., Vasileva A. N. Guidelines for conducting pre-clinical trials of medicines. Part one. Moscow: Grif and K. 2012.
22. Olsen R. W. Picrotoxin-like channel blockers of GABAA receptors. Proceedings of the National Academy of Sciences of the United States of America 2006; 103(16), 6081–6082.
23. Santos M. S., Gonçalves P. P., Carvalho A. P. Effect of ouabain on the gamma-[3H]aminobutyric acid uptake and release in the absence of Ca(+)+ and K(+)-depolarization. J. Pharmacol. Exp. Ther. 1990; 253(2), 620–627.
24. Salazar P., Tapia R. Epilepsy and hippocampal neurodegeneration induced by glutamate decarboxylase inhibitors in awake rats. Epilepsy Res. 2015; 116, 27–33.
25. Otter J., D’Orazio J. L. Strychnine Toxicity. In StatPearls. StatPearls Publishing 2021.
26. Park T. J., Seo H. K., Kang B. J., Kim K. T. Noncompetitive inhibition by camphor of nicotinic acetylcholine receptors. Biochem. Pharmacol. 2001; 61(7), 787–793.
27. Narayan S., Singh N. Camphor poisoning-An unusual cause of seizure. Medical Journal, Armed Forces India 2012; 68(3), 252–253.
28. Zadvornov А. А., Golomidov А. V., Grigoriev E. V. Clinical pathophysiology of cerebral edema (part 2). Messenger of Anesthesiol. Resus. 2017; 14(4), 52–60.
29. Wei D., Peng J. J., Gao H., Li H., Li D., Tan Y., et al. Digoxin downregulates NDRG1 and VEGF through the inhibition of HIF-1α under hypoxic conditions in human lung adenocarcinoma A549 cells. Int. J. Mol. Sci. 2013; 14(4), 7273–7285.
30. Löscher W. The holy grail of epilepsy prevention: Preclinical approaches to antiepileptogenic treatments. Neuropharmacology 2020; 167, 107605.
31. Perucca E. Antiepileptic drugs: evolution of our knowledge and changes in drug trials. Epileptic disorders: international epilepsy journal with videotape 2019; 21(4), 319–329.
32. Löscher W., Klein P. The Pharmacology and Clinical Efficacy of Antiseizure Medications: From Bromide Salts to Cenobamate and Beyond. CNS Drugs 2021; 35(9), 935–963.
33. Waller D., Sampson A. Medical Pharmacology and Therapeutics. 5th ed. U.K.: Elsevier 2017.
34. Sills G. J., Rogawski M. A. Mechanisms of action of currently used antiseizure drugs. Neuropharmacology 2020; 168, 107966.
35. Patocka J., Nepovimova E., Wu W., Kuca K. Digoxin: Pharmacology and toxicology-A review. Environmental toxicology and pharmacology 2020; 79, 103400.
36. de Lores Arnaiz G. R., Ordieres M. G. Brain Na(+), K(+)-ATPase Activity In Aging and Disease. Int. J. Biomed. Sci. 2014; 10(2), 85–102.
37. Funck V. R., Ribeiro L. R., Pereira L. M., de Oliveira C. V., Grigoletto J., Della-Pace I. D., et al. Contrasting effects of Na+, K+-ATPase activation on seizure activity in acute versus chronic models. Neuroscience 2015; 298, 171–179.
38. Krishnan G. P., Filatov G., Shilnikov A., Bazhenov M. Electrogenic properties of the Na+/K+ ATPase control transitions between normal and pathological brain states. J. Neurophysiol. 2015; 113(9), 3356 – 3374.
Labels
Pharmacy Clinical pharmacology
Article was published inCzech and Slovak Pharmacy

2022 Issue 2-
All articles in this issue
- Verejní lekárnici a správna aplikácia inhalačných liekov
- Capillary zone electrophoresis in combination with UV detection for simultaneous determination of tramadol and paracetamol in pharmaceutical and biological samples
- Beliefs and knowledge related to nutritional supplements among pharmacy students
- Digoxin at sub-cardiotonic dose modulates the anticonvulsive potential of valproate, levetiracetam and topiramate in experimental primary generalized seizures
- Czech and Slovak Pharmacy
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Capillary zone electrophoresis in combination with UV detection for simultaneous determination of tramadol and paracetamol in pharmaceutical and biological samples
- Verejní lekárnici a správna aplikácia inhalačných liekov
- Digoxin at sub-cardiotonic dose modulates the anticonvulsive potential of valproate, levetiracetam and topiramate in experimental primary generalized seizures
- Beliefs and knowledge related to nutritional supplements among pharmacy students
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

