-
Medical journals
- Career
Preparation of feed premix for veterinary purposes
Authors: Aleš Franc; Róbert Lehocký; Jan Muselík; David Vetchý; Radka Dobšíková; Helena Modrá
Authors‘ workplace: Veterinary and Pharmaceutical University in Brno, Faculty of Pharmacy, Department of Pharmaceutics 1; Veterinary and Pharmaceutical University in Brno, Department of Veterinary Public Health and Animal Welfare, Faculty of Veterinary Hygiene and Ecology 2
Published in: Čes. slov. Farm., 2014; 63, 213-216
Category: Original Articles
Overview
This experimental study describes the preparation of a veterinary medicated premix containing tetracycline hydrochloride for oral administration to aquatic animals. For the manufacture of the premix, commercially produced animal feed is used, which is intended for consumption in the form of pellets that were coated with a mixture of chlortetracycline hydrochloride and other excipients. Feed pellets were combined with a mixture of an active substance and excipients with a large specific surface (colloidal silica – Aerosil® 200) allowing an easy adhesion to the surface of the pellets, and a solid polymer with a low glass transition point (Eudragit® E) which ensures the formation of a hard coat. A mixture of these substances has been applied to the surface of the pellets either A) in the solid state simply by dry adhesion; B) by coating the pellets with the mixture and additional impregnation with ethanol; or C) the polymer was subsequently applied in solution. In the final stage, the pellets were heated in order to achieve the glass transition point of the polymer to create a solid and mechanically resistant coating. Coated pellets prepared by three methods described above are almost identical in their physical properties. With this technology it is possible to produce a feed mixture with a very low content of the active substance in situ without the need for a complex technological equipment.
Keywords:
medicated feed premix • pellets • impregnation • adhesion • fishIntroduction
Fish in aquaculture can suffer from various diseases that complicate their farming and yield. These diseases can cause increased mortality, thus having impact on production yield1).
Treatment of fish has gone through gradual development. Progress in treatment is accompanied by progress in pharmaceutical technology as well. The most frequent ways of administration are application in water, oral administration and injectables2). Drugs purposed for oral administration to fish are manufactured by various technologies leading to four dominant dosage forms: feed premixes, pellets, coated pellets, and microparticles3).
One of most frequent ways of administration is represented by feed premixes that are applied on the surface of the farming reservoir, e.g. a pond. The dose is calculated with respect to the number and weight of fish in a particular reservoir; it is alleged that fish will consume the premix evenly. If the premix has to contain a small amount of an active substance, there is a problem because insufficient uniformity can be expected, leading to uneven dosage to fish. In such cases, dry coating method developed by the Department of Pharmaceutics has been proved to be suitable4). The method is based on feed pellets that are coated with a mixture of an active substance, a substance with a large surface area and a polymer with a low glass transition temperature. These substances are subsequently heated, the polymer undergoes glass transition and forms a hard coat. Alternatively, prior to heating, the mixture can be impregnated with a small volume of liquid, or the polymer can be used in the form of a solution in an organic liquid. Even when using these modifications based on liquids, the mixture remains solid and can be mixed in a standard blender. The technology does not demand any complicated technical equipment and the feed premix can be prepared “on site” with the amount of active substance calculated with respect to the amount of fish in a particular reservoir.
In our case, three types of feed premixes intended for oral administration to fish were prepared, according to the three possible technologies described above. For the formulation of the premix, commercially available pellets made from a mixture of fish and wheat meal purposed for fish were used. Chlortetracycline hydrochloride was used as the active substance, colloidal silica (Aerosil® 200) was used as the substance with a large surface area, and a cationic poly(meth)acrylate polymer (Eudragit® E) was used as the polymer. The preparation proper is done in a common gravitation homogenizer, e.g. a drum blender, or by manual mixing in sacks4). Prepared feed premixes were tested for standard physical and chemical properties, i.e. density, flow properties, friability, hardness, and dissolution profile.
The experiment was aimed at a medicated feed premix in the form of pellets containing a small amount of chlortetracycline, using the standard technical device, i.e. a gravitational blender. The premix should have suitable physical properties as hardness and friability, the active substance should dissolve evenly and be distributed in pellets with required content uniformity.
Experimental part
Raw material
The pellets contain: chlortetracycline hydrochloride (Meditek, Germany); Eudragit® E PO (Evonik, Germany); Eudragit® E 100 (Evonik, Germany); Aerosil® 200 (Evonik, Germany); ethanol 85%, (Dr. Kulich, Czech Republic); acetone (Merck, USA); feed pellets Inicio® 918 (Biomar, Denmark) – mean particle size 2 mm, proteins 46%, fats 23%, saccharides 16.9%, fibre 1.1%, ash 7.0 %).
Preparation of pellets
Coated pellets containing chlortetracycline were prepared using three different technologies. Batch A: the polymer was a part of the solid phase. Batch B: a solid mixture containing a polymer was wetted by ethanol. Batch C: the polymer was dissolved in acetone and only then added to the mixture. Compositions of particular batches are listed in Table 1.
Procedure
Formulation A
Chlortetracycline hydrochloride, Aerosil 200®, and Eudragit E PO® were sieved through a 0.250mm sieve. These additional components as well as feed pellets were put in a homogenizer Turbula T2C (GlenMills, Switzerland) and mixed at 40 rpm for 15 minutes. After mixing, the coated pellets were put in a hot air dryer Horo 38 A (Hofman, Germany) and dried at 80 °C for 2 hours.
Formulation B
Chlortetracycline hydrochloride, Aerosil 200®, and Eudragit E PO® were sieved through a 0.250mm sieve. These additional components as well as feed pellets were put in a homogenizer Turbula T2C and mixed at 40 rpm for 15 minutes. Ethanol 85% was added and the blending continued for additional 5 minutes. After mixing, the coated pellets were put in a hot air dryer Horo 38 A and dried at 80 °C for 2 hours.
Formulation C
Eudragit® E 100 was dissolved in acetone while being mixed for 60 minutes. Chlortetracycline hydrochloride and Aerosil 200® were sieved through a 0.250mm sieve. These additional components as well as feed pellets were put in a homogenizer Turbula T2C and mixed at 40 rpm for 15 minutes. Then, the mixture was evenly wetted by an acetone solution of Eudragit® E 100 and blending continued for additional 15 minutes. After mixing, the coated pellets were put in a hot air dryer Horo 38 A and dried at 80 °C for 2 hours.
Evaluation of pellets
Pellets were tested for bulk and tapped density and the related parameters – Hausner ratio (HR) and compressibility index (CI), according to CP 2009. An Erweka SVM 102 was used (Erweka, Germany). Pellet friability was measured using an Erweka TAR 10 (Erweka, Germany), 20 rpm, 10 minutes, using 10 g of pellets mixed with 200 4mm glass beads. Flow properties were measured with the use of a Flow Properties Tester (Medipo, Czech Republic), aperture size 10 mm. Hardness (CP 2009) was measured using a Tablet Hardness & Compression Tester C50 (Engineering Systems, United Kingdom). “On-line” dissolution test (CP 2009) was measured using the SOTAX AT 7 Smart On-line system (Donau Lab, Switzerland) with UV/VIS detection on a spectrophotometer Lambda 25 (Perkin Elmer, USA). 6 samples of coated pellets (1.0 g) of all batches were measured, using placebo (original uncoated pellets) as blank, using the paddle method, 20 rpm, and distilled water as the medium (25 °C, 1000 ml). The amount of the released active substance was measured spectrophotometrically at the wavelength of 276 nm, using the calibration curve. Samples were taken automatically at 5, 10, 15, 30, 45, 60, 90, and 120 minutes. At endpoint, with all of the active substance dissolved, content uniformity was measured (a non-pharmacopoeial method).
Results and discussion
Three batches of pellets purposed for administration to fish were prepared. They contained 0.9 % chlortetracycline hydrochloride (concentration in coated pellets). For preparation, “the powder coating” method was used, i.e. application of a dry powder mixture on pellets. This powder mixture consisted of the active substance, a substance with a large surface area, and a polymer with a low glass transition temperature. Colloidal silica (Aerosil® 200) was used as the substance with a large surface area, declared approximately as 150 m2.g-1; colloidal silica has also a strong electrostatic charge that enables the mixture to get absorbed on the surface of pellets. The large volume of Aerosil® 200 helps to triturate the active substance and apply it uniformly to the surface of pellets4). The cationic copolymer of dimethylaminoethyl metacrylate, butyl metacrylate, and methyl metacrylate, Eudragit® E has a low glass transition temperature (45 °C); after heating above this temperature it forms hard coating on the pellet surface. It is commercially available in two sorts – as the fine powder Eudragit® E PO or as the coarse granulate Eudragit® 100 that is suitable for preparation of solutions5).
Powder coating with subsequent heating to activate the polymer component was introduced by Cerea et al. In 2004, they used Eudragit® E PO and a heated spheronizer to prepare tablets coated by dry powder without the need of liquids6).
We modified the Cerea method by not using tablets but feed pellets and instead of a spheronizer we used a Turbula mixer. Furthermore, we employed the ability of Aerosil® 200 to triturate the low content of the active substance and to adhere the mixture onto the pellet surface (sample A). Another modification of the method consisted in the impregnation by a small amount of liquid in which the polymer is soluble (ethanol 85%) before heating (sample B). And another method was based on impregnating the pellets with a small volume of a solution of the polymer in acetone (sample C). Due to an enormous absorption capacity of Aerosil® 200, coated pellets retain their solid character and during the impregnation process, neither agglomeration nor sticking to the walls of the blender was observed. This modification combines “powder coating” and “impregnation,” a method that is used in the preparation of dosage forms with a low content of the active substance and a high content uniformity7). Our intention was to find out what will be the influence of impregnation, either by the solvent or polymer solution, on the properties of prepared coated pellets.
Physical properties (Table 2) show that hardness of coated pellets manufactured by three different technologies (A, B, C) is almost identical, i.e. about 7 N. Nevertheless, there are differences in friability. Pellets with the polymer added in the solid state without impregnation (A) have a friability of 0.193% of the wear, pellets impregnated with ethanol (B) have a friability of 0.144%, and pellets impregnated with a polymer solution (C) have a friability of only 0.081%. This proves that impregnation, or using a dissolved polymer, has a significant and positive impact on the mechanical properties of prepared pellets. Other values, e.g. bulk and tapped density and related values like Hausner ratio and compressibility index, are of less importance. When impregnation with ethanol (sample B) was employed, both the density values and compressibility index were lower than in the other methods (samples A, C). We have no acceptable answer to this difference.
2. Properties of coated pellets 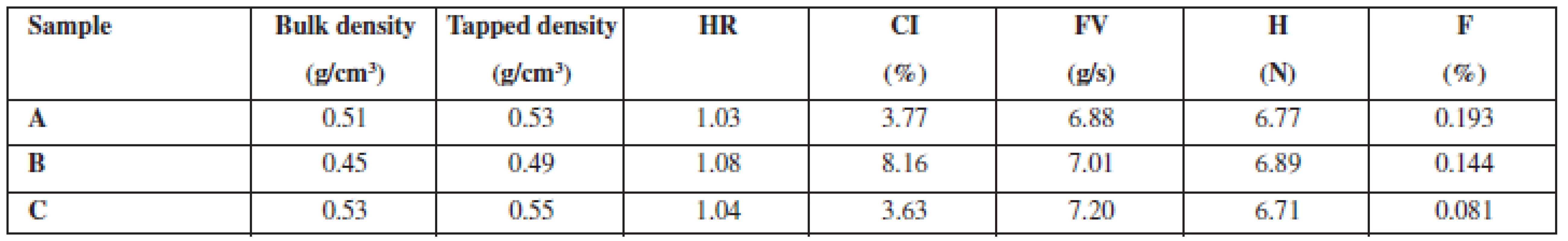
HR – Hausner ratio, CI – compressibility index, FV – flow velocity, H – hardness, F – friability The evaluation of dissolution profiles (Fig. 1) showed that the fastest release was observed in powder coated pellets (sample A), ethanol impregnated pellets were a little bit behind (sample B) and the last to release the active substance were polymer solution impregnated pellets (sample C). This may be proportionally related to friability values as the pellets with a higher friability released the active substance faster. Coating that is less mechanically resistant seems to be able to release the active substance sooner. Low measurement errors (low relative error at all measurement points) show that the active substance was absorbed onto the pellet surface uniformly.
1. Dissolution profiles of chlortetracycline 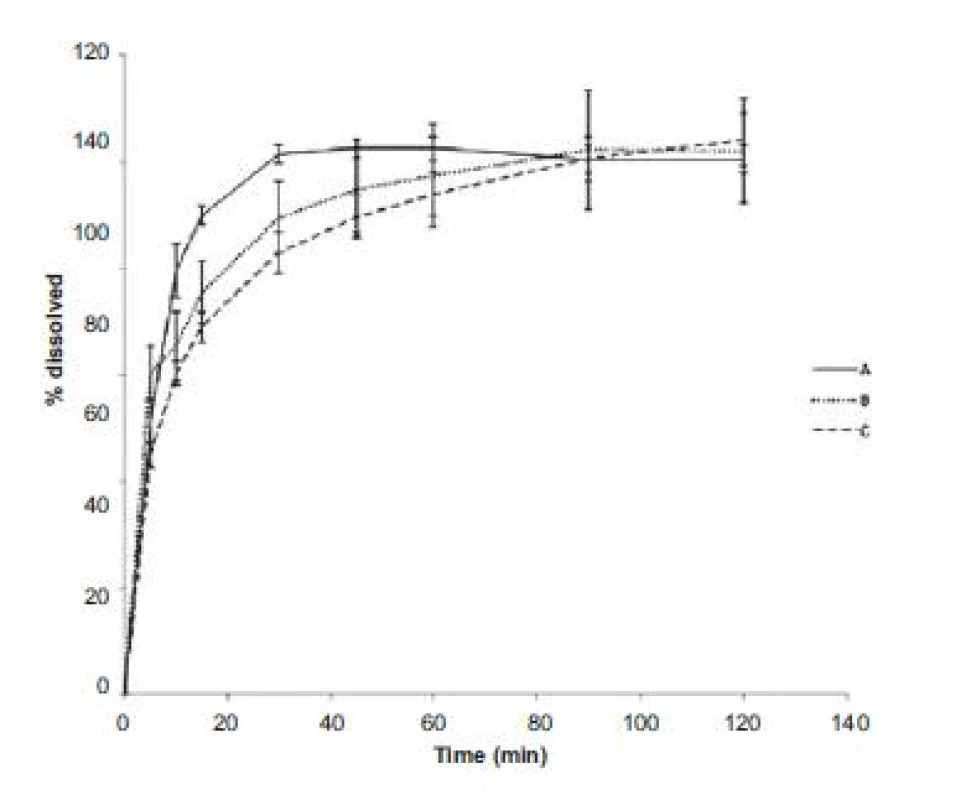
This is supported by the content uniformity results (Table 3) based on the concentration of the dissolved active substance at the dissolution endpoint. In all the samples of coated pellets that were tested for dissolution, the content of chlortetracycline was 95–105% of the declared content and in any batch the relative standard deviation (RSD) did not exceed 10%.
3. Content uniformity of pellets 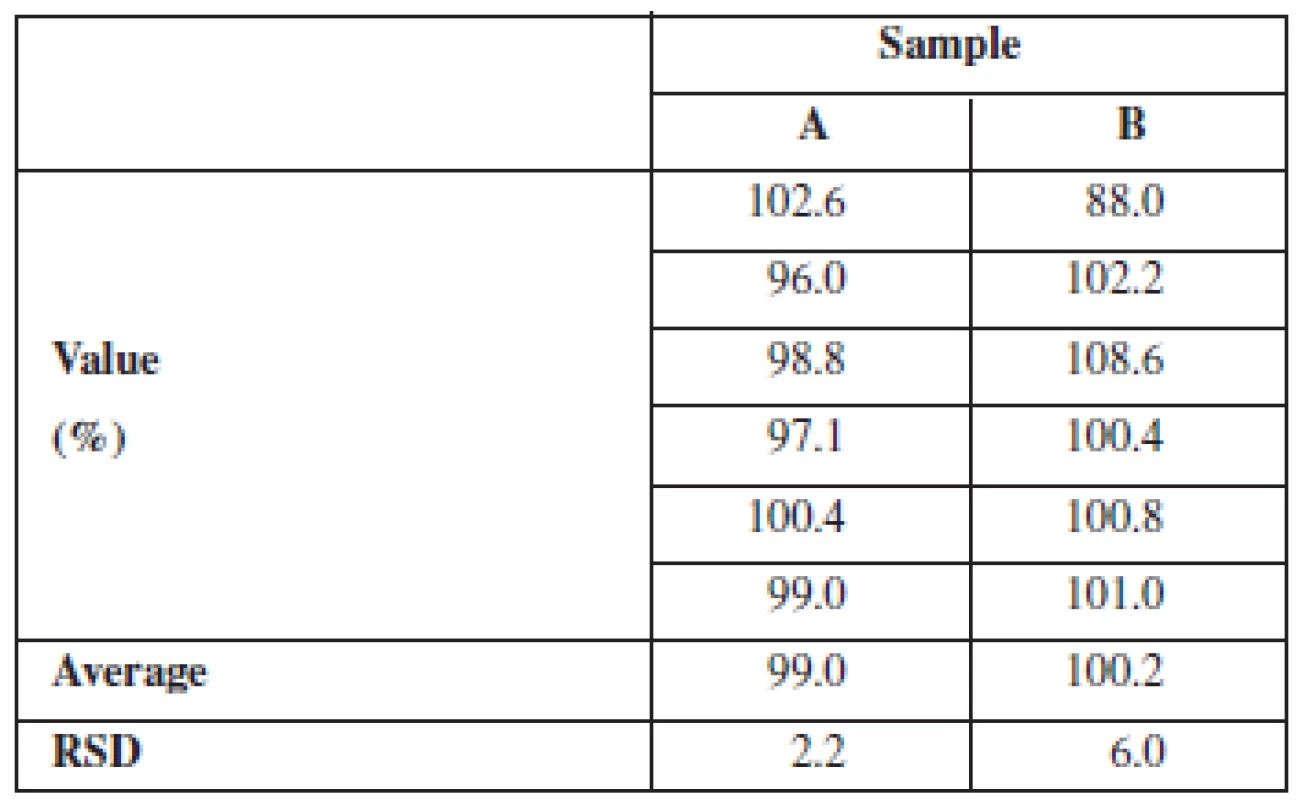
In all three cases, mechanical properties of pellets as well as their dissolution profiles are sufficient. Feed premixes with a low content of the active substance can be produced by any of the three described methods – “powder coating” (sample A), powder coating combined either with solvent impregnation (sample B), or with polymer solution impregnation (sample C).
Conclusion
Three technologies of feed premixes for fish containing a low amount of an active substance, chlortetracycline hydrochloride (0.9%), with good content uniformity were tested. The technologies included powder coating (A), powder coating with subsequent impregnation with a solvent prior to heating (B), and powder coating with subsequent impregnation with a polymer solution prior to heating (C). These methods resulted in mechanically resistant coated pellets with good content uniformity. Mechanical resistance increased in the line A→B→C; on the contrary, the velocity of release by dissolution increased in the line C→B→A. The dosage form retained its solid character during the preparation. All these methods allow for very easy preparation of coated pellets in conventional gravitational blenders.
Conflicts of interest: none.
Received 11 September 2014 / Accepted 18 September 2014
PharmDr. Aleš Franc, Ph.D. (✉) • R. Lehocký • J. Muselík • D. Vetchý
Veterinary and Pharmaceutical University in Brno, Faculty of Pharmacy
Department of Pharmaceutics
Palackého tř. 1/3, 612 42 Brno, Czech Republic
e-mail: franca@vfu.cz
R. Dobšíková • H. Modrá
Veterinary and Pharmaceutical University in Brno, Czech Republic
Department of Veterinary Public Health and Animal Welfare, Faculty of Veterinary Hygiene and Ecology
Sources
1. Woo P. T. K, Leatherland J. F. Fish diseases and disorders: protozoan and metazoan – viral, bacterial and fungal infections: CABI, 2006.
2. Noga J. Fish disease: diagnosis and treatment. Iowa: University press, 2010.
3. Treves-Brown K. M. Applied fish pharmacology: Kluwer academic publishers, 2000.
4. Franc A., Muselík J., Rabišková M., Dobšíková R. CS Patentová přihláška PV 2011 – 698.
5. Rowe R. C., Sheskey P. J. Quinn A M. E. Handbook of pharmaceutical excipients. 6th ed. London: Pharmaceutical Press, 2009.
6. Cerea M., Zheng W. J., Young C.R., McGinity J.W. A novel powder coating process for attaining taste masking and moisture protective films applied to tablets. Int. J. Pharm. 2004; 279, 127–139.
7. Franc A., Rabišková M., Goněc R. Impregnation: a progressive method in the production of solid dosage forms with low content of poorly soluble drugs. Eur. J. Parent. Pharm. Sci. 2011; 16, 85–93.
Labels
Pharmacy Clinical pharmacology
Article was published inCzech and Slovak Pharmacy

2014 Issue 5-
All articles in this issue
- Lyophilisation of protein-based drugs
- Optimization of technological processes for the preparation of tablets with a low content of warfarin by direct compression
- Continuing/life-long education of pharmacists in the Czech Republic 4th cycle 2008–2011
- History of diabetes treatment in Czechoslovakia prior to 1989
- Formulation of cores for the controlled release of glucose for prevention of hypoglycemia in diabetes patients
- Preparation of feed premix for veterinary purposes
- Czech and Slovak Pharmacy
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- History of diabetes treatment in Czechoslovakia prior to 1989
- Lyophilisation of protein-based drugs
- Continuing/life-long education of pharmacists in the Czech Republic 4th cycle 2008–2011
- Formulation of cores for the controlled release of glucose for prevention of hypoglycemia in diabetes patients
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career