-
Medical journals
- Career
Formulation of cores for the controlled release of glucose for prevention of hypoglycemia in diabetes patients
Authors: Aleš Franc; Kateřina Dvořáčková; Jan Muselík; Martina Žvaková; Vladimíra Slováková; David Vetchý; Dana Sabadková; David Neumann; Roman Goněc
Authors‘ workplace: Veterinary and Pharmaceutical University in Brno, Faculty of Pharmacy, Department of Pharmaceutics 1; Masaryk Memorial Cancer Institute, Brno 2; University Hospital, Pediatric Clinic, Hradec Králové 3
Published in: Čes. slov. Farm., 2014; 63, 206-212
Category: Original Articles
Overview
Hypoglycemic episodes are a frequent and serious complication in both types of diabetes mellitus. The risk of hypoglycemic conditions can be managed by a coated pellet dosage form, which can release glucose in a delayed regime to achieve the maximum estimated effect of antidiabetics. The pellet cores, intended for coating with ethylcellulose, were prepared consisting of four osmotically active excipients: crosscarmellose (Ac-Di-Sol®), a mixture of microcrystalline cellulose and carmellose sodium (Avicel® RC 591), carboxymethyl starch sodium (Vivastar® P 5000) and macrogol 6000, respectively. The aim of this study was to increase the glucose content in the pellets to minimize their volume and to improve the administration to the patients. The content of glucose in the pellet cores was increased from 45 to 75 or 80%, respectively, for all compositions. All pellet samples had satisfactory mechanical and flow properties required for the coating process. The highest values of sphericity were achieved in the lower mean particle size sample containing 80% of glucose, 15% of Avicel® PH 101 and 5% of carboxymethyl starch sodium and the higher mean particle size sample containing 75% of glucose and 25% of Avicel® RC 591.
Key words:
hypoglycemia - delayed release - glucoseIntroduction
Both diurnal and nocturnal hypoglycemia are common complications in both types of diabetes mellitus (DM). They can occur almost in any patient treated with insulin or particular oral antidiabetics. Glycemia under 3.3 to 3.9 mmol/l (60–70 mg/dl) is considered generally to be dangerous. The American Diabetes Association recommends the value of 3.9 mmol/l to define hypoglycemia, irrespective of the age of the patient1).
Hypoglycemia episodes are a significant obstacle against reaching optimal and controlled glycemia. This symptom has a negative impact on the attention and thus on the ability to drive, work or learn. The patients may get in socially embarrassing situations. Hypoglycemia is the consequence of a wrong ratio between the effect of administered insulin (or oral antidiabetics), released energy and the intake of saccharides.
Although hypoglycemia may occur at any time during the day, there is some dependence on the insulin protocol, intake of meals, activities and habits of the patient. Usually, it emerges between breakfast and lunch, often because of delayed lunch or missed late morning meal. The risk is higher in children as well as in old people, in the latter case probably because of comorbidities and comedications that may suppress the intensity of the symptoms and so suppress the chance for quick and correct diagnosis of hypoglycemia. DM1 patients on the intensified insulin protocol encounter serious hypoglycemia episodes three times more often than patients on the convention protocol2). In spite of the fact that the frequency of hypoglycemia in DM1 patients varies to a great degree, large cohort studies have shown that DM1 patients face two light hypoglycemia episodes per week and one third of DM1 patients face one or more severe hypoglycemia episodes per year. Hypoglycemia itself is the cause of death in 2–4% DM1 patients3).
Hypoglycemia manifests itself by vegetative symptoms caused by the activation of the peripheral nervous system by a decreased glucose serum level. The recognition of these symptoms is a very important signal to the patient that his or her sugar blood level decreased under the threshold level. The symptoms include attention, memory, and decision making disorders, etc. More serious episodes can include convulsions or coma. Low glycemia can also cause tachycardia, increased ejection fraction and myocardial contractility. These changes are related to the stimulation of the sympaticus, catecholamine secretion and can result in arrhythmia, ischemia and cardiac failure. All these factors can be the cause of “dead in bed” syndrome, i.e. sudden, unpredictable death of a young DM1 patient during his or her sleep4). If hypoglycemia episodes are serious or repeat themselves, they may result in permanent changes in the sympaticus and neuroendocrine regulation mechanisms, eventually, in the absence of warning symptoms of decreasing glycemia. (“hypoglycemia unawareness”). Rarely, permanent deficiency of intellectual abilities is possible. In a large number of diabetics, hypoglycemic episodes induce fear and anxiety. This fact has a negative impact on the therapy because the patients avoid hypoglycemic episodes by ignoring any suggestions to reach controlled glycemia. Incessant fear of possible hypoglycemic episode has an impact on the social life of the patient, causing changes in mood and affecting all family members3). A hypoglycemic state can be prevented in the case of adherence to preventative measures. It is very important that the patient masters glycemia “self-monitoring” and practises it regularly. When the patient exerts physical activity, the dose of insulin should be decreased by 30-50% or the intake of saccharides should be increased. The nutrition of a DM patient has to be balanced and contain an increased amount of energy5).
Hypoglycemic episodes that can be treated only with difficulty include: nocturnal hypoglycemia characterised by a peak effect in the time of a physiologically lowest need of insulin (Somógyi phenomenon), hypoglycemia during sport activity that cannot be interrupted to measure glycemia (most collective sports), hypoglycemia in small children that eat irregularly and reluctantly. Possible solutions can be hoped for in new insulin analogues that are now being developed.
Recently developed food containing control-released glucose may also be a solution as it compensates hypoglycemia and enables to decrease the amount of saccharides that the patients eat “for preventive reasons”. Glucose-containing food releases a defined amount of glucose over a defined time period, maintaining its blood level and balancing the effect of insulin, or oral antidiabetics. However, there has not been any efficient dosage form that would enable controlled release of glucose. In 1994, The Estee Corporation applied for a patent application on a dietary supplement containing nanoparticles with saccharides that are released and modify the blood glucose level6). The described solution is based on a slow release of glucose without a lag time in which glucose would be released in a minimal amount or not at all. In 1995, a similar patent application by Hercules Inc. claims a special manufacture of food with controlled release of glucose, however, without defined forms and without achieving the desired profile with a lag time7).
This study aimed to prepare pellet cores containing as much glucose as possible by extrusion/spheronisation and to evaluate them. The pellet cores would be coated by semi-permeable membrane (not described in this paper) to allow for a delayed release of glucose (lag time of 2, 3, 4, or 5 hours) to achieve the optimal glucose blood level for a sufficiently long time (e.g. all night) without disturbing the daily regime of patients and preventing them from hypoglycemia. A single dose of pellets containing glucose should be low enough to allow for easy administration. This experimental work is based on previously published papers8,9,10) describing the preparation and evaluation of coated cores containing glucose in a lower amount. The previous work included the selection of suitable excipients and some manufacture parameters.
Experimental part
Raw material
Glucose monohydrate (Dr. Kulich Pharma, Czech Republic); microcrystalline cellulose – MCC Avicel® PH 101 (FMC Europe, Belgium); sodium cross-carmellose – Ac-Di-Sol® (FMC Europe, Belgium); mixture of microcrystalline cellulose and sodium carmellose – Avicel® RC 591 (FMC Europe, Belgium); sodium carboxymethyl starch – Vivastar® P 5000–CMS (JRS Pharma, Germany); macrogol 6000 – PEG 6000 (Merck Schuchardt, Germany).
Preparation of cores
Individual raw materials from Table 1 were mixed in dry state on a Tefal, Kaleo (France) mixer at 400 rpm for 60 seconds. Purified water (CP 2009) was added in such an amount so as to allow the formation of dough-like consistence. The amount of water necessary for the preparation of the extrudate was based on previous tests and experiments that are not described in this paper8–10). Wet mass was mixed for 60 seconds under identical conditions. Then, the wet mass was transferred to the extruder and extruded axially through the partition (aperture size 1.0 mm, screw 110 rpm). Resulting ropes were broken and spheronized in a spheronizer at 1000 rpm for 5 minutes. Extrusion and spheronization were performed using an integrated unit Pharmex 35 T (Switzerland). The pellets were dried in a hot air dryer Horo 38 A (Germany) at 50 °C. To allow for the best possible impact of pellet composition on their properties, process parameters remained identical for all batches (Table 1).
1. Composition of pellet cores samples 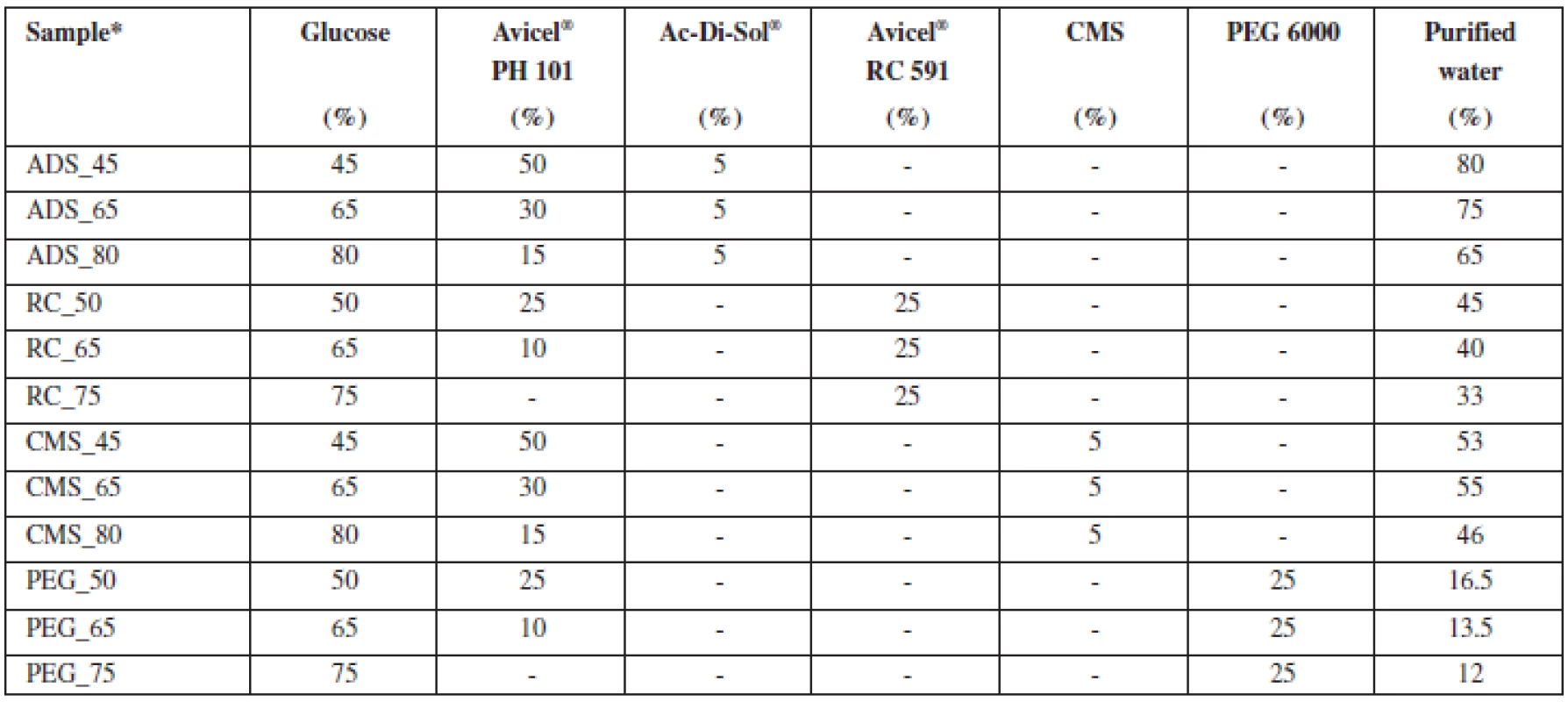
*X_Y… , where X = osmotically active substance: ADS – Ac-Di-Sol®, RC – Avicel® RC 591, CMS – sodium carboxymethyl starch, PEG 6000 – polyethylene glycol 6000; Y – percentage of glucose in pellets Pellet characteristics
Sieve analysis of pellets (Czech Pharmacopeia, CP) was performed on an AS 200 basic (Retsch, Germany) device; mean particle size was calculated according to Equation 1:
where ¯d stands for mean pellet size, xi stands for average aperture size of the upper and lower sieve of i fraction, di stands for mass fraction percentage, and n stands for the number of sieves. In prepared pellets, bulk and tapped density were measured as well as the related values - Hausner ratio and compressibility index (CP 2009). Bulk and tapped density were measured using an Erweka SVM 102 (Germany) device. Friability of pellets was measured with the use of an Erweka TAR 10 (Germany) apparatus. 10 g of pellets were mixed with 200 glass beads with a diameter of 4 mm and rotated at 20 rpm for 10 minutes. Flow properties (CP 2009) were measured using a Medipo analyzer (Czech Republic), aperture size 10 mm. Microscopic evaluation of pellets (20 particles) was measured by an optical microscope Intarcho-micro DN 25 Lambda (Czech Republic), using a CCD camera Nikon Alphaphot-2 (Japan) and the optical analysis operation programme Leco Instruments Leco IA 32 (USA). Sphericity was evaluated as the sphericity factor SF (Equation 2) and the aspect ratio AR (Equation 3):
where A stands for pellet area in v μm2 and p stands for pellet perimeter in μm.
where dmax stands for the highest Feret diameter in v μm (the Feret diameter is the distance between the two parallel planes restricting the object perpendicular to that direction) and dmin stands for the lowest Feret diameter in μm. Pellets were photographed with a Nikon Coolpix 4500 (Japan) apparatus.
Results and discussion
10 g of glucose are the exchange unit in diabetology. Therefore we wanted to prepare pellet cores with suitable physical parameters (density, size, shape, mechanical resistance) that would contain as much glucose as possible. If the cores contained a lower amount of glucose, the total volume would be higher and administration, namely swallowing, more difficult. The intended route of administration is coated pellets mixed with liquid or semisolid food or dispersed in a drink.
Choice of excipients
Based on the literature, microcrystalline cellulose was chosen as the main excipient, as there is a lot of experience with this substance in pellet preparation. Microcrystalline cellulose has a large surface area and porosity, enabling it to hold and distribute water in the wet mixture during the preparation of the extrudate, thus increasing its plasticity and improving the spheronization process. Pellets of microcrystalline cellulose are relatively hard, with low breakability, smooth surface and high density, and are able to hold a relatively high amount of the active substance11).
To achieve hydrostatic pressure, pellets have to contain osmotically active substances. In our experiments we tested sodium croscarmellose Ac-Di-Sol®, a mixture of microcrystalline cellulose and sodium carmellose Avicel RC 591®, macrogol 6000, and sodium carboxymethyl starch Vivastar® P 5000. The ratio of osmotically active ingredients remained the same, whereas the ratio of microcrystalline cellulose and glucose did not (see Table 1). Osmotically active substances in the core cause water to migrate through the semi-permeable insoluble coating. When threshold pressure is reached, the coat ruptures apart and the active substance is released. With respect to the type and amount of the osmotically active substance that is used, the release time (lag time) of the active substance is different. This experiment is based on a claim by a similar patent that was applied for by the Department of Pharmaceutics12).
Avicel® RC 591 is used in the pelletization process also due to its ability to support spheronization. That is the reason why in one case Avicel® PH 101 was completely supplemented with Avicel® RC 591 (sample RC_75).
In the experiment, purified water was used as the wetting agent. Purified water is able to form an extrudate of desired properties, it is cheap and environmentally friendly. The amount of the added wetting agent for particular batches was set experimentally in previous trials. In all cases, the amount of water needed to prepare a plastic matter decreased proportionally to the increasing ratio between glucose that is easily soluble and microcrystalline cellulose (Avicel® PH 101) that is insoluble and able to bind water on its surface.
Properties of glucose pellets
Produced pellets were dried and tested for flow properties, particle size distribution, sphericity, aspect ratio, and friability (see Table 2).
2. Characteristics of pellet cores 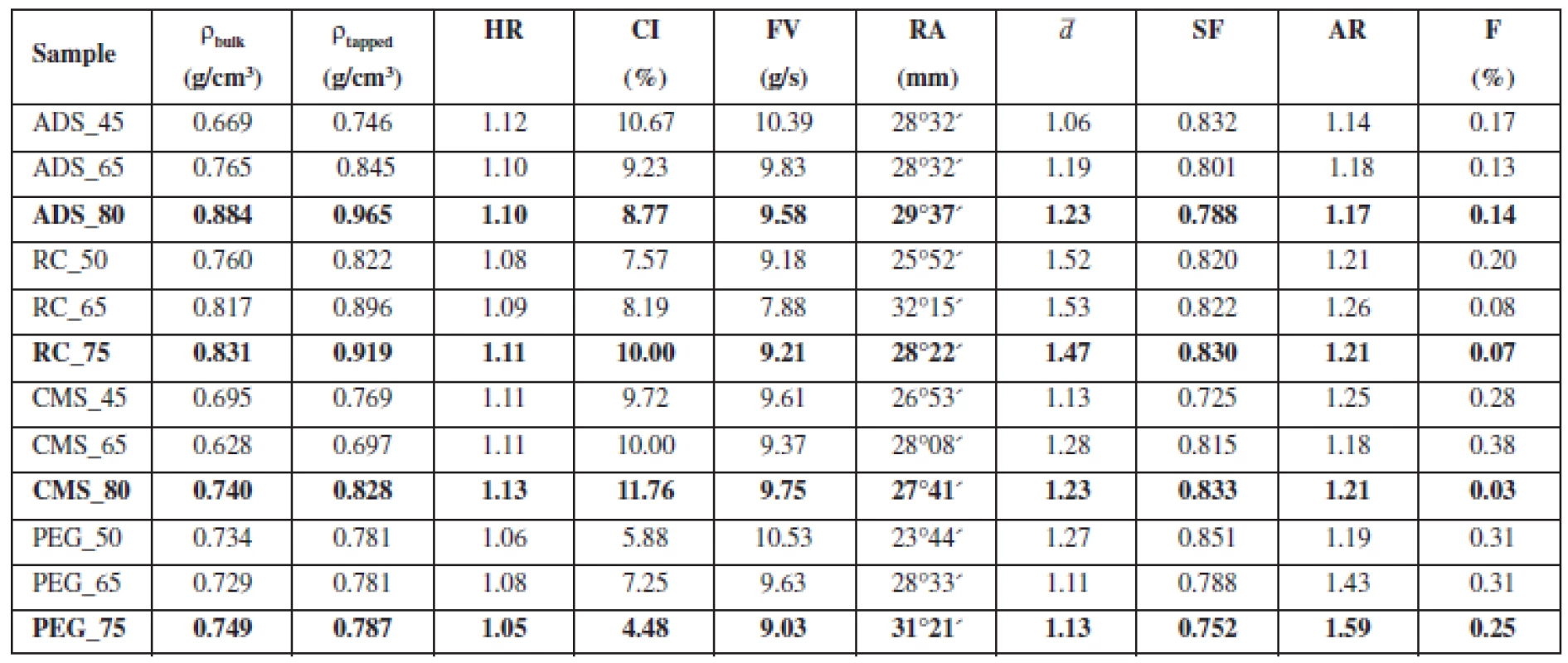
HR – Hausner ratio. CI – compressibility index, FV – flow velocity, RA – angle of repose, ¯d – mean particle size, SF – sphericity factor, AR – aspect ratio, F – friability There were hardly any differences in flow properties. With the exception of batches ADS_45 and CMS_80, HR of all samples was ≤ 1.11 and CI was ≤ 10. Apart from those two limit batches, prepared pellets can be described as having excellent flow properties (CP 2009). The literature suggests that for excellent flow properties flow velocity has to be higher than 9.5–10.5 g/s, which corresponds to glass beads of a diameter of 0.5–1.0 mm13). If we assess flow properties of pellets with respect to this criterion, only the samples with Ac-Di-Sol® would have excellent flow properties. However, flow velocity of other batches was near this value. The angle of repose was measured to be 25–30°, which corresponds to excellent flow properties. In general, all samples can be described as having excellent flow properties, typical of pellets prepared by extrusion/spheronisation14).
The results of sieve analysis (Fig. 1) showed that in the pellets containing Ac-Di-Sol®, sodium carboxymethyl starch and macrogol 6000, the 1–1.25 mm fraction was the most frequent. In pellets containing Avicel® RC 591, the 1.25–2 mm fraction was most frequent. Similarly, the mean particle size was the highest in samples containing Avicel® RC 591. In all samples, the fraction of particles larger than 2 mm amounted at most to 1%, which means that agglomerates were almost absent. The fraction of particles smaller than 0.25 mm amounted at most to 1%, so the amount of powder was small (Fig. 1).
1. Size distribution of pellet cores containing different osmotically active substances; a) Ac-Di-Sol<sup>®</sup>, b) Avicel<sup>®</sup> RC 591), c) CMS, d) PEG 6000 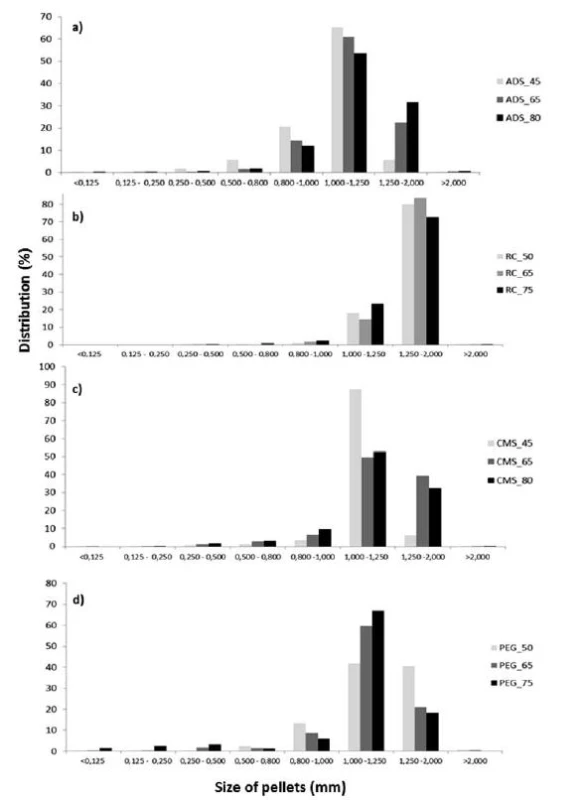
The shape of prepared pellets was evaluated by microscopic analysis, the sphericity factor (SF) was calculated according to Equation 2 and the aspect ratio (AR) was calculated according to Equation 3. Sphericity of pellets ranged between 0.725–0.851. Podczeck et al. opine that sphericity should be higher than 0.8 to be considered sufficient15). If this criterion is used to evaluate the pellets containing glucose, only samples containing Avicel® RC 591 had required sphericity in all samples. The worst results were found for pellets containing macrogol 6000, where only one sample passed, the sample with the highest content of Avicel® PH 101.
The literature suggests that the aspect ratio of pellets should be lower than 1.216). Based on this criterion, all samples containing Ac-Di-Sol® meet the requirements. Surprisingly, samples containing Avicel® RC 591 showed the worst results.
In pellets containing Ac-Di-Sol® and macrogol 6000, the increasing ratio glucose/Avicel® PH 101 was proportional to the decreasing sphericity factor. Low sphericity is the limiting factor for pellets containing a higher amount of glucose, which is in accordance with the data from the literature17). In pellets containing Avicel® RC 591, sphericity values were similar for different glucose/Avicel® PH ratios, which is probably caused by the ability of Avicel® RC 591 to support pellet spheronization and suppress the impact of the decreasing content of Avicel® PH 10118). The only exception were the pellets containing sodium carboxymethyl starch, where sphericity increased with the increasing ratio of glucose/Avicel® PH 101.
Friability is a significant factor concerning mechanical properties of pellets. Low friability is essential for coating process, during which the pellets hit one another and the walls of the coater and any chipped parts have a negative impact on the quality of coating19). In all samples, friability was lower than 1.7%, which is the value required by the literature; all samples have suitable mechanical properties in this area20).
Pellet cores with the highest content of glucose were photographed (Fig. 2). At first sight, the highest portion of regular particles is contained in the sample ADS_80 (with one exception of the barbell-shaped pellet); on the contrary, the lowest portion of regular particles is contained in the sample PEG_75. These observations correspond to the results of microscopic analysis.
2. Optical microscope images of pellet cores with the highest glucose content (magnification 25×) 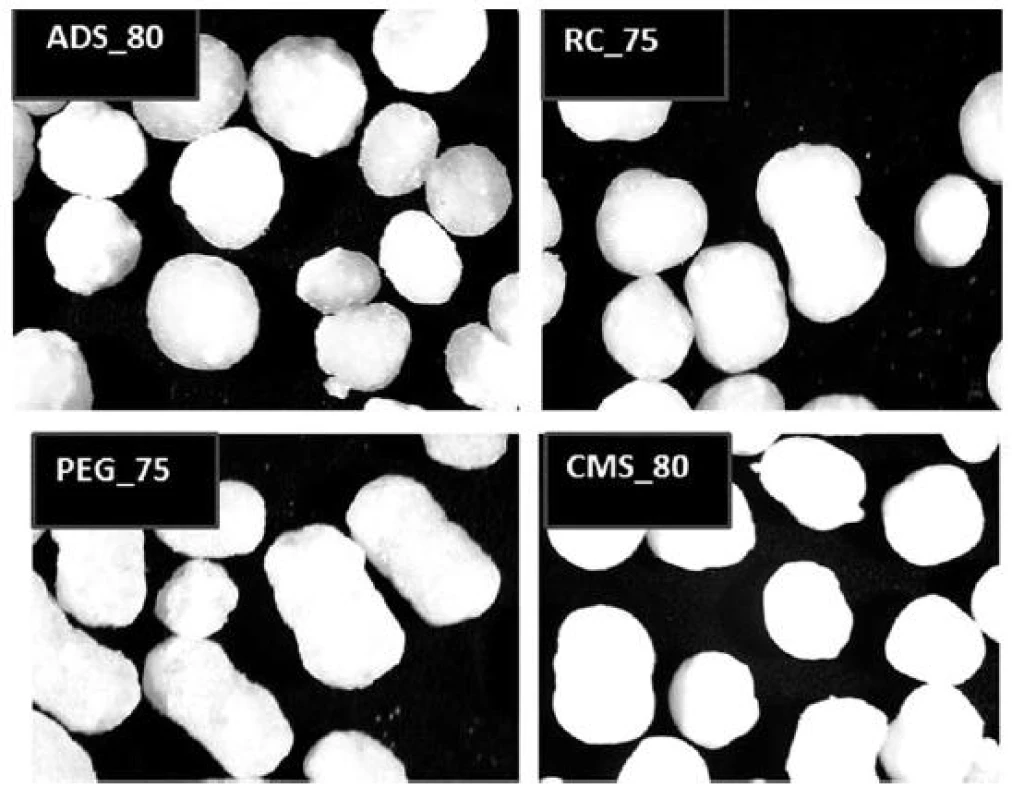
Conclusion
High glucose content (75–80%) pellets were prepared, containing also one of four osmotically active substances (Ac-Di-Sol®, Avicel® RC 591, sodium carboxymethyl starch, macrogol 6000). Pellets are intended for further coating with a semi-permeable membrane that together with osmotically active excipients should allow for a delayed release of glucose during the passage through the gastrointestinal tract because of the coat rupture mechanism. All samples had suitable mechanical and flow properties that are necessary for the coating process. Highest sphericity was found in lower mean particle size pellets containing 80% of glucose, 15% of Avicel® PH 101, and 5% of sodium carboxymethyl starch with and in higher mean particle size pellets containing 75% of glucose and 25% of Avicel® RC 591.
This paper was supported by the project IGA MZ No 14479/2013 Preparation of dosage forms with controlled release of glucose to prevention of hypoglycemic states and the project IGA VFU No 61/2014/FaF Development of pH-dependent dissolution method for control of pellets with controlled release of glucose for the prevention of hypoglycemia.
Conflicts of interest: none.
Received 8 September 2014 / Accepted 16 September 2014
PharmDr. Aleš Franc, Ph.D. (✉) • K. Dvořáčková • J. Muselík • M. Žvaková • V. Slováková • D. Vetchý • D. Sabadková • R. Goněc
Veterinary and Pharmaceutical University in Brno, Faculty of Pharmacy,
Department of Pharmaceutics
Palackého tř. 1/3, 612 42 Brno, Czech Republic
e-mail: franca@vfu.cz
D. Neumann
University Hospital, Pediatric Clinic,
Hradec Králové, Czech Republic
M. Žvaková • R. Goněc
Masaryk Memorial Cancer Institute, Brno, Czech Republic
Sources
1. American Diabetes Association Workgroup on Hypoglycaemia, 2005.
2. Hopkins D. Exercise-induced and other daytime hypoglycemic events in patients with diabetes: prevention and treatment. Diabetes. Res. Clin. Pr. 2004; 65, 35–39.
3. Frier B. M. Morbidity of hypoglycaemia in type 1 diabetes. Diabetes. Res. Clin. Pr. 2004; 65, 47–52.
4. Fisher M., Heller S. R. Hypoglycaemia in clinical diabetes. Chichester, UK. 1999, 167–186.
5. Ryan Ch. N., Gurtunca A. D. Hypoglycaemia: a complication of diabetes therapy in children. Semin. Pediatr. Neurol. 2005; 12, 163–177.
6. The Estee Corp. US Patent, 5545410.
7. Hercules Inc. US Patent, 5795606.
8. Sabadková D., Franc A., Muselík J., Neumann D.. Pelety s riadeným uvoľňovaním glukózy k prevencii hypoglykemií, Čes. Slov. Farm. 2014; 63, 101.
9. Franc A., Muselík J., Sabadková D., Neumann D., Minaříková I. Different size of pellets with controlled release of glucose and dissolution profiles. World meating on pharmaceutics, biopharmaceutics and pharmaceutical technology. Lisbon Portugal 31. March – 3. April 2014.
10. Franc A., Muselík J., Sabadková D., Neumann D. Pellets with controlled release of sugar for hypoglycaemia prevention in diabetes. 9. World meating on pharmaceutics, biopharmaceutics and pharmaceutical technology. Lisbon Portugal 31. March – 3. April 2014.
11. Rowe R. C., Sheskey P. J.. Quinn A. M. E. Handbook of pharmaceutical excipients. 6th ed. London: Pharmaceutical Press, 2009.
12. Neumann D., Franc A., Muselík J. CS Patentová přihláška PV 2013 – 338.
13. Rabišková, M. Technologické parametry lékových mikroforem, jejich význam a metody jejich stanovení. Čes. Slov. Farm. 1996; 45, 177–179.
14. Celine V. L., Siang M. Ch., Heng P. W. S. Elucidation of spheroid formation with and without the extrusion step. AAPS PharmSciTech. 2007; 8, E70–E81.
15. Podczeck F., Rahman S. R., Newton J. M. Evaluation of a standardised procedure to assess the shape of pellets using image analysis. Int. J. Pharm. 1999; 192, 123–138.
16. Chopra R. F., Podczeck J. M., Alderborn G. The influence of pellet shape and film coating on the filling of pellets into hard shell capsules. Eur. J. Pharm. Biopharm. 2002; 53, 327–333.
17. Gajdziok J., Bernatoniene J., Muselík J., Masteiková R., Dvořáčková K., Petkeviciute Z., Lazauskas R., Kalveniene Z., Bernatoniene R. The evaluation of formulations for the preparation of new formula pellets, Pharm. Dev. Technol. 2011; 16, 520–528.
18. Rabišková M., Häring A., Minczingerová K., Havlásek M., Musilová P. Mikrokrystalická celulosa v perorálních lékových formách. Chem. Listy, 2007; 101, 70–77.
19. Wlosnewski J. C., Kumpugdee-Vollrath M., Sriamornsak P. Effect of drying technique and disintegrant on physical properties and drug release behavior of microcrystalline cellulose-based pellets prepared by extrusion/spheronization. Chem. Eng. Res. Des., 2010; 88, 100–108.
20. Rabišková M., Masteiková R., Chalupová Z., Dvořáčková K. Lékové formy a biofarmacie: II. Brno: Veterinární a farmaceutická univerzita 2009.
Labels
Pharmacy Clinical pharmacology
Article was published inCzech and Slovak Pharmacy

2014 Issue 5-
All articles in this issue
- Lyophilisation of protein-based drugs
- Optimization of technological processes for the preparation of tablets with a low content of warfarin by direct compression
- Continuing/life-long education of pharmacists in the Czech Republic 4th cycle 2008–2011
- History of diabetes treatment in Czechoslovakia prior to 1989
- Formulation of cores for the controlled release of glucose for prevention of hypoglycemia in diabetes patients
- Preparation of feed premix for veterinary purposes
- Czech and Slovak Pharmacy
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- History of diabetes treatment in Czechoslovakia prior to 1989
- Lyophilisation of protein-based drugs
- Continuing/life-long education of pharmacists in the Czech Republic 4th cycle 2008–2011
- Formulation of cores for the controlled release of glucose for prevention of hypoglycemia in diabetes patients
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career




