-
Medical journals
- Career
Study of biocompatibility of peritoneal dialysis solutions measured as in vitro cells viability
Authors: Nataliia Hudz; Anna Filipska; Nataliia Stepaniuk; Nataliia Dmytrukha; Raisa Korytniuk; Piotr P. Wieczorek
Published in: Čes. slov. Farm., 2019; 68, 161-172
Category: Original Article
Článek představuje výsledky srovnatelných testů životaschopnosti buněk HepG2 a Vero v přítomnosti tradičních roztoků pro peritoneální dialýzu (PD) provedené třemi metodami, a to pomocí (3-[4,5-dimethylthiazol]--2-yl-2,5-difenyltetrazolium bromidu (MTT), neutrální červeně (NČ) a sulforhodaminu B, se stanovením různých korelací mezi životaschopností a indexy kvality testovaných roztoků pro PD.
Overview
This paper presents the comparable viability study results of the HepG2 and Vero cells in the presence of traditional peritoneal dialysis (PD) solutions determined by three methods (3-[4,5-dimethylthiazol]-2-yl-2,5-diphenyl tetrazolium bromide (MTT), neutral red (NR) and sulforhodamine B assays) with establishing different correlations between viability and quality indexes of the tested PD solutions. The obtained results confirmed cytotoxicity of the PD solutions even compared with an isotonic solution of sodium chloride. PD solutions action resulted in a similar reduction in the HepG2 and Vero cells. Moreover, this research found that metabolic cellular activity is more vulnerable to the action of PD solutions measured in the MTT-test. One more point is that cytotoxicity is related to pH of a solution and other unknown mechanisms, while glucose degradation products, glucose or lactate did not exert an exceptional negative action on PD solutions cytotoxicity. It is concluded that MTT-test is the best suitable for comparative studies of PD solutions which differ in pH values
Keywords:
solutions for peritoneal dialysis – viability – HepG2 – Vero cells – neutral red – MTT – sulforhodamine B
Introduction
Issues of biocompatibility studies of solutions for peritoneal dialysis (PD) are essential from a point of view of saving peritoneum functions and finding factors influencing biocompatibility of PD solutions1–7). The bioincompatibility of PD solutions is associated with glucose degradation products (GDPs), which are formed during thermal sterilization of solutions, an absence of nutrients for cells in these solutions, unphysiological glucose concentrations, low pH values, and high osmolarity of PD solutions for the peritoneum (5.0–6.5), etc.2, 3, 8, 9). As a result of such bioincompatibility, there is an increased vulnerability of the peritoneal cavity cells, an increased chronic inflammation, pain when administering solutions to the peritoneal cavity, damaged immune defence of patients suffering from the fifth stage of chronic kidney disease2, 10). Peritonitis and peritoneal sclerosis are complications because of the PD solutions bioincompatibility3, 10, 11). Therefore, the issues of biocompatibility of PD solutions are important during the development of these solutions, their production and medical administration. One of the modern directions of studying the PD solutions safety is basal cytotoxicity investigation of these solutions, a comparison of basal cytotoxicity of different PD solutions, and establishing correlations between measured basal cytotoxicity and physical and chemical parameters of these solutions as well1–11).
In vitro viability studies are expected to remain vital laboratory tools when multiple medicinal products are tested for antifibroblastic and cytostatic effects, xenobiotic-induced cytotoxicity using mouse peritoneal macrophages12), human leukemia T cell13), HepG2 cells14), human hepatocytes15), normal human keratinocytes16), the human colorectal cell-line HT2917), P19 embryonic stem cells18), NIH3T3, human cancer (AGS, HT-29, MCF-7 and MDA-MB-231)19), Vero cell lines19, 20), L929 lines, etc.21–25).
The first technique uses a yellow tetrazolium dye (3-[4,5-dimethylthiazol]-2-yl-2,5-diphenyl tetrazolium bromide (MTT) that is converted into insoluble purple formazan crystals in metabolically active cells13, 18, 21, 24); namely MTT reduction is used to assess enzymes activity of viable cells22, 24). The higher the amount of formazan detected, the higher the number of viable and metabolically active cells21).
The second assay utilizes neutral red (2-methyl-3-amino-7-dimethyl-amino-phenazine) (NR), a weak basic dye which is selectively concentrated in living cells lysosomes. Lysosomes are cytoplasmic membrane-enclosed organelles containing hydrolytic enzymes that degrade macromolecules and cell components. This acidic milieu of lysosomes (pH ≤ 5) is maintained by a vacuolar ATPase that pumps protons from the cytoplasm into the lysosomal lumen12, 23, 26). The NR dye penetrates through cell membranes by nonionic passive diffusion and concentrates in the lysosomes binding by electrostatic hydrophobic bonds to anionic and/or phosphate groups of the lysosomal matrix. The NR assay allows to assess membrane permeability, lysosomal integrity and activity, making it possible to differentiate viable, damaged, or dead cells. Alterations of the cell surface or the sensitive lysosomal membrane lead to lysosomal fragility and result in a decreased uptake and binding of NR14, 16, 23, 26, 27). The sulforhodamine B (SRB) assay relies on the ability of SRB to bind to amino groups of cells proteins22, 24).
Some publications provide more than one test for the evaluation of chemicals cytotoxicity with the purpose of studying action mechanisms of tested compounds, influence of different factors which can interfere with results of cells viability and/or a comparison of results obtained by means of different techniques, etc.13, 25, 24, 28).
Currently, the development of the composition and technology of PD solutions or a comparison of the safety of PD solutions of different manufactures is often carried out in combination with the study of cells viability in the presence of PD solutions1, 3, 5, 10, 29, 30).
The purpose of our work was to study the viability of the HepG2 cells and compare it with the viability of Vero cells in the presence of traditional PD solutions containing glucose and sodium lactate in single-chamber containers determined by three methods (MTT, NR and SRB assays) and establish correlations between cell viability and the values of analytical indexes of the tested solutions and between cell viability measured by different tests.
Experimental part
Materials
The objects of the study were PD solutions with the contents of lactate ions of 35 and 40 mmol/L and 1.5%, 2.5% and 4.25% of glucose monohydrate in glass and polyvinyl chloride (PVC) containers. The composition of the tested PD solutions is shown in Table 1.
1. Composition of the PD solutions 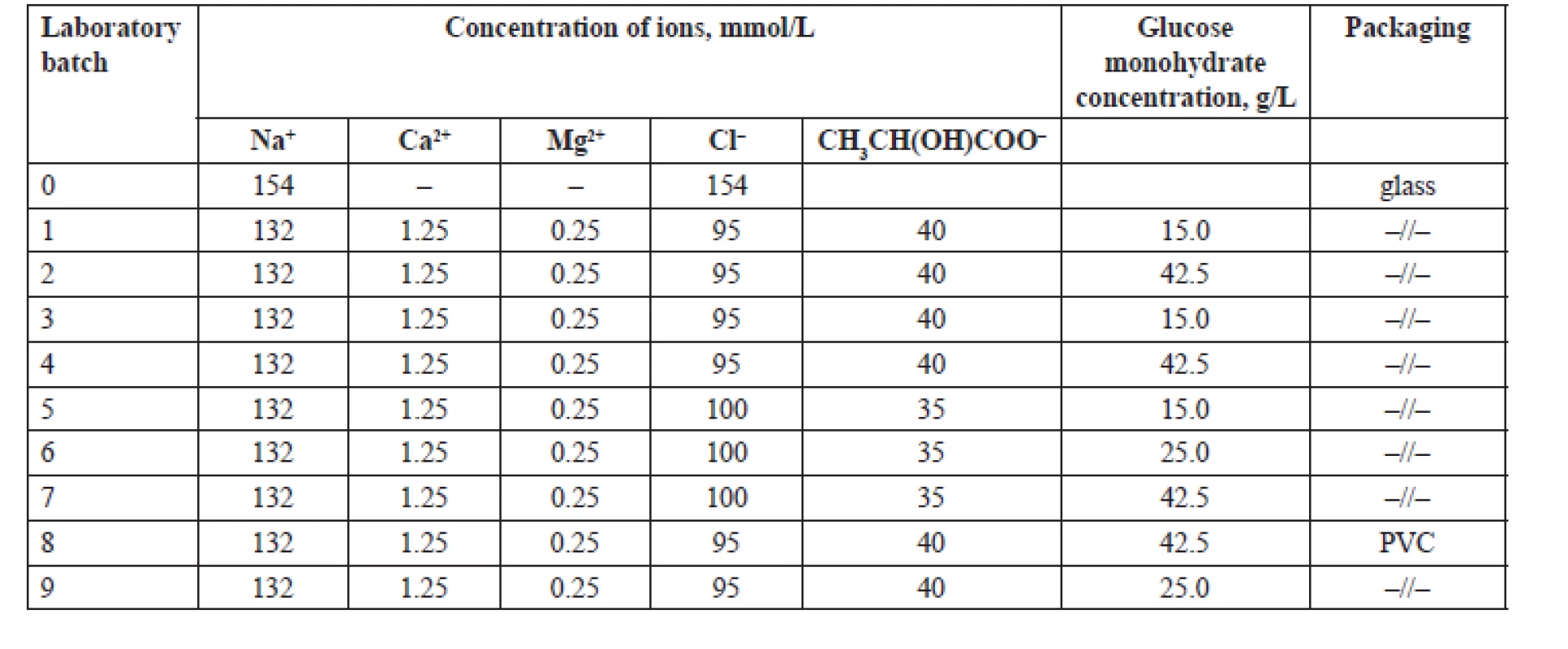
Methods
The following research methods were used: analytical methods for the determination of pH, GDPs and chloride contents, and biological methods for the determination of cell viability using the HepG2 and Vero cells, and NR-, MTT - and SRB-tests to evaluate the cytotoxic effect of the tested PD solutions using also the methods of statistical processing of results8, 31).
Analytical equipment used in this study was the following: a рН meter: “рН-150 М“ (Gomel Plant of Measuring Instruments, Belarus), burettes of I accuracy class (volume, division and measurement accuracy of burette were of 25.0 ml, 0.05 ml, and 0.03 ml, respectively), and a spectrophotometer Optizen POP (Mecasys Co. Ltd., Korea) with a 1-cm quartz cell used in the range of 200 to 350 nm.
The determination of chloride ions was performed according to the analytical procedure developed and validated by Hudz et al.8, 31): 10 ml of a PD solution (or 5 ml of an isotonic solution of sodium chloride) were titrated with a 0.1 M solution of silver nitrate using 0.8 ml of potassium chromate solution as the indicator (0.4 ml of potassium chromate solution for an isotonic solution of sodium chloride) to an orange-yellow colour, stirring constantly. The content of chloride ions should be from 95% to 105% of the stated amount32).
Spectrophotometry and pH-metric measure
Glucose degradation processes were estimated by changing the pH values, the absorbance at 228–230 nm and an absorption maximum in the wavelength range of 273 to 286 nm before and after sterilization3, 8, 31). The pH of the PD solutions was measured in the temperature range of 20 °С to 25 °С. Before measurements, the pH-meters were calibrated using buffer solutions with the values of pH 4.01, 6.87 and 9.18.
Cytotoxicity (cell viability) tests
The evaluation of the cytotoxic activity of the tested samples was carried out using three cell-based assays: MTT-test, NR uptake (NR-test), and SRB-test.
The cells of the African monkey kidney (Vero line) and HepG2 were obtained from the Institute of Microbiology and Virology of the National Academy of Sciences of Ukraine and from the cell bank of the Kavetsky Institute of Experimental Pathology, Oncology and Radiobiology of the National Academy of Sciences of Ukraine, respectively. Special equipment was used for cell culture: a Class II Biological Safety Cabinet (Thermo Scientific MSC Advantage, Germany), a binocular microscope (MC 300× MET Invert, Austria), a Thermo Scientific Revco Ultima II Midi Dry Wall CO2 Incubator (USA), a plate rotary centrifuge (Biosan LMC 300, EU), a Tecan Sunrise absorbance microplate reader (Tecan, Austria), single-dose and multichannel variable-volume dispensers from 1–1000 μl, a Goryaev camera, an electronic laboratory scale, a refrigerator with a freezer compartment, and a potentiometric analyzer with a pH measurement error within ± 0.01.
The HepG2 and Vero cells were cultured, respectively, in a medium of DMEM (Sigma) or RPMI 1640 (Sigma, USA), containing 4 mmol/L L-glutamine, 10% fetal calf serum (Sigma, USA), and 40 μg/ml gentamicin (Hemofarm AD, Serbia), in a humidified atmosphere at 37 °C (5% CO2 + 95% air). 100 μl of a suspension of cells at a density of 1 × 105 cells per ml were placed into each well of a 96-well plate. The cells were incubated for cell attachment and monolayer formation in wells. Then, the cells were treated with 100 μl of the PD solutions, their appropriate dilutions, an isotonic solution of sodium chloride and its appropriate dilutions at 37 °C for 24 hours. 100 μl of the culture medium were added to the control wells instead tested samples. The plates were incubated in a humidified atmosphere (5% CO2 + 95% air) in a thermostat at 37 °C for 24 hours. Thereafter, the cells were treated with MTT, NR or SRB for the determination of cells viability21).
Procedure of the MTT-test
A 0.5% solution of MTT in a Hanks solution (Sigma, USA) was filtered through a sterile cellulose acetate membrane filter. 10 μl of MTT solution were injected into each well. Then incubation was conducted in a thermostat for 3 hours. After the incubation the plates were centrifuged at a rate of 1500 rpm for 5 minutes, and then the supernatant fluid was removed from the wells. 50 μl of dimethyl sulfoxide (Sigma, USA) were added to each well to dissolve formazan crystals. The plates were kept at room temperature for 30 minutes, and then the absorbance of the well contents was determined at a wavelength of 540 nm using a Tecan Sunrise absorbance microplate reader (Tecan, Austria)21, 31).
Procedure of the NR-test
After incubation, the medium was carefully removed from each well. The cells were washed with 150 μl of a warm buffer solution. Then 100 μl of NR solution were added to each well. Incubation was carried out in a thermostat at 37 °C for 3 hours. After incubation the NR solution was removed and the wells were washed by 150 μl of phosphate buffer. After removing the buffer, 150 μl of extraction solution was added to each well and the plate was gently shaken on a plate shaker for 10 minutes. The absorbance of the well contents was determined at a wavelength of 540 nm using a Tecan Sunrise absorbance microplate reader (Tecan, Austria)14, 31).
Procedure of the SRB-test
After incubation the medium was carefully removed from each well. The cells were washed with a warm buffer solution with the purpose of removing all proteins which could interfere with results24). Later, a 10% solution of trichloracetic acid was added for cells fixation and a 96-well plate was kept in a refrigerator at 4 °C for 1 hour. After fixation, the wells were rinsed with water and dried in air. A 0.4% solution of SRB was added into each well and a 96-well plate was kept at room temperature for 60 min. For removing of SRB, wells were rinsed with a 1% solution of acetic acid, 100 μl of a 10 mM solution of Tris-base buffer for SRB dissolution and a 96-well plate was kept on a shaker at a rate of 250 rotations per min for 15 min. The absorbance of the well contents was determined at a wavelength of 540 nm using a Tecan Sunrise absorbance microplate reader (Tecan, Austria).
In three tests, the percentage of viability (%viability) was calculated using the formula:
- %viability = Viabilitytested/Viabilitymedium × 100%, where
- %viability – the number of viable cells in %,
- Viabilitymedium – the absorbance in the wells with a culture medium,
- Viabilitytested – the absorbance in the wells with the tested samples.
Correlation analysis
Achim Buyul and Peter Tsefel classification was used to evaluate correlation coefficients (r): up to 0.2 – very weak, up to 0.5 – weak, up to 0.7 – medium, up to 0.9 high, and over 0.9 is a very high correlation33). Similar classification was used in the paper of L. Vajrabhaya L. and S. Korsuwannawong24).
Results and discussion
The issue of improving biocompatibility of traditional PD solutions is essential as significantly expensive newer solutions demonstrated only greater urine volumes and residual renal function without other clinical advantages7).
Physicochemical tests
The pH of the tested PD solutions was in the range of 5.25–5.77 and the actual content of chlorides was in the range from 99.61% to 105% of the stated amount which met the requirements of the British Pharmacopeia for PD solutions32). The absorbance of the solutions at 228 nm ranged from 0.294 to 1.587 indicating strong fluctuations of the 3,4-DGE content. According to Kjellstrand et al.29), 3,4-DGE has an absorption maximum at 228–230 nm, and 5-HMF at 228 and 284 nm. Moreover, ultraviolet absorbance and pH are considered to be general indicators of glucose degradation3, 6, 8, 29, 31). The results of the analytical studies and cells viability of tested PD solutions are presented in Table 2.
2. Physicochemical parameters of the tested PD solutions after sterilization and viability of the HepG2 and Vero cells, X ± SD 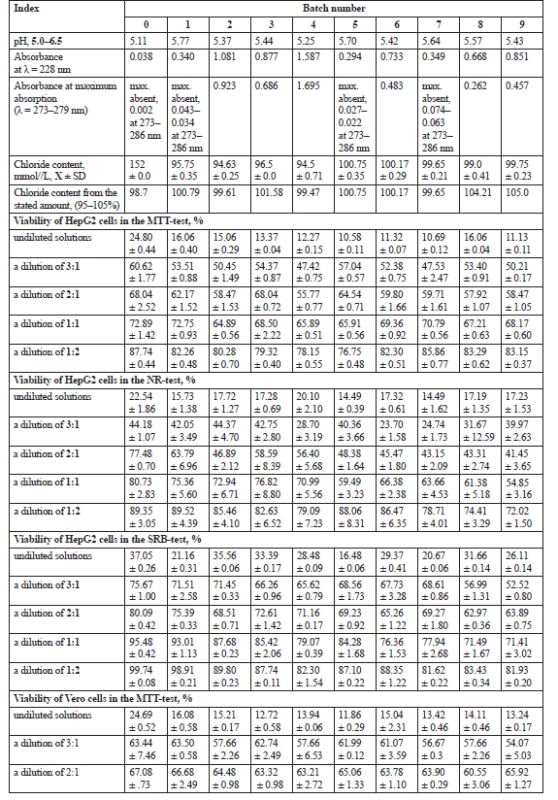
Cell-based cytotoxicity assays in vitro promise to be useful, reliable and rapid methods for demonstrating activity of medicines or detecting their toxicity24). It could be considered the standard for evaluating innovative and generic PD solutions during pharmaceutical development, existing solutions after improving their composition and manufacture or accumulation of different data by manufactures in dependence of cell viability on solutions pH, absorbance at 228 nm, 284 nm, etc. According to published data, L-929 fibroblasts, mouse fibroblast cells NIH 3T3, human peritoneal mesothelial cells (HPMC), and human leukocytes are used in the studies of PD solutions cytotoxicity measured by NR - or MTT tests1, 3, 5, 6, 30).
Under proper culture conditions, HepG2 cells show limited hepatocyte-like features and are therefore often used as an alternative in vitro model for human hepatocytes14). Vero cells are derived from the kidney of an African green monkey. They are one of the most commonly used mammalian continuous cell lines in microbiology, molecular and cell biology research, evaluation of the effects of substances on mammalian cells20).
Metabolic activity of cells is evaluated by the MTT-test. MTT tetrazolium salts determine the activity of oxidoreductases, including NADH - and NAD(P)H-dependent cellular oxidoreductase enzymes, in the cytoplasm, various mitochondrial dehydrogenase enzymes (succinate dehydrogenase), and dehydrogenases associated to the endoplasmic reticulum, endosome/lysosome vesicles, and cytoplasmic membrane6, 13, 21, 24, 28, 34).
The uptake of NR depends on the capacity of the cells to maintain pH gradients through the production of ATP. At physiological pH, the dye presents a net charge close to zero, enabling it to penetrate cell membranes by diffusion. Inside lysosomes, there is a proton gradient to maintain pH lower than that of the cytoplasm. Thus, the dye is trapped by protonation inside lysosomes, becomes charged and is accumulated there as membranes are impermeable or very slightly permeable for the protonated form of NR. NR is only taken up and retained by lysosomes present in healthy cells as misbalancing pH in lysosome matrix of died cells does not retain NR there. Consequently, the amount of the retained dye is proportional to the number of viable cells12, 14, 23, 27). The pH dependence of uptake is of great interest as a change in pH induces a change in the ratio of the free base to the protonated form12).
SRB-method relies on the property of SRB to bind stoichiometrically to amino groups of cell proteins under mild acidic conditions and then it can be extracted under basic conditions. Thus, the amount of the bound dye can be used as a marker of cell mass22, 24, 35).
This study established the influence of some factors on the biocompatibility of PD solutions in vitro: type of cells, test of the viability determination, pH, etc. The action of PD solutions regardless of the site or action mechanism results in a reduction in the HepG2 and Vero cells number. Our study confirms the data on cytotoxicity of tested undiluted PD solutions on cell cultures even in a comparison with an isotonic sodium chloride solution. Our results are in line with data of Wieslander et al.3), Erixon et al.5), Witowski et al.6), Distler et al.30). Additionally, our study demonstrates the cells viability in the presence of the undiluted and diluted PD solutions and an isotonic solution of sodium chloride in three tests compared to studies of the above-mentioned authors who performed studies with diluted solutions using only one method (NR - or MTT-test).
The tested solutions have been found to exert a negative effect on the membrane permeability and lysosome activity, metabolic activity of the cells and cells ability to synthetize proteins. The viability levels of both cells types can be ranked in the descending order depending on the test as follows: SRB > NR > MTT. Our findings suggest that such a rank indicates that metabolic activity of the cells is the most vulnerable to PD solutions and/or Vero and HepG2 cells have relatively low enzyme levels or activity in vitro. Our studies are in line with Perez et al.35), who stated that the IC50 values of compounds tested using the SRB - and MTT-tests were slightly higher in the first-mentioned test. Probably, this phenomenon could be explained by saying that MTT detects only viable cells, whereas the SRB method does not distinguish between viable and dead cells17, 22).
As can been seen from Table 2, the cells viability does increase at the solutions dilutions. Such regularity was found: the larger the dilution, the higher the viability of both types of the cells in three tests.
The tested solutions at a dilution of 3 : 1 showed a slightly less damaging effect on the membrane - and metabolic cellular activity and ability to protein synthesis. The HepG2 cell viability was in the range of 47.42% ± 0.75% to 57.04% ± 0.57% in the MTT-test, 23.70% ± 1.58% to 44.37% ± 4.70% in the NR-test, and 52.52% ± 0.80% to 71.51% ± 2.58% in the SRB-test. The number of cells was from 54.07% ± 5.03% to 63.50% ± 0.58% in the MTT-test, from 36.39% ± 1.91% to 59.82% ± 0.72% in the NR-test31), and from 32.65% ± 0.42% to 56.27% ± 0.69% in the SRB-test. The viability levels of the cells can be ranked in the descending order depending on the test as follows: SRB > MTT > NR and MTT > NR > SRB for the HepG2 and Vero cells, respectively.
The number of the cells gradually increased at the further dilutions of the tested PD solutions with the medium and it was the highest at a dilution of 1 : 2. The tested solutions at the last dilution of 1 : 2 showed the least damaging effects on the cells. The HepG2 cell viability was in the range of 76.75% ± 0.55% to 85.86% ± 0.77% in the MTT-test, 72.02% ± 1.5% to 89.52% ± 4.39% in the NR-test, and 81.62% ± 0.22% to 98.91% ± 0.21% in the SRB-test. The same effects were observed in the Vero cells using three viability tests. The number of these cells was from 87.67% ± 1.16% to 97.04% ± 0.58% in the MTT-test, 87.00% ± 0.66% to 97.47% ± 0.07% in the NR-test31), and 53.49% ± 1.53% to 88.85% ± 4.10% in the SRB-test. The viability levels of the cells can be ranked in the descending order depending on the test as follows: SRB > MTT ≥ NR for HepG2 cells, which almost conforms with undiluted solutions and MTT ≥ NR > SRB for the Vero cells.
Assessing the HepG2 viability and comparing the results with the Vero cells viability, there were weak but predicted positive relationships (0.35 and 0.32) between viability and pH in the MTT-test (the higher pH of the solutions, the higher cells viability) and negative unpredicted relationships in the NR - (–0.88 and –0.50) and SRB-tests (–0.72 and –0.50), respectively (Fig. 1). The estimation of the Vero cells viability depending on the pH solutions was presented previously31).
1. Estimation of the HepG2 cells viability depending on the pH of the solutions 
As demonstrated by Fig. 1 and a similar dependence of the Vero cells viability depending on the pH solutions8), only the MTT-test gave a predicted but non-significant relationship between an increase in both cell types viability and an increase in solutions pH. An increase in the viability at a pH decrease in the NR-test could be explained by various reasons, especially that stress caused by exposure to PD solutions could induce an increase in lysosome production to help combat and protect cells from cell death23) and/or lower pH in lysosomes, the cells of which were treated by solutions with lower pH and, respectively, increased in NR accumulation there12). It could be also postulated that an increase in the viability at pH decrease in the SRB-test could be explained that a degree of binding SRB with protein is a slightly higher at lower values of intracellular pH not expressing increased cells mass22).
Our studies are in line with those of Wieslander et al. who established that there was 100% death of cells at pH of PD solutions of 5.53). However, it is very hard to state to what degree inhibition effects could be attributed to pH alone, or may be partly due to a combination of low pH and other known (GDPs, unphysiological concentrations of lactate and glucose, high osmolarity, etc.) and not known factors3, 10, 36), including duration of end-stage renal disease, diabetes mellitus, and time on PD in vivo studies2).
The issue of pH and lactate influence on cells is very complicated. According to Liberek et al.36), the incubation of polymorphonuclear leukocytes in PD solutions containing 35 mM lactate at pH 5.2 resulted in an immediate and profound lowering in intracellular pH and inhibition of respiratory burst activation. However, lowering in intracellular pH was not observed in lactate-containing solutions at neutral pH or at low pH in the absence of lactate36). It could be supposed that lowering in intracellular pH led to an increased diffusion of NR through the biological membranes and, respectively, enhancing accumulation in lysosomes that is related to an increased absorbance, but not related with a plausible amount of viable cells. Moreover, the necessity of measuring pH of samples in the NR-test is indicated in reference16). It is could be also postulated that lowering in intracellular pH led to an increased degree of binding SRB with proteins and, respectively, enhancing amount of extracted SRB that is not related with a plausible amount of viable cells as well. Some authors offer to study neutralized PD solutions in vitro studies in order to eliminate inhibitory effects related to acidic pH3).
There were non-significant unpredicted relationships (–0.12 and –0.20) between viability and GDPs concentration expressed by the absorbance at 228 nm in the MTT-test and strong and middle positive (the more was the concentration, the higher was the viability) unpredicted relationships in the NR-test (0.96 and 0.50) and middle and weak unpredicted relationships (0.66 and 0.33) in the SRB-test, respectively, with the HepG2 and Vero cells (Figs. 2 and 3).
1. Estimation of the HepG2 cells viability depending on the absorbance solutions at 228 nm 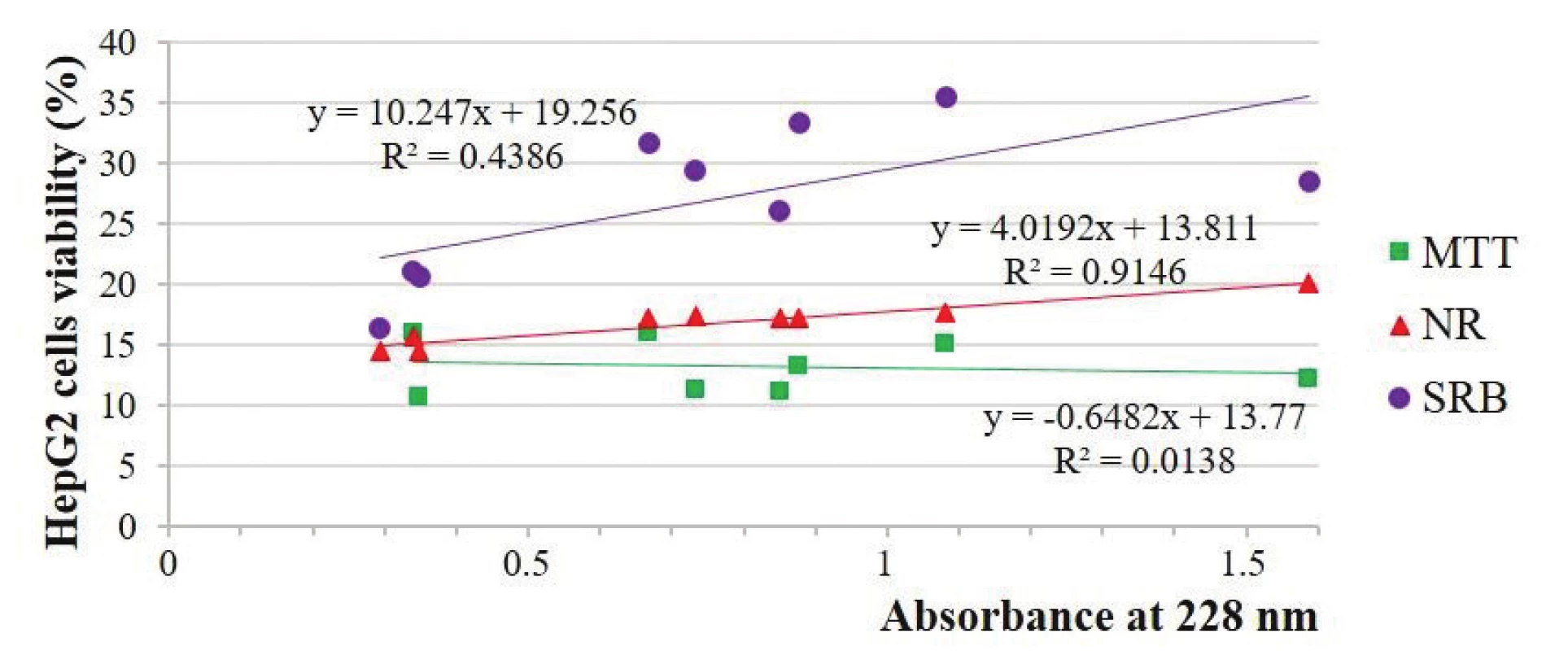
2. Estimation of Vero cells viability depending on the absorbance solutions at 228 nm 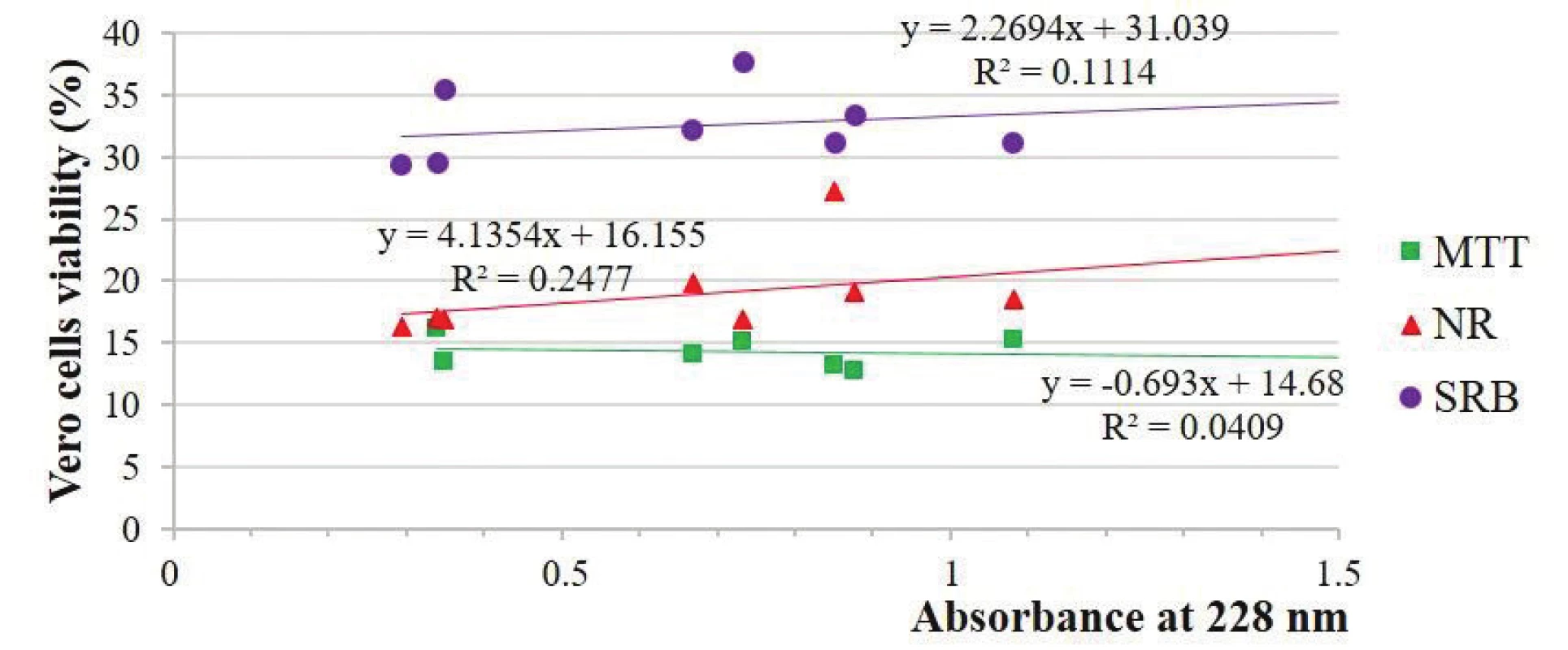
Our studies are partly in line with those of Dioos et al.1) who established that there was a negative correlation between the inhibition of cell growth and GDPs concentration for three solutions of one manufacturer. These authors did not reveal an exceptionally strong effect of PD solutions on the basal cytotoxicity1). Moreover, Distler et al. found that after one day incubation, all tested solutions (sterile-filtered, heat sterilized and sterile-filtered with adding glyoxal and methylglyoxal, 3-deoxyglucosone, 3-deoxygalactosone, glucosone, and 3,4-dideoxyglucosone-3-en) showed cell growth30).
There were positive unpredicted relationships between the viability of the HepG2 and Vero cells and glucose concentration: 0.12 and 0.20 in the MTT-test, 0.37 and 0.14 in the NR-test and 0.37 in the two SRB-tests, respectively (Figs. 4 and 5).
3. Estimation of HepG2 cells viability depending on the glucose concentration of the solutions 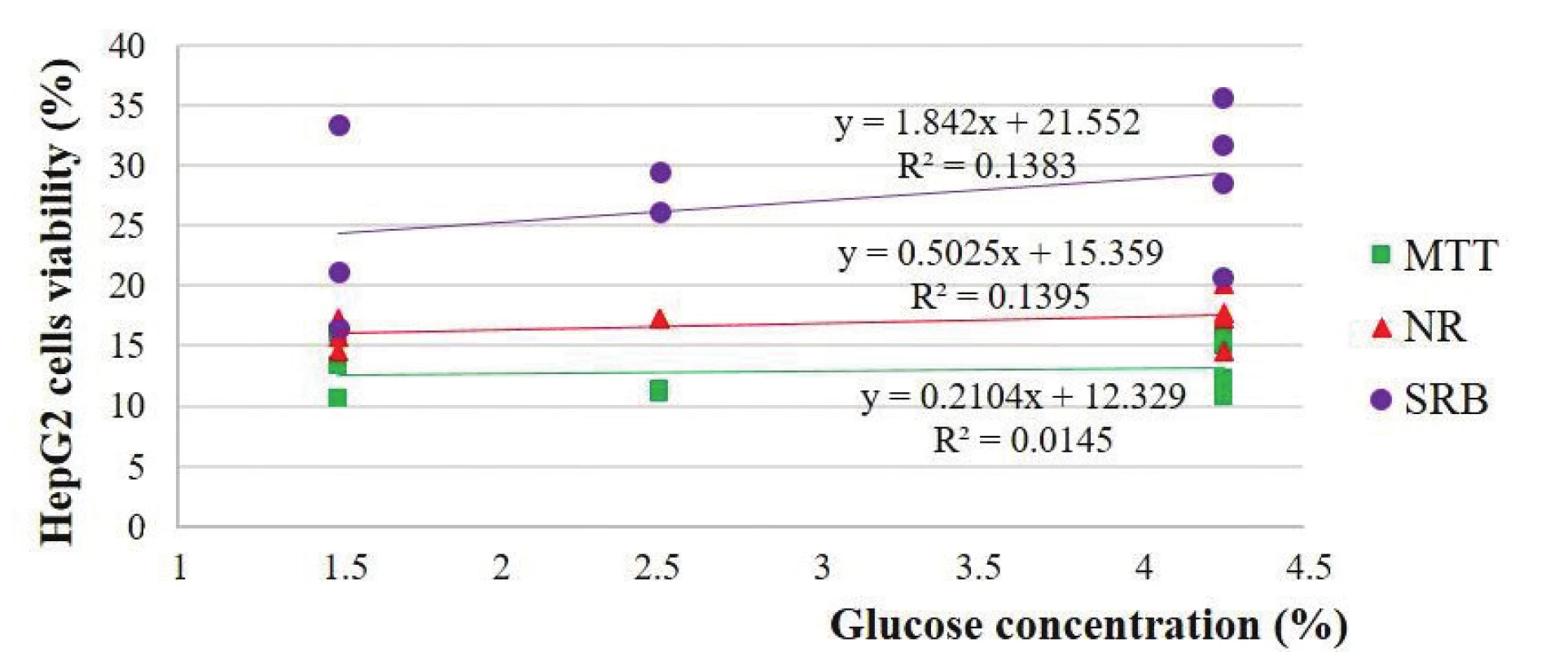
4. Estimation of Vero cells viability depending on the glucose concentration of the solutions 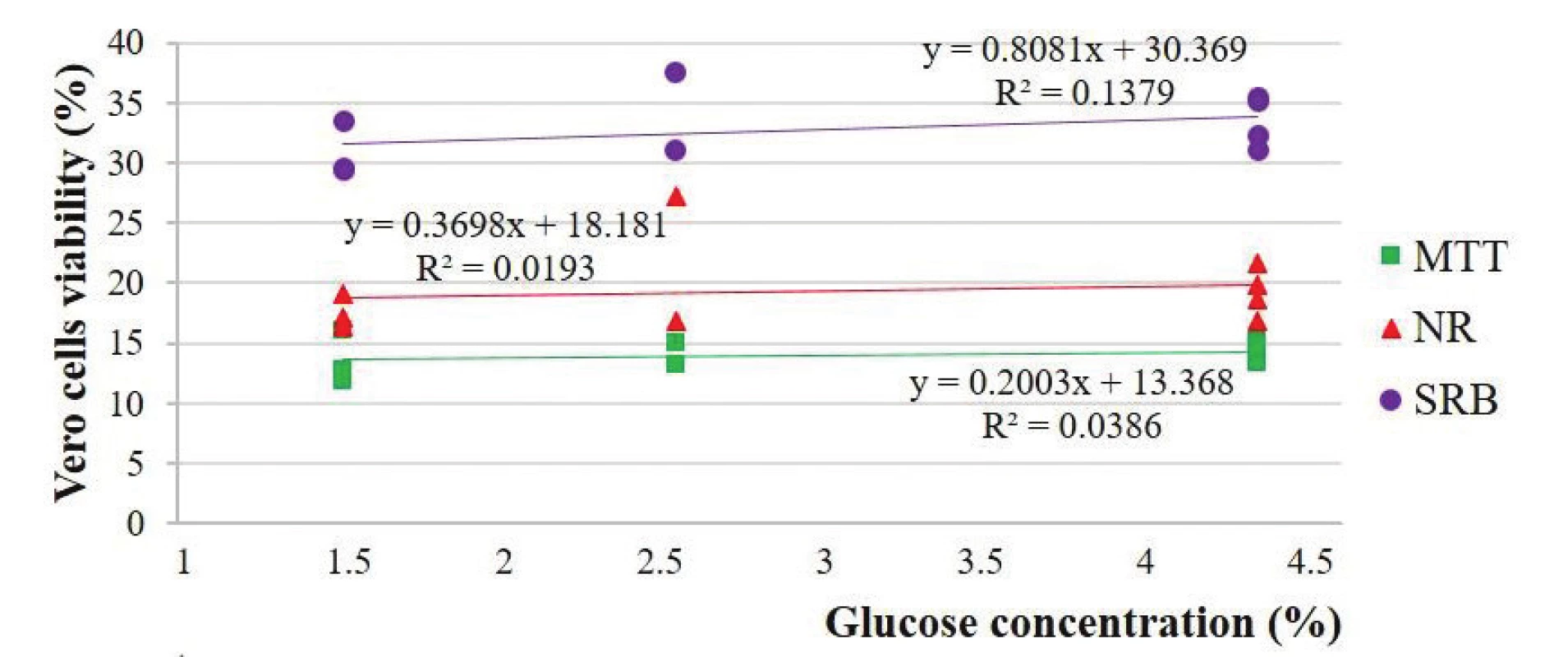
Our studies are in partial agreement with those of Dioos et al., who showed a negative correlation between inhibition of cell growth and glucose concentration for three solutions of one manufacturer1). According to Distler, all the tested solutions showed continuous cell growth after one day incubation. These authors explained the observed cell proliferation by the possible high glucose concentration in the samples30). This could explain slightly positive correlations between the viability of the HepG2 and Vero cells and glucose concentration.
There were also unpredicted relationships between the viability of the HepG2 and Vero cells and lactate concentration: 0.69 and 0.29 in the MTT-test, 0.60 and 0.56 in the NR-test and 0.56 and –0.36 in the two SRB-tests, respectively (Figs. 6 and 7).
5. Estimation of the HepG2 cells viability depending on the lactate concentration of the solutions 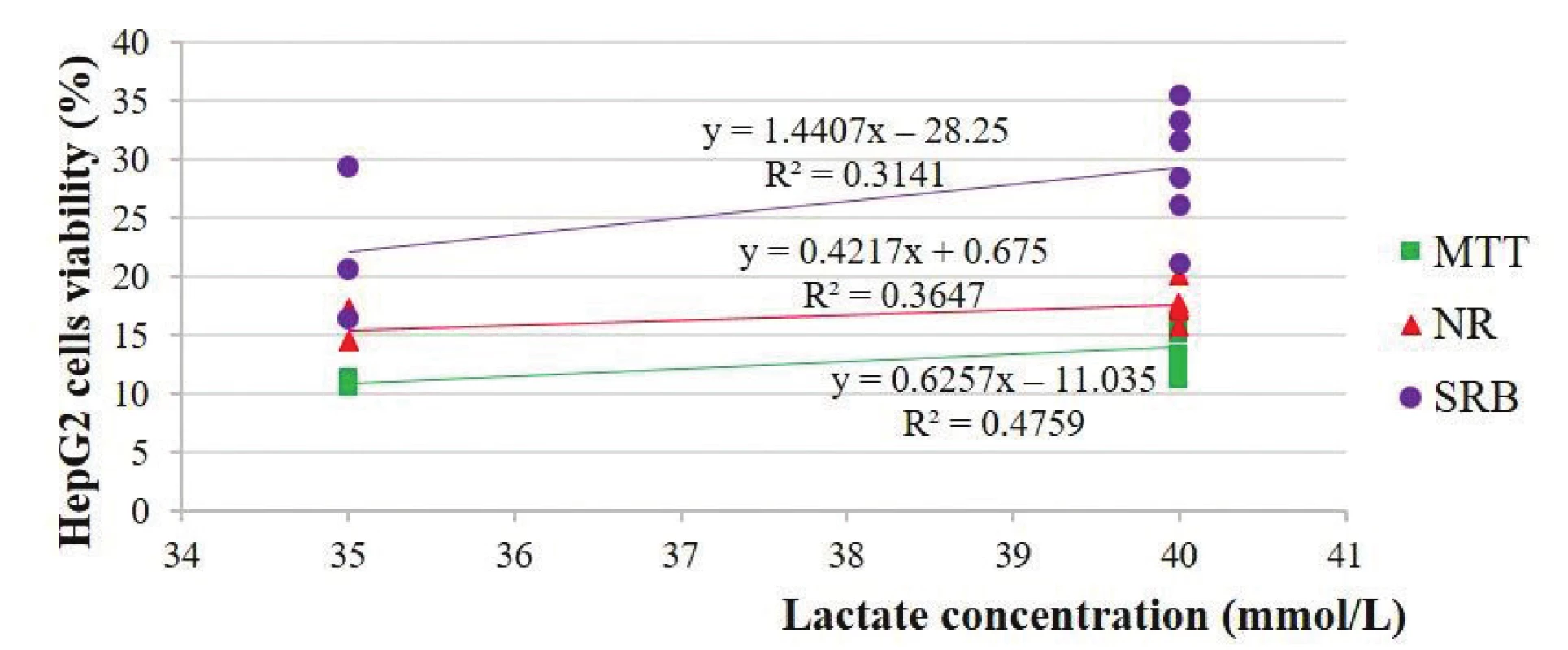
6. Estimation of the Vero cells viability depending on the lactate concentration solutions 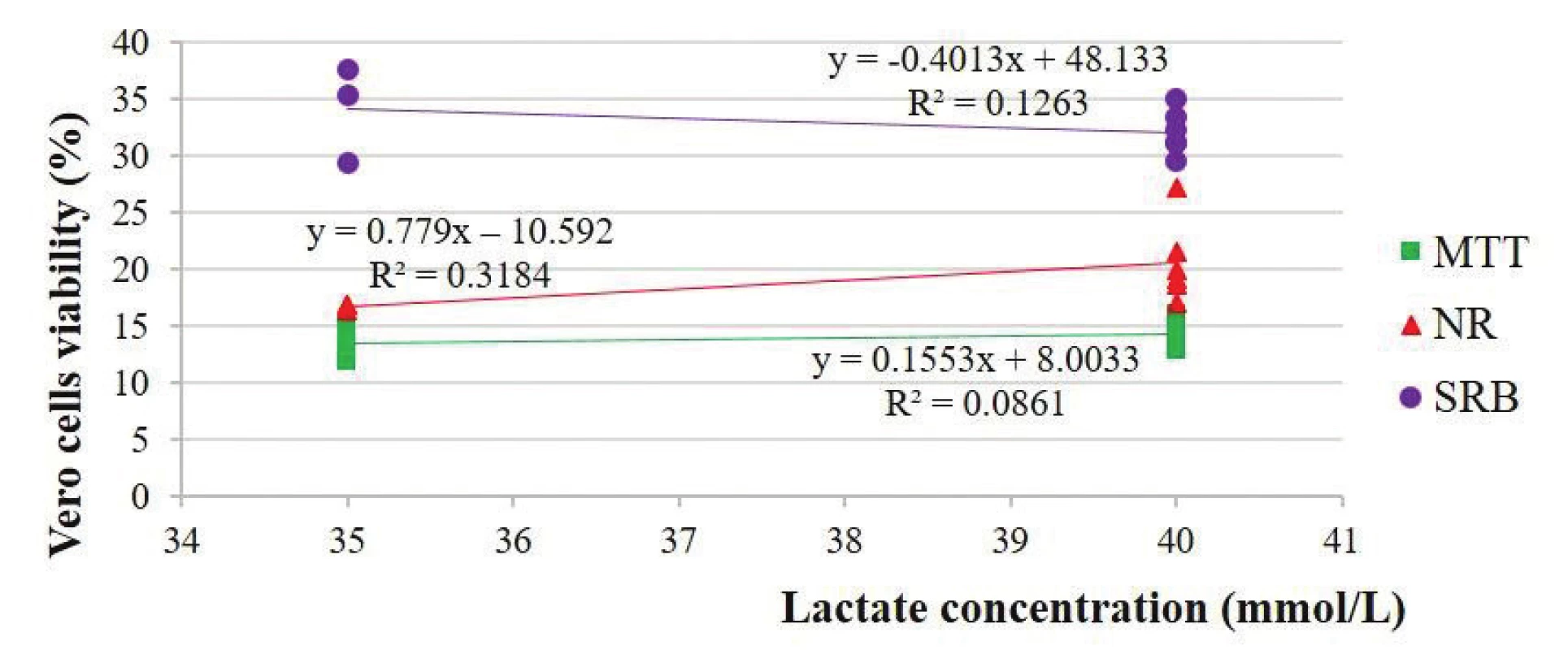
Our studies are also in line with the studies of Al-Hwiesh et al.2) who demonstrated that both solutions (lactated buffered solution with high levels of GDPs and bicarbonate/lactate-buffered PD solution with a low level of GDPs) had similar effects on the peritoneal structure and function in vivo. According to Wieslander et al.3), lactate at neutral pH did not cause a significant change in proliferation.
Erixon et al.5) also determined growth inhibition of HPMC and mouse L-929 fibroblast cells by PD solutions using the NR-test. However, our studies are not in line with the data of these authors concerning correlations because the cell viability even at a weak correlation increases with a pH decrease, an increase in the absorbance at 228 nm of the solutions and an increase in the sodium lactate concentration in the NR test. It could be supposed that such complicated correlations are related with acidic pH of the solutions6). One of the drawbacks of the NR absorption assay is its pH-dependence12), which, in the case of solutions with different pH, interferes with establishing accurate correlations between pH of these solutions and cell viability. The fact that NR accumulates in lysosomes more at lower pH can also be explained by the increased permeability of living cell membranes in the presence of lower pH values of the solutions and, respectively, an increased retention of NR inside the lysosomes of living cells at lower pH of solutions. Our assumption is also based on the data of Noh et al.37) that reactive oxygen species (ROS) generated by conventional PD solutions are, in large part, responsible for peritoneal membrane hyperpermeability, neoangiogenesis, accumulation of extracellular matrix, and eventual peritoneal fibrosis regardless of whether ROS are produced by high glucose, angiotensin II, or GDPs. One more explanation can be that stress induced by exposure to PD solutions can cause an increase in lysosome production to help combat and protect cells from cell death2). Therefore, one of the learning points of our study is that the NR-test is not suitable for comparative studies of PD solutions which differ in pH, as it is pH-dependent and does not enable a comparison of plausible cell viability after changes in solution pH or adjusted manufacturing of solutions.
Comparing the correlations between different tests for the Vero cells, unusual negative correlations were revealed: –0.47 and –0.27, –0.47 and –0.29 between the MTT-test and NR-test, the MTT-test and SRB-test, the NR-test and MTT-test and the NR-test and SRB-test, respectively. Comparing the HepG2 cells, it was established that there were these positive correlations: 0.22 and 0.45 between the MTT-test and NR-test and the MTT-test and SRB-test, respectively, and 0.22 and 0.71 between the NR-test and MTT-test and the NR-test and SRB-test, respectively. Comparing the connection between the viability for two types of the cells measured by the same test, these unusual correlations were revealed: 0.30, 0.50 and 0.62 for SRB-, NR - and MTT tests, respectively. To our knowledge, there were no published data with such connections. Therefore, it is impossible to compare the obtained correlations with the data of other authors. The higher correlations in the MTT-test confirmed our assumption about its rational usage for comparison of different PD solutions.
Our study has found several important learning points around biocompatibility. It confirms the data on cytotoxicity of PD solutions in cell cultures even in a comparison with an isotonic sodium chloride solution in the three tests. One more point is that the cytotoxic activity of the tested PD solutions and an isotonic solution of sodium chloride was greatest when cells were incubated with undiluted PD solutions. When the solutions were diluted by the medium, the amount of viable cells was increased, and at a dilution of 1 : 2 it was close to the values in the control wells where the tested solutions were absent. Another point is that cytotoxicity is mainly related to pH, because GDPs, glucose or lactate did not exert an exceptionally strong negative effect upon PD solutions cytotoxicity. One more point indicates that cellular metabolic activity measured in the MTT-test is more vulnerable to the action of PD solutions compared to membrane permeability and lysosome activity, and ability to proliferation measured in the NR - and SRB tests, respectively. Therefore, NR - and SRB tests are not very suitable for comparative studies of PD solutions which differ in pH, as these tests are pH-dependent and do not enable a comparison of plausible cell viability of solutions differing in pH. There are two important limitations of our study. The first is a small number of PD solutions. The second limitation is that in vitro tests measure only basal toxicity on the cells, not peritoneal origin. Notwithstanding, from the one hand, the selection of cells for measuring basal toxicity is less important as basic cellular mechanisms are similar in both specialized and non-specialized cells, including L-929 fibroblasts and HPMC5, 7). However, on the second hand, the issue of choosing cells for the study of cell viability is very disputable in the field of PD solution research, as Witowski et al.6) stated that the exclusive use of L-929 cells may underestimate the full extent of the toxicity associated with GDPs and therefore it would be more correct to employ primary cell cultures of peritoneal origin in the future research. Moreover, risks factors other than PD solutions composition need considering when evaluating the damage of the peritoneal cavity cells2).
Conclusion
The results of this research showed the inhibition of HepG2 and Vero cells under influence of traditional PD solutions. Our study showed several important learning points around biocompatibility. The cytotoxic activity of the tested PD solutions and an isotonic solution of sodium chloride was greatest when cells were incubated with undiluted PD solutions. The number of viable cells was increased at solutions dilution. Cytotoxicity is mainly related to pH, as GDPs, glucose and lactate did not exert an exceptionally strong effect upon PD solutions cytotoxicity. Cellular metabolic activity measured in the MTT-test is more vulnerable to the action of PD solutions compared to membrane permeability and lysosome activity, and ability to proliferation measured in the NR - and SRB tests. Correlations were found between cell viability and the values of analytical indexes of the tested solutions, between cell viability measured by three tests for the same type of the cells, and between cells viability measured by the same test for two types of the cells. The best correlation (0.62) was revealed between the cells viability measured by the MTT-test for the two types of cells. Finally, the results of this study show the necessity of careful selection of the viability test evaluating cytotoxicity of PD solutions. We suppose that the MTT-test is the best suitable for comparative studies of PD solutions which differ in pH.
Acknowledgments
Co-author Nataliia Hudz is grateful to the International Visegrad Fund (contract No. 51700107) for providing the scholarship for the studies related to solutions for dialysis therapy.
Conflict of interest: none.
Doctor of Pharmacy, саndidate of pharmaceutical sciences Nataliia Hudz (∗)
A. Filipska
N. Stepaniuk
Danylo Halytsky Lviv National Medical University
Pekarska Street 69, 79000 Lviv, Ukraine
e-mail: natali_gudz@ukr.net
N. Dmytrukha
State Institution "Kundiev Institute of Occupational Health of National Academy of Medical Sciences of Ukraine", Kyiv, Ukraine
R. Korytniuk
Shupyk National Medical Academy of Postgraduate Education, Kyiv, Ukraine
P. P. Wieczorek
University of Opole, Poland
Sources
1. Dioos B., Paternot G., Jenvert R.-M., Duponchelle A., Marshall M. R., Nakajima M., Ganoza E. R., Sloand J. A., Wieslander A. P. Biocompatibility of a new PD solution for Japan, ReguanealTM, measures as in vitro proliferation of fibroblasts. Clinical and experimental nephrology 2018; https://doi.org/10.1007/s10157-018-1602-2
2. Al-Hwiesh A. K., Shawarby M. A., Abdul-Rahman I. S., Al-Oudah N., Al-Dhofairy B., Divino-Filho J. C., Abdelrahman A., Zakaria H., El-Din M. A. N., Eldamati A., El-Salamony T., Al-Muhanna F. A. Changes in peritoneal membrane with different peritoneal dialysis solutions: Is there a difference? Hong Kong Journal of Nephrology 2016; 19, 7–18; http://dx.doi.org/10.1016/j.hkjn.2016.03.001
3. Wieslander A., Linden T., Kjellstrand P. Glucose degradation products in peritoneal dialysis fluids: PD Solutions how they can be avoided. Perit. Dial. Int. 2001; 23, 119–124.
4. Schmitt C. P., Aufrich C. Is there such a thing as biocompatible peritoneal dialysis fluid? Pediatr. Nephrol. 2017; 32, 1835–1843; https://doi.org/10.1007/s00467-016-3461-y
5. Erixon M., Lindén T., Kjellstrand P., Carlsson O., Ernebrant M., Forback G. PD fluids contain high concentations of cytotoxic GDPs directly after sterilization. Peritoneal. Dial. Int. 2004; 4, 392–398.
6. Witowski J., Korybalska K., Wisniewska J., Breborowisz A., Gahl G. M., Frei U., Passlick-Deetjen J., Jorres A. Effect of glucose degradation products on human peritoneal mesothelial cell fuction. J. Am. Soc. Nephrol. 2000; 11, 729–739.
7. Cho Y., Johnson D. W., Badve S. V., Craig J. C., Strippoli G. F. M., Wiggins K. J. The impact of neutral-pH peritoneal dialysates with reduced glucose degradation products on clinical outcomes in peritoneal dialysis patients. Kidney International 2013; 84, 969–979; https://doi.org/10.1038/ki.2013.190
8. Hudz N., Korytniuk R., Vyshnevska L., Wieczorek P. P. Complex technological and biological research of solutions for peritoneal dialysis, International Journal of Applied Pharmaceutics. 2018; 10(4), 59–67; https://doi.org/10.22159/ijap.2018v10i4.24823
9. Erixon M., Wieslander A., Linden Т., Carlsson O., Forsbäck G., Svensson E., Jönsson J. A., Kjellstrand P. Take care in how you store your PD fluids: actual temperature determines the balance between reactive and non-reactive GDPs. Peritoneal Dial. Int. 2005; 25(6), 583–590.
10. Diaz-Buxo J., Sawin D.-A., Himmele R. PD solutions: new and old. Dial. Transplant. 2011; 356–361; https://doi.org/10.1002/dat.20601
11. Liao С-Т., Andrews R., Wallace L. E., Khan M. W. A., Kift-Morgan A., Topley N., Fraser D. J., Taylor P. R. Peritoneal macrophage heterogeneity is associated with different peritoneal dialysis outcomes. Kidney International 2017; 91, 1088–1103; http://dx.doi.org/10.1016/ j.kint.2016.10.030
12 Ohkuma S., Poole B. Cytoplasmic vacuolation of Mouse peritoneal macrophages and the uptake into lysosomes of weakly basic substances. The Journal of Cell Biology 1981; 90(3), 656–664.
13. Angius F., Floris A. Liposomes and MTT cell viability assay: An incompatible affair. Toxicol. In Vitro 2015; 29, 314–319; https://doi.org/10.1016/j.tiv.2014.11.009
14. Gilbert D. F., Friedrich O. Cell Viability Assays: Methods and Protocols. Methods in Molecular Biology, vol. 1601; https://doi.org/10.1007/978-1-4939-6960-9_2
15. Ponsoda X., Jover R., Nunez C., Royo M., Castell J. V., Gomez-Lechon M. J. Evaluation of the cytotoxicity of 10 chemicals in human and rat hepatocytes and in cell lines: correlation between in vitro data and human lethal concentration. Toxicol. In Vitro 1995; 9(6), 959–966.
16. Test Method Protocol for the NHK Neutral Red Uptake Cytotoxicity Assay Phase III – Validation Study: November 4, 2003.
17. Keepers Y. P., Pizao P. E., Peters G. J., Ark-Otte J. V., Winograd B., Pinedo H. M. Comparison of the Sulforhodamine B Protein and Tetrazolium (MTT) Assays for in vitro Chemosensitivity Testing. Eur. J. Cancer 1991; 27(7), 897–900.
18. Perez M. G., Fourcade L., Mateescu M. A., Paguin J. Neutral Red versus MTT assay of cell viability in the presence of copper compounds.Analytical Biochemistry 2017; 535, 43–46.
19. Akter R., Uddin S. J, Tiralongo J., Grice I. D., Tiralongo E. A new cytotoxic steroidal glycoalkaloid from the methanol extract of Blumea lacera leaves. J. Pharm. Sci. 2015; 18(Suppl 4), 616–633.
20. Ammerman N. C., Beier-Sexton M., Azad A. F. Growth and Maintenance of Vero Cell Lines. Curr. Protoc Microbiol. 2008 APPENDIX: Appendix–4E; https://doi.org/10.1002/978047172 9259.mca04es11
21. Bahuguna A., Khan I., Bajpai V. K., Kang S. C. MTT assay to evaluate the cytotoxic potential of a drug. Bangladesh J. Pharmacol. 2017; 2(12), 115–118; https://doi.org/10.3329/bjp.v12i2.30892
22. Vichai V., Kirtikara K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nature Protocols 2006; 1, 1112–1116; https://doi.org/10.1038/nprot.2006.179
23. Miller M. A., Bankier C., Al-Shaeri M., Hartl M. C. J. Neutral Red cytotoxicity assays for assessing in vivo carbon nanotube ecotoxicity in mussels – Comparing microscope and microplate methods. Marine Pollution Bulletin 2015; 101(2), 903–907; https://doi.org/10.1016/j.marpolbul.2015.10.072
24. Vajrabhaya L., Korsuwannawong S. Cytotoxicity evaluation of a Thai herb using tetrazolium (MTT) and sulforhodamine B (SRB) assays. Journal of Analytical Science and Technology 2018; 9, 15; https://doi.org/10.1186/s40543-018-0146-0
25. Kobylinska L., Patereha I., Finiuk N., Mitina N., Riabtseva A., Kotsyumbas I., Stoika R., Zaichenko A., Vari S. G. Comblike PEGcontaining polymeric composition as low toxic drug nanocarrier. Cancer Nano. 2018; 9, 1–13; https://doi.org/10.1186/s12645-018-0045-5
26. Boja P. Lysosomal Function and Dysfunction: Mechanism and Disease. Antioxidants & Redox signaling Volume 2012; 17(5), 766–774; https://doi.org/10.1089/ars.2011.4405
27. Repetto G., Peso A. D., Zurita J. L. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nature Protocols 2008; 3(7), 1125–1131; https://doi.org/doi:10.1038/nprot.2008.75
28. Lim S-W., Loh H-S., Ting K-N., Bradshaw T. D., Allaudin Z. N. Reduction of MTT to Purple Formazan by Vitamin E Isomers in the Absence of Cells. Trop. Life Sci. Res. 2015; 26(1), 111–120.
29. Kjellstrand P., Erixon M., Wieslander A., Lindén T., Martinson E. Temperature: the single most important factor for degradation of glucose fluids during storage. Periton. Dialysis Int. 2004; 24(4), 385–391.
30. Distler L., Georgieva A., Kenkel I., Huppert J., Pischetsrieder M. Structure - and concentration-specific assessment of the physiological reactivity of α-dicarbonyl glucose degradation products in peritoneal dialysis fluids. Chem. Res. Toxicol. 2014; 27, 1421–1430; https://doi.org/10.1021/tx500153n
31. Hudz N., Kobylinska L., Dmytrukha N., Korytniuk R., Wieczorek P. P. Biological and analytical studies of peritoneal dialysis solutions. Ukr. Biochem. J. 2018; 90, 34–44; https://doi.org/10.15407/ubj90.02.034
32. British Pharmacopoeia. Edition 2009. London: The Stationery Office 2009; 10952 p.
33. Bühl A., Zöfel P. SPSS Version 10. Einführung in die modern Datenanalyse unter Windows, 7, überarbeitete und erweiterte Auflage. Diasoft: 2005; 602 p. (in Russian).
34. Stockert J. C., Blazquez-Castro A., Canete M., Horobin R. W., Villanueva A. MTT assay for cell viability: Intracellular localization of the formazan product is in lipid droplets. Acta Histochemika 2012; 14, 785–7976; https://doi.org/10.1016/j.acthis.2012.01.006
35. Perez R. P., Godwin A. K., Handel L. M. and Hamilton T. C. A comparison of Clonogenic, Microtetrazolium and sulforhodamine B assays for determination of cisplatin cytotoxicity in human ovarian carcinoma cell lines. Eur. J. Cancer. 1993; 3(29A), 395–399.
36. Liberek T., Topley N., Jörres A., Petersen M. M., Coles G.A., Gahl G. M., Williams J. D. Peritoneal dialysis fluid inhibition of polymorphonuclear leukocyte respiratory burst activation is related to the lowering of intracellular pH. Nephron. 1993; 65(2), 260–265; https://doi.org/ 10.1159/000187485
37. Noh H., Kim J. S., Han K-H., Lee G. T., Song J. S., Chung S. H., Jeon J. S., Ha H., Lee H. . Oxidative stress during peritoneal dialysis: Implications in functional and structural changes in the membrane. Kidney International 2006; 69, 2022–2028; https://doi.org/10.1038/sj.ki.5001506
Labels
Paediatric nephrology Pharmacy Clinical pharmacology Nephrology
Article was published inCzech and Slovak Pharmacy

2019 Issue 4-
All articles in this issue
- Plant α-amylase inhibitors and their effect on the utilization of polysaccharides contained in the diet
- Theory and practice of pharmacopoeial control of quality of drugs and excipients X. Number of parallel determinations, processing of results and their use in the assessment of the content of active substances and excipients in the European Pharmacopoeia (Ph. Eur.)
- New approach for detoxification of patients dependent on benzodiazepines and Z-drugs for reduction of psychogenic complications
- Study of biocompatibility of peritoneal dialysis solutions measured as in vitro cells viability
- Nephroprotective effect of N-acetylglucosamine in rats with acute kidney injury
- Czech and Slovak Pharmacy
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- New approach for detoxification of patients dependent on benzodiazepines and Z-drugs for reduction of psychogenic complications
- Plant α-amylase inhibitors and their effect on the utilization of polysaccharides contained in the diet
- Nephroprotective effect of N-acetylglucosamine in rats with acute kidney injury
- Theory and practice of pharmacopoeial control of quality of drugs and excipients X. Number of parallel determinations, processing of results and their use in the assessment of the content of active substances and excipients in the European Pharmacopoeia (Ph. Eur.)
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career


