-
Medical journals
- Career
Selected natural phenolic compounds – potential treatment for peripheral neuropathy?
Authors: Karel Šmejkal
Authors‘ workplace: University of Veterinary and Pharmaceutical Sciences Brno
Published in: Čes. slov. Farm., 2014; 63, 55-70
Category: Review Articles
Overview
Neuropathic pain is a syndrome comprising pain caused by a lesion or dysfunction of the nervous system, or resulting from lesions or diseases of the somatosensory system. Neuropathic pain is often connected with adverse effects of chemotherapy administered because of cancer, infiltration of the nervous tissue with cancer cells, neurodegeneration and diabetes mellitus. Disbalance in the production of various cytokines plays an important role in the pathogenesis of many of the diseases connected with neuropathies. These cytokines comprise in particular interleukins IL-1β, IL-15, and IL-6, tumour necrosis factors, and prostaglandins. The biochemistry of the production of cytokines is directed by nuclear factors, which affect the expression of the mRNA for the respective cytokines or enzymes metabolizing the cytokines. The main nuclear factor which regulates the expression of cytokines is NF-κB. Because of insufficient effectiveness or adverse effects of the pharmacological treatment of peripheral neuropathy, many patients seek supportive or adjuvant therapy. Natural compounds which modulate the production of inflammatory cytokines may reduce the symptoms of neuropathies. Many natural phenolic compounds belong to substances affecting the activity of NF-κB and consequently the activity of cytokines which are regulated by this substance. The aim of this mini-review is to present information about three natural phenols which are potentially usable for the treatment of neuropathies: curcumin, resveratrol and mangiferin, and bring attention to the practical usability thereof. Curcumin and mangiferin are active constituents of plants; they have been used for centuries in traditional medicine. Biological effects of resveratrol have been known for a relatively short time; since the discovery of the so-called French paradox, attention has been focused on resveratrol. This summary includes particularly the information related to the influence on the activity of NF-κB, expression of anti-inflammatory cytokines, and antiradical activity, because imbalance between the creation and degradation of free radicals plays an important role in the activation of NF-κB and in inflammatory processes. It also briefly summarizes basic information concerning bioavailability, metabolism and practical application of the aforementioned substances.
Keywords:
phenols • curcumin • mangiferin • NF-κB • peripheral neuropathy • resveratrolIntroduction
Neuropathic pain is defined as pain which is initiated or caused primarily by a lesion or dysfunction of the nervous system, or directly resulting from lesions or diseases affecting the somatosensory system. Cytokines play the key pathogenetic role in several preclinical models of neuropathic and inflammatory pain. An example can be lipopolysaccharide-induced (LPS-induced) hyperalgesia, which can be blocked by antagonists of interleukin IL-1. Similarly, activated glial-fibrilary-acidic-protein-positive astrocytes, which are the source of TNF (tumour necrosis factor), and interleukins IL-1β, IL-15 and IL-6, are present in increased amounts in spinal segments with the projection of nerves affected by peripheral neuropathy. Cytokines cause neuropathic pain by direct action on nociceptors, and also by changing the synthesis of growth factors in nerve fibres. These effects manifest themselves by changes in phenotype of sensorineural endings, changes in support of nerve functions provided by glial cells, or by induction of neuronal degeneration and a slowdown/deterioration of cellular processes1).
Neuropathic pain is commonly reported in various types of cancer processes, as an adverse effect of chemotherapy, or as a direct consequence of nervous tissue infiltration by cancer cells. The role of inflammatory mediators in the induction of neuropathic pain connected with cancer was proven by several studies. Neuropathic pain is also reported in connection with peripheral neuropathy caused by diabetes mellitus2). Neuropathy and microvascular impairment are often observed in diabetics. Insufficient oxygenation of the tissues in the limbs leads to abnormal functioning of nerve axons, and to non-physiological response to trauma and/or infection. Accumulation of inflammatory cells and liquid, and activation of the coagulation cascade contribute to reduced blood supply to neurons. Moreover, advanced glycation products and substances that are released due to the stimulation by these glycation products (TNF-α, interleukins IL-1 and IL-6) cause gradual degeneration of all types of peripheral nerve fibres1).
Reactive oxygen species (ROS) are signalling molecules which are important for many cellular processes3). Disbalance or overproduction of ROS are thought to be related to many pathologic conditions. Peripheral neuropathy and inflammation of nervous tissue are also connected with ROS and their overproduction in impaired tissue and also with a condition called oxidative stress. Increased production of ROS and an effort to compensate the emerging oxidative stress was observed in the spinal cord after peripheral innervation impairment. Various studies showed reduction of accentuated pain and an anti-allodynic effect after the administration of various ROS scavengers. For instance, in tests on rats with induced peripheral neuropathy, vitamin E (a well-known ROS scavenger) had an analgesic effect due to desensitization of neurons and inactivation of NMDA receptors. Increased levels of ROS play a role in the phosphorylation of NMDA receptors (pNR1 subunit, via activated protein kinase). ROS are closely connected with cytokines. TNF-α contributes to increased production of NO (nitric oxide), ROS, eicosanoids and glutamate, and in C-fibres, it induced long termed potentiation (LTP) of signal transmission (again in tests on rats with induced nerve impairment). This effect of TNF-α can be blocked by NF-κB inhibitors. Furthermore, TNF-α and IL-1β may cause impairment of glial cells through the suppression of their ability to remove glutamate molecules from the synapses4–6).
The aforementioned facts suggest that compounds affecting the inflammation in various manners, or compounds affecting oxidative stress, could be useful in the treatment of peripheral neuropathies. Due to insufficient effectiveness or frequent adverse effects of pharmacological treatment of peripheral neuropathy, many patients seek supportive or adjuvant therapy7). Natural compounds which modulate the production of inflammatory cytokines may reduce the symptoms of neuropathies. Such compounds usually have a whole range of targets in the cell (pleiotropic effect), their price is not exaggerated with respect to the potential effect, their toxicity in doses to be administered is low and their availability is relatively good8). However, it remains questionable, if the concentrations of such active substances after usual oral administration are sufficient if the aforementioned effect is to be achieved9).
These substances include also phenolic compounds. Phenolic compounds belong to relatively common secondary metabolites; their structure varies, but they all have a so-called phenolic hydroxyl group. They include shikimates and acetates, or substances biosynthesized via various pathway combinations, e.g. flavonoids, chalcones, xanthones, stilbenes, coumarins, quinones, phthalides and aromatic organic acids. Their biological activity is generally wide and strongly influenced by the character of their skeleton and also by potential substitution on the phenolic hydroxyl group (commonly leading to the formation of glycosides). The most commonly described effects of natural phenolic compounds comprise anti-inflammatory, anti-bacterial, chemoprotective, antidiabetic and other effects. The following text describes the anti-inflammatory and anti-oxidative effects of a number of typical natural phenolic compounds and their potential therapeutic use.
Curcumin
Curcuma (Curcuma longa L.), a plant belonging to the family of Zingiberaceae, is widely used in traditional Indian medicine for the treatment of various inflammatory and other diseases. The typical rhizome and the massive root of a characteristic smell and typical yellow color are used in traditional medicine. The Curcuma family comprises more than 100 species of different use and content of active substances. They are used and planted in particular in China, India, Indonesia, Jamaica and Peru. The medicinal properties have been known for centuries, Ayurvedic medicine uses these plants internally as stomach medications, tonics, and for blood cleansing, externally in prevention and for the treatment of skin diseases. Furthermore, traditional Indian medicine uses curcuma for the treatment of bile production disorders, for anorexia, rhinitis, sinusitis, cough, diabetic lesions, liver function disorders and rheumatism. The current interest in curcuma and the substances contained therein can be tracked back into the seventies, when its anti-inflammatory and anti-bacterial properties and anti-oxidative action have been described10, 11).
The curcuma aroma is caused by a whole range of compounds from a group of sesquiterpenes, which are simple volatile compounds consisting of fifteen carbon atoms. Typical examples are turmerone, curcumene or curdione. Their ratio and the presence of a wide range of other volatile compounds (e.g. monoterpenes) in various curcuma cultivars affect the taste and aroma of curcuma if used as a spice10).
Another group of substances isolated from curcuma is called the curcuminoids. The main representative of curcuminoids is bis-α,β-unsaturated diketone curcumin (diferuoyl methane). Its structure was described already in 1910. Curcumin shows keto-enol tautomerism with the predominance of the keto form in acidic environment and stable enol form in basic media. Curcuma contains 2–5% of curcumin, according to the origin of the source plant. After extraction, it is characterized as yellow crystalline powder, practically insoluble in water, showing good solubility in fats or in ethanol12).
Pharmacodynamic properties
Curcumin was studied in many experiments with the intention to prove its biological activity. Curcumin showed anti-oxidative, anti-inflammatory and anti-cancer effects12). Furthermore, its anti-microbial, hepatoprotective, nephroprotective and antithrombotic effects have also been proved, as well as its protective effect on infarcted myocardium. The hypoglycemic and antirheumatic effects were also observed10, 13, 14). The purpose of this text is not to summarize all biological effects of curcumin ever explored, but to describe effects which might have a relationship to the use of curcumin in the therapy of peripheral neuropathy. The anti-inflammatory and anti-oxidative effects might fulfil this condition.
Curcumin and its derivatives belong to the so-called free radical scavengers15, 16). Phenolic hydroxyl groups, methoxy groups, and the presence of α,β-diketones contribute to their scavenger activity. Curcumin is considered to be a dimer of two molecules of ferulic acid. Ferulic acid also showed scavenging activity in tests in vitro using a whole range of methods. However, if tested on cells, curcumin was more active than ferulic acid17).
Curcumin showed the ability to induce endogenous antioxidative defence mechanisms18). It belongs to multipotent antioxidants19). It is probable that the redox-dependent nuclear transcription factor Nrf2 (nuclear factor (erythroid-derived 2)-like 2) significantly contributes to this effect. Figure 1 shows a diagram of the signalling pathway connected with Nrf2 and the target of curcumin action14). Nrf2 is a transcription factor, which regulates gene expression of antioxidative defence elements and detoxification enzymes of phase 2. In its basic form which is present in cytoplasm, Nrf2 is bound to the Keap1 repressor. Proteasomal degradation of the protein is initiated through polyubiquitination via the action of the Cul3/Rbx1/E3 ubiquitin ligase complex. The response to an inductor, like curcumin, is release of Nrf2 bound to Keap120). Nrf2 is then internalized into the nucleus and in the nucleus, in a complex with the sMAF protein and the CBp/p300 cofactor, it binds to the ARE (antioxidant responsible element) region of the DNA and induces expression of genes for substances of HO-1 type and others. Phosphorylation of serine and threonine residues in Nrf2 via various kinases also allows for Nrf2 cleaving and internalization into the nucleus21). Nrf2 induces expression of a wide range of phase 2 enzymes and components of antioxidative defence mechanisms14). Nevertheless, it has been described that curcumin can e.g. cause inhibition of GST activity in vitro or in vivo22, 23). Table 1 contains an overview of the antioxidative defence enzymes which are affected by curcumin.
Fig. 1. The target of antioxidative action of curcumin and the signaling pathway of Nrf2 (adapted from the literature)<sup>14</sup>) 
1. Overview of the antioxidative defence enzymes affected by curcumin 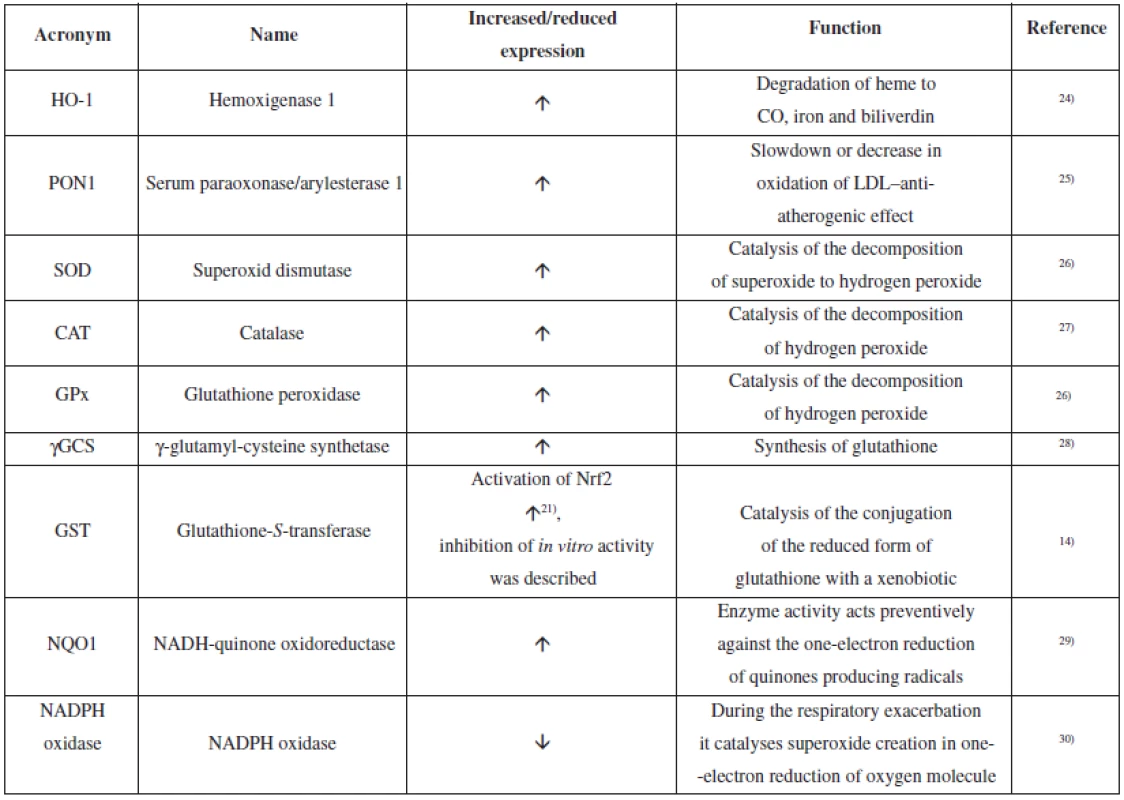
Studies in cellular and animal models demonstrated neuroprotective effects of curcumin14). As shown above, curcumin is an antioxidant and it can protect neurons from oxidative stress, which may occur e.g. due to mitochondrial dysfunction caused by ischemia (and related decrease in the supply of oxygen). Curcumin acts protectively against the loss of mitochondrial membrane integrity and leak of cytochrome c into the cytoplasm and prevents apoptosis31). Curcumin also prevents the formation of β-amyloid peptide and reduces the density of amyloid plaques32). It probably reduces the excitotoxicity by influencing the increased calcium ion influx into the synapses33). Furthermore, curcumin reduces the activation of microglial cells by decreasing the translocation of NF-κB into the nucleus and most likely by preventing the interaction of NF-κB with DNA (Fig. 2)14). NF-κB pathway may be activated in various ways; the tests frequently use LPS stimulation of TLR4 (Toll-like receptor 4). By interaction of LPS with TLR4, the adaptation proteins TRAF 2 and 6 are activated; subsequently, they activate the IKK enzyme complex. Activated IKK enzyme complex catalyses the phosphorylation of the IκBα unit, which, if unphosphorylated, keeps the heterodimer p50 and p65 subunits in the cytoplasm. After the interaction with CBP and the p/300 cofactor, the internalized heterodimer p50/65 binds to a binding site in the DNA and triggers the expression of the corresponding proteins. Curcumin interacts with the NF-κB pathway in several phases, causes its inhibition and, consequently, suppresses the expression of pro-inflammatory enzymes like iNOs (inducible NO synthase) or COX-2 (cyclooxygenase 2), and pro-inflammatory cytokines (e.g. TNF-α, IL-1β). This is an explanation of curcumin-induced suppression of inflammation14, 34).
Fig. 2. Antioxidative action of curcumin on the nerve tissue (adapted from the literature)<sup>14)</sup> 
The treatment of peripheral neuropathy also comprises pain suppression. According to the tests on animals, curcumin suppresses neuropathic pain via inhibition of monoamine system, which is connected with spinal β2-adrenoreceptors and 5-HT1A receptors35, 36).
Pharmacokinetics
Studies completed approximately in the recent 30 years proved a relatively low oral bioavailability of curcumin. Generally, the reasons of the low bioavailability of any substance may include poor absorption, high metabolism rate, inactivity of metabolic products and/or very quick elimination of the substance from the body37).
Various studies demonstrated a relatively low concentration of curcumin in blood serum. Similarly low values were established both in tests on animal models and in clinical studies on humans38–41). The doses up to grams/kg of body weight after oral administration to experimental animals resulted in regularly measurable concentration values after oral administration. However, the tests completed proved that it is not possible to make direct comparisons between the results of studies in humans and rats37).
Absorption and distribution of curcumin in tissues have crucial impact on biological activity and effects of curcumin. Determination of curcumin in tissues was performed basically in mice and rats. Curcumin was monitored in the liver, kidneys, brain, heart and other internal organs. The distribution of curcumin in tissues has been established and according to tests, the intake of curcumin in diet (insufficient doses = low concentrations) cannot be compared with therapeutical administration of curcumin. Thanks to its lipophilicity, curcumin crosses the hematoencephalic barrier (observation from tests on rodents)37).
After absorption, curcumin is quickly metabolized. In tests on rats, predominantly conjugation – i.e. sulphatation and glucuronidation – have been described. After oral administration, the major portion is metabolized in the liver42). The principal metabolites of curcumin are glucuronides and sulphates of tetrahydrocurcumin and hexahydrocurcumin. Minority metabolites are dihydroferulic acid and ferulic acid. Reduction of curcumin is probably catalyzed by alcohol dehydrogenase43).
After absorption and metabolization, curcumin is excreted mostly into the intestine via bile and subsequently removed in the stool. This means that only a minor portion is eliminated via the kidneys and urine. Curcumin is generally well tolerated; phase I clinical testing showed good tolerance up to the dose of 12 g of curcumin per day43).
It is not clear if the metabolites of curcumin are as biologically active as curcumin. Studies dealing with this topic are contradictory; some of them show lower activity, other show comparable or even increased activity. For instance, tetrahydrocurcumin showed better antidiabetic and antioxidant activity in rats with type 2 diabetes mellitus, while its anti-inflammatory and anti-proliferating activity was lower. Reduced and conjugated derivatives of curcumin inhibit the expression of COX-2 to a lesser extent than curcumin. Unlike curcumin, curcumin glucuronides caused inhibition of microtubule protein formation. However, thorough verification of the activity of curcumin metabolites requires further testing44).
Clinical studies
As demonstrated by the aforementioned, curcumin is a very interesting bioactive molecule with remarkable pharmacological potential. Therefore, curcumin has been evaluated in a whole range of minor or major clinical studies, with the aim to analyze its bioavailability and safety, or to verify the presumed therapeutic effects45, 46). The overview of clinical studies relevant for inflammatory diseases is presented in Table 2.
2. The overview of clinical studies of curcumin (verification of bioavailability and safety, relevant for anti-inflammatory activity) 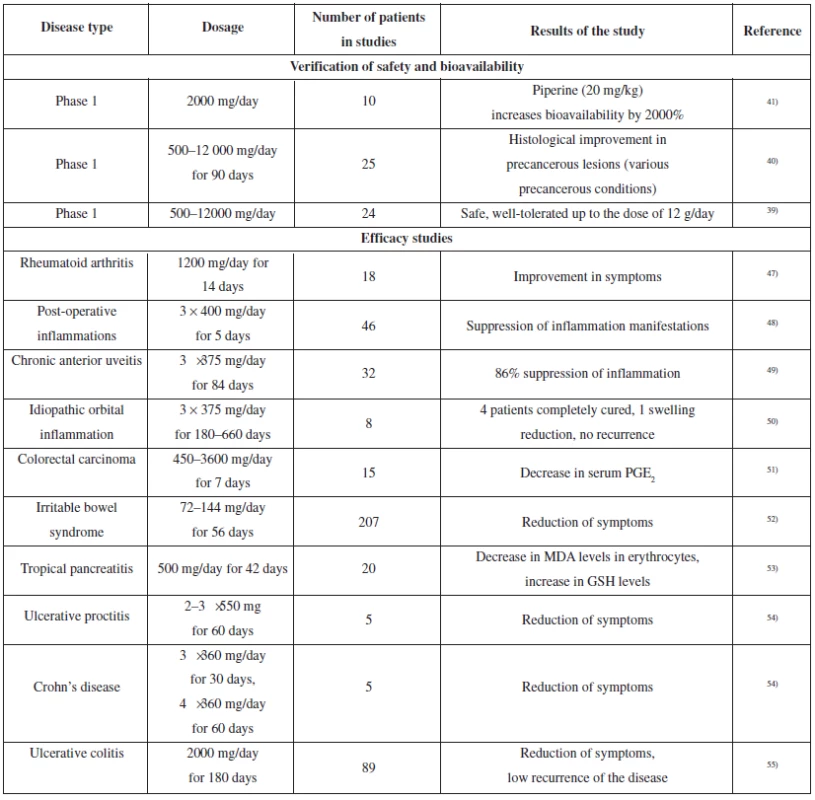
In clinical studies, curcumin showed anti-inflammatory effects comparable with pharmaceutical drugs used. Its antirheumatic effect was compared with phenylbutazone in a double-blinded study. Another study compared effects of curcumin and phenylbutazone on the course of postoperative inflammation.
The anti-inflammatory effect of curcumin was also assessed in two clinical studies performed in patients suffering from rare diseases: chronic anterior uveitis and idiopathic inflammatory orbital pseudotumors. Both studies demonstrated the contribution of curcumin to patient’s condition and symptom improvement, which was comparable with the only therapy used, i.e. corticosteroids.
A few other studies confirmed the effect of curcumin, which comprised improvement in the symptoms of various forms of intestinal inflammation. The improvement was also documented by a decrease in C-reactive protein levels or by the adjustment of erythrocyte sedimentation rate. In a few cases, the patients were able to reduce the dose or cease the treatment with 5-aminosalicylic acid.
The results of various studies including clinical studies suggest that there is some hope for curcumin usability in the treatment of various diseases connected with the inflammatory process. In a few previous years, several dozens of further clinical studies have started (both phase I, phase II and III), which should complete the research into pharmacokinetic parameters and confirm the therapeutic effect of curcumin46).
Resveratrol
Resveratrol is a compound which was an object of enormous interest in the recent years. It belongs to the group of stilbenoids, which are secondary metabolites often produced by plants as a response to a pathogenic attack (which are called phytoalexins). Resveratrol is probably the most popular stilbenoid; from the chemical point of view it is 3,4´,5-trihydroxystilbene. It exists in the form of two geometric isomers cis and trans. It can be found either as a free molecule, or in the form of a glycoside (e.g. as piceid – resveratrol-3-O-β-D - -glucoside). Trans-resveratrol is considered to be the active form56).
It is relatively common in plants. There are several plants containing relatively large amounts thereof, which can be used as the source of this substance. The most known sources are the skins and seeds (5–10%) of grapevine (Vitis vinifera) berries57). In wine grapes, it is produced in response to the presence of the pathogenic fungus Botrytis cinerea. Depending on the technology of grape processing, it can pass into red and white wines, as described in the literature. Red wine usually contains higher amounts of resveratrol due to longer maceration of skins and seeds; the highest concentrations are found in a special cultivar called muscadine, which comes from the south of Northern America (Vitis rotundifolia)58). It is likely that resveratrol is formed during the processing, via hydrolysis of piceid59). In relatively high amounts, resveratrol can be also found in peanuts (Arachis hypogaea, in particular in sprouts)60), and in seeds of black currant (Ribes nigrum), blueberries (Vaccinium myrtillus), mulberries (Morus alba) and grapefruit (Citrus paradisis)56). High amounts are present in Japanese knotweed (Polygonum cuspidatum), which is a well-known invasive plant species61).
Resveratrol was described in the fourth decade of the 20th century, but the interest in this plant has increased since the discovery of its pharmacological properties62).
Pharmacodynamic properties
Attention has been focused on resveratrol since the discovery of so-called French paradox. A popular epidemiological study discovered that the relatively low incidence of cardiovascular diseases in inhabitants of Southern Europe (in particular in French) does not correlate with their relatively high dietary intake of saturated fats. It was discovered that trans-resveratrol might be the factor responsible for this paradox. This molecule attracted attention due to the fact that it showed several biological effects, e.g. anti-inflammatory, anti-apoptotic, antioxidant, antidiabetic, antiviral and cardioprotective activity63, 64).
Resveratrol has been considered one of the substances suitable for the treatment of neurodegenerative diseases (Fig. 3)65). In tests on rats, resveratrol proved effective on the formation of amyloid plaques; it also reduced the secondary impairment of spinal cord after spinal injuries. Effects of this type were connected with the inhibition of COX-1, inhibition of lipoperoxidation and increase in reduced gluthatione65).
Fig. 3. Schematic description of the effects of resveratrol on the nerve tissue 
Effects of resveratrol could also have a relationship to neuropathy. Because oxidative stress is a primary pathogenetic mechanism, which mediates the effects of hypoxia and advanced glycation on biological systems, it is possible to assume that resveratrol, which is a potent antioxidant, could have a therapeutic effect on diabetic peripheral neuropathy. Tests on diabetic rats showed that extracts from grapefruit seeds (which are rich in resveratrol) reduced demyelinization and improved conduction velocity in motor nerves and also improved morphology of Schwann cells. Another neuroprotective effect of resveratrol could be mediated by activation of SIRT1 and subsequent increase in the levels of glutathione and glutamine in neurons65). The aforementioned facts suggest that neuroprotective effects of resveratrol could be connected with its antioxidant and anti-inflammatory activity.
Some of the neuroprotective effects of resveratrol could be mediated by microglial cells. Figure 4 shows the diagrams of potential mechanisms: uptake or inhibition of ROS production, suppression of MAPK signaling pathway, activation of SIRT1-mediated pathway connected with the suppression of NF-κB, and inhibition of NF-κB. These actions subsequently reduce the production of pro-inflammatory substances and alternatively exhibit a neuroprotective effect66).
Fig. 4. Resveratrol prevents the impairment of neurons via several mechanisms 
Excessive production of reactive forms of oxygen, e.g. superoxides, hydroxyl radicals, peroxyl radicals and hydrogen peroxide, may cause lipoperoxidation, oxidation of proteins and DNA fragmentation, which may lead to cell death67). The nervous tissue is relatively sensitive to oxidation stress due to its weak antioxidation defence. Moreover, the reaction of NO with superoxide leads to the formation of peroxynitrite, which also impairs the cells via nitration of various structures66). Antioxidant effects of resveratrol can be easily tested in vitro. Resveratrol is a potent in vitro scavenger of ROS, because the structure containing three phenolic hydroxyls readily binds the radicals, e.g. DPPH or ABTS, even better than the typical antioxidants as Vitamin C, Vitamin E or propyl gallate68). In the inflammatory environment, the key enzyme in free radical formation is NADPH oxidase. The activation thereof requires translocation of the phosphorylated cytosol subunits (p47, p67 and p40) on the cell membrane and binding to cytochrome b558, which consists of p22 and gp91 subunits. Resveratrol, apart from its direct antioxidant effect, inhibits the translocation of the p47 subunit to the membrane, and blocks the NADPH oxidase, which leads to a decrease in ROS formation during the inflammation69).
Mitogen-activated protein kinases (MAPK), which are activated e.g. by LPS or by oxidative stress, in particular ERK1/2, p38 and c-Jun-N-terminal kinases (JNK), are involved in one of the principal signaling cascades, which are responsible for increased expression of TNF-α and iNOS in glial cells. Intracellular ROS products of H2O2 superoxide conversion induce the activation of MAPK cascade. Inhibitors of NADPH oxidase and ROS scavengers prevent activation of MAPK and NF-κB and this mechanism interferes with the inflammatory cascade. Resveratrol interferes with the phosphorylation of ERK 1/2, p38 and JNK and hereby suppresses the inflammatory reaction68).
NF-κB is the most important transcription factor involved in the inflammatory reaction. It regulates the production of the whole range of inflammatory factors, including NO, TNF-α and interleukin IL-1β. Several studies showed that resveratrol suppresses the activation of NF-κB through the inhibition of phosphorylation and degradation of IκBα, and hereby reduces the expression of genes coding the production of TNF-α, iNOS, IL-1β, and IL-6. Apart from this, resveratrol activates SIRT 1. SIRT 1, a member of the family of sirtuins, is an enzyme widely present in the neural tissue, fat tissue, kidneys, liver and muscles. The increase in the activity of SIRT 1 is connected with the defence against oxidation impairment, which has been described in neurodegerative diseases. SIRT 1 is also connected with the suppression of the NF-κB pathway. Therefore, resveratrol-mediated activation of SIRT1 can serve as a key to its action on inflammation-induced neurological diseases70, 71).
Pharmacokinetics
Resveratrol is a relatively lipophilic molecule with phenolic hydroxyls; as such, it easily passes through membranes. It is absorbed after oral administration. According to current findings, the absorption of resveratrol is not only passive, but it is also mediated by specific transporters. Metabolites of resveratrol (glucuronides and sulphates) are actively excreted into the intestine. Following absorption, resveratrol is extensively metabolized and interacts with and modulates a whole range of enzymes, which belong both to phase I and II of biotransformation (P450, glutathione-S-transferase, catechol-O-methyltransferase). The principal process is performed using the cytochrome oxidase system P450 (isoforms CYP1B1, CYP1A2, CYP3A4, and CYP2D6)65). One of its principal metabolites is the monohydroxylated compound piceatannol. Piceatannol belongs to the active metabolites of resveratrol – it inhibits the activity of various proteinkinases, inhibits the activity of NF-κB, and acts as an apoptosis inductor72). Other resveratrol metabolites comprise the products of conjugation reactions – 3-O-sulphate and 3-O-β-D-glucuronide. Resveratrol-3-O-β-D-glucuronide has been identified as the principal metabolite in tests on rats; after oral administration to humans, the major metabolites also include resveratrol sulphate and resveratrol glucuronides. Sulphatation is the limiting factor for the bioavailability of resveratrol. However, higher concentrations of resveratrol cause inhibition of sulphotransferases (e.g. 1A1), which are responsible for the sulphatation of resveratrol. On the other hand, resveratrol sulphates are also bioactive, although probably to a lesser extent than resveratrol itself. They are created on a large scale e.g. in Caco-2 human bowel cancer cells73). Due to quick metabolization, the bioavailability of resveratrol is approximately 1%74).
Clinical studies
Several clinical studies dealing with resveratrol have been conducted; the aim was to verify the effects which had been observed in laboratory tests in vitro or in animals in vivo. Most of the studies describe pharmacokinetics and metabolism of resveratrol75). Selected studies are summarized in Table 3.
3. The overview of clinical studies of resveratrol (verification of bioavailability and safety, relevant for anti-inflammatory activity) 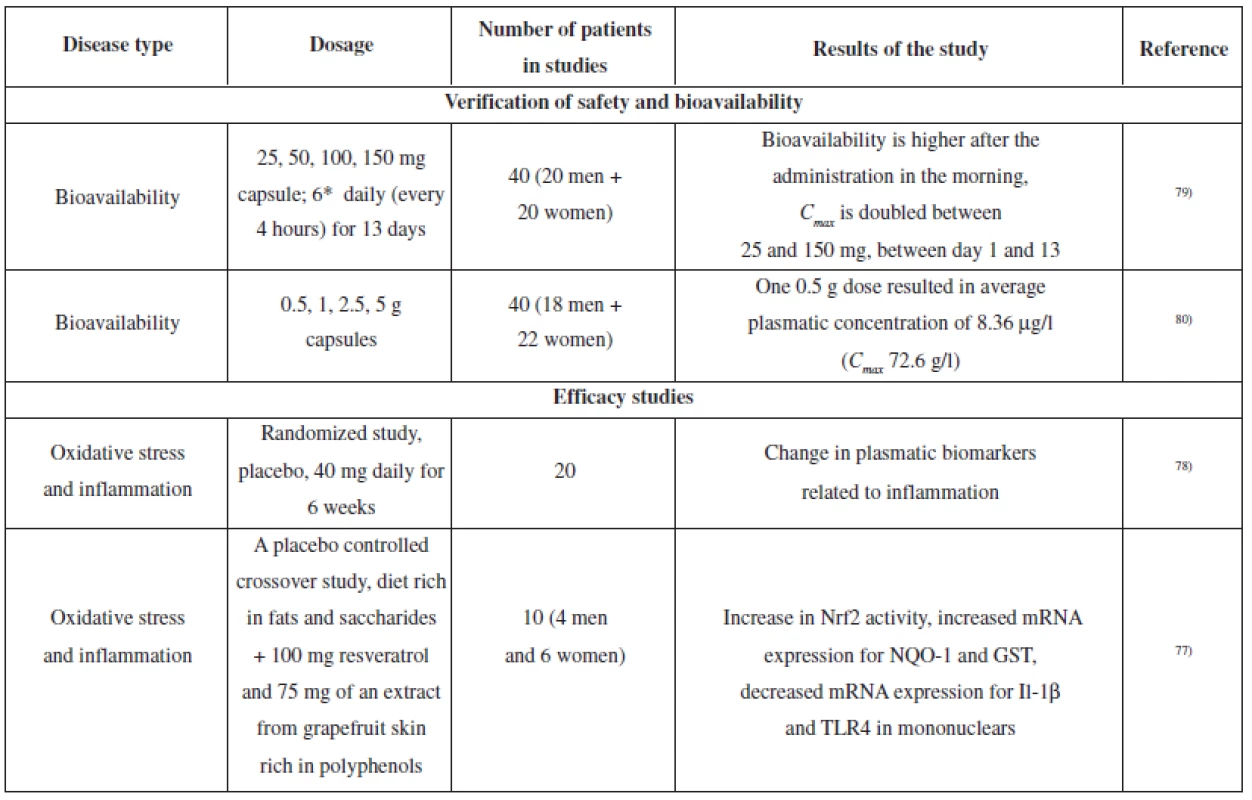
In relation to the protective effects of resveratrol on the nervous tissue, an exercise test on healthy volunteers revealed the efficacy of a 500 mg dose on increased blood flow in the prefrontal cerebral cortex76). Another study reported oxidative stress reduction in healthy volunteers. Volunteers were on a diet which was rich in fats and saccharides and used placebo or a product containing 100 mg of resveratrol and 75 mg of polyphenols extracted from grapefruit skin. The product containing resveratrol and grapefruit polyphenols suppressed the onset of oxidative stress and stimulated the activity of Nrf2 and subsequently induced expression of genes involved in antioxidation defence (NQO1 and glutathione-S - -tranferase)77).
A study on healthy volunteers explored the influence of 6-week administration of an extract from P. cuspidatum (containing 40 mg of resveratrol) on plasmatic cholesterol levels (in the fasting state, LDL, HDL, and total cholesterol were measured). The study did not prove any influence on these parameters; however, the mononuclears from the “resveratrol group” showed suppression of NF-κB activity and decreased levels of ROS, TNF-α and IL-6. Furthermore, there was a decrease in the levels of plasmatic TNF-α and C-reactive protein78).
These results suggest the potential for resveratrol use in the treatment of inflammatory diseases.
Mangiferin
Mangiferin (C-2-β-D-glucopyranosyl-1, 3, 6, 7-tetrahydroxyxanthon) belongs to xanthones. It belongs to C-glycosides, which are relatively resistant to hydrolysis. Crystalline mangiferin is a light yellow substance, well-soluble in water and other polar solvents, less soluble in medium-grade polar solvents and practically insoluble in lipophilic media. Mangiferin is a stable compound; however, it easily reacts with oxidizing agents. It is relatively common in nature, some plants are relatively richer in mangiferin. Mangiferin frequently occurs together with isomangiferin (4-C-β-D-glucopyranosyl-1,3,6,7-tetrahydroxyxanthone), a methoxy analogue called homomangiferin (2-C-β-D-glucopyranosyl-1,6,7--tetrahydroxy-3-methoxyxanthone) and an O-glycosylated derivative neomangiferin (mangiferin-7-O-β-D-glucopyranoside)81).
The most common method of mangiferin production is extraction from mango (Mangifera spp., Anacardiaceae), from the leaves (2–5%)82), fruit (0.3 % in the skin, less than 1% in the pulp) or peel (1.2–1.8%)83). Other sources are irises: Iris unguicularis (Iridaceae), which comes from Greece and Eastern Mediterranean (rhizome 0.7%)84), rhizome of Anemarrhena asphodeloides (Asparagaceae) (1–5%)85), a plant used in traditional Chinese medicine (zhi mu), Salacia spp., Celastraceae, a traditional plant used in Ayurvedic medicine (roots of S. chinensis – 1.6%, S. reticulata 1.4%)81), and also Cyclopia spp., Fabaceae – honeybush, a traditional plant used in the medicine of Southeast and Southwest Africa (leaves 1.6–3.6%)81).
Mangiferin as a bioactive compound is often reported in connection with the so-called Vimang. Vimang is a name for standardized watery extract from the peel of selected mango varieties (Mangifera indica L.), which contains defined amounts of various components, including polyphenols (mangiferin), triterpenes, phytosterols, fatty acids and microelements. Vimang containing formulations are registered and administered in particular in Cuba4).
Pharmacodynamic properties
Many studies consider mangiferin to be the potential active principle of the extracts from mango peel and leaves; it definitely contributes to their biological activity. Various studies demonstrated that mangiferin and mangiferin-rich extracts show, among others, anti-oxidative, anti-cancer, anti-microbial, anti-atherosclerotic, anti-allergic, anti-inflammatory, analgesic and immunomodulatory effects86).
Mangiferin showed neuroprotective effect in tests on models of glutamate-induced impairment of glial cells or neurons. Furthermore, mangiferin can reduce the inflammatory lipopolysaccharide-stimulated microglial activation, which is manifested through a decrease in ROS production, suppression of COX-2 expression and a decrease in PGE2 synthesis81).
Mangiferin is a potent antioxidant. Its antioxidation effect is mediated in particular via the Fe2+ chelating activity. Ortho-dihydroxy group in mangiferin structure clearly shows the potential of mangiferin for metal ion chelating87). The unstable complex mangiferin-Fe2+ is subsequently oxidized to a more stable complex mangiferin-Fe3+, which is no more capable of lipoperoxidation initiation or support. This complex also prevents the “regeneration” of Fe3+ to Fe2+, e.g. via ascorbate, and through this mechanism, it eliminates Fe2+ ions from reactions necessary for the production of e.g. hydroxyl radicals (inhibition of Fenton’s reaction). Oxidative stress suppression subsequently manifests itself through decreased ROS production, which is connected e.g. with the phosphorylation of the pNR1 subunit of NMDA receptors83).
Furthermore, mangiferin is involved in the pathway of glutamate induced neurotoxicity (Fig. 5). Stimulation of the presynaptic neuron leads to the release of all excitation mediators, e.g. glutamate. Interaction of glutamate with the postsynaptic NMDA receptor leads to a cascade of reactions, and, consequently, increases the concentration of NO (stimulation of nNOS and increased expression and activation of iNOS) and PGE2 (stimulation of phospholipase A2, release of arachidone acid and its metabolization via COX-2)88). NO passing through membranes may influence the adjacent neurons and nerve endings, or, potentially, glial cells, and induce a system of secondary messengers. This causes an increase in cGMP levels and activation of cGMP-dependent kinases, which may further increase the release of glutamate. Sensitization of nociceptors caused by prostaglandins is an important factor for the development of inflammatory hyperalgesia. PGE2, via the interaction with the EP2 receptor (prostaglandin E2 receptor), activates adenylyl cyclase (AC), and increases the production of cAMP, which may activate cAMP-dependent protein kinase A (PKA), which, in turn, stimulates further release of glutamate89). Mangiferin inhibits glutamate release, inhibits the expression of nNOS and iNOS and, consequently, NO production, inhibits the expression of COX-2 and, consequently, the production of PGE2 and other prostaglandins. Mangiferin inhibits the activation of NF-κB mediated by TNF-α, its translocation into the nucleus, and expression of genes involved in the synthesis of pro-inflammatory cytokines4).
Fig. 5. Mangiferin influences glutamate-induced neurotoxicity (adapted from the literature)<sup>4)</sup> Legend: C-fibre ending – Glutamate – Synapse – Postsynaptic neuron – Arachidonic acid – Pro-dynorphine 
Anti-nociceptive activity of mangiferin was confirmed by pain perception testing in mice, with pain induction by application of capsaicin, formalin or acetic acid. The results of the tests suggested that the mechanism of action is connected with anti-inflammatory and antioxidant effects of mangiferin. Moreover, the analgesic effect was reversible after naloxone administration, which suggests the interaction between mangiferin and the peripheral action of endogenous opioids via NO/cGMP K+ATP channels. Furthermore, the action of mangiferin was connected with the interaction with adenosine receptors90, 91).
Peripheral neuropathy is often reported as a symptom of diabetes mellitus. Mangiferin has both pancreatic and extrapancreatic antidiabetic effects. It has been proved that the administration of mangiferin decreases glycaemia by decreasing intestinal absorption of glucose. Mangiferin inhibits glucosidase (saccharase, isomaltase and maltase) in tests on rats92), which affects glucose level via a slowdown of intestinal absorption. Mangiferin may contribute to overall antidiabetic action via the influence on the levels of blood triglycerides and decrease in atherogenic index (tests on rats). Increased levels of triglycerides provide a source of free fatty acids which may be preferentially metabolized by oxidation and may decrease the sensitivity of cells to insulin action93). Mangiferin inhibits PTPB1 (protein tyrosin phosphatase B1), an enzyme responsible for the dephosphorylation (deactivation) of the active insulin receptor in muscles and the liver, and for the deactivation of JAK2 (Janus kinase, protein tyrosin kinase) in the hypothalamus. Both mechanisms play important roles in regulation of glycaemia94).
Pharmacokinetics
The chemical structure of mangiferin meets several conditions for good oral bioavailability (Lipinski rules): molecular mass less than 500, presence of less than 5 donors for the formation of hydrogen bridges, less than 10 acceptors for the formation of hydrogen bridges, log P lower than 5 (log P for mangiferin is 2.73). After all, these conditions are met by all compounds described herein86).
Only a few studies have tested pharmacokinetic parameters of mangiferin. HPLC analysis of mangiferin plasmatic concentrations after oral administration in a study on human volunteers showed dependency of absorption on dose, plasmatic distribution, and presence in extracellular liquids, without specific distribution in the tissues. Pharmacokinetics of mangiferin is non-linear, which suggests certain saturation of the organism and binding on plasmatic proteins. Important differences in pharmacokinetics were observed after the comparison between intravenous and oral administration. According to some findings, mangiferin may inhibit the activity of selected CYP isoforms, which may cause certain interaction with other xenobiotics81).
Several experiments showed low bioavailability of mangiferin after oral administration. The plasmatic levels were in nanograms per millilitre. This means that mangiferin is absorbed; however, it is subjected to the first-pass effect in the liver95). Mangiferin is probably metabolized by intestinal microflora – an in vitro study suggested that C-glycosidic bond might be subjected to cleavage and aglycone is released96).
Therefore, mangiferin metabolites might also participate in biological effects of mangiferin. Tests on rats identified 1,3,7-trihydroxyxanthone, 1,3,6,7-tetrahydroxyxanthone (trivial name norathyriol), 1,3,6-trihydroxy-7-methoxyxanthone and 1,7-dihydroxyxanthon in plasma97). E.g. its aglycone, norathyriol, has anti-oxidative or anti-inflammatory effects86).
Clinical studies
So far, mangiferin has not been subject to a genuine clinical study as a substance with an anti-inflammatory effect. However, bioavailability of mangiferin has been tested; the aim was to optimize the methods for monitoring of mangiferin plasmatic levels95).
Two in vivo studies in humans assessed the effects of mangiferin-rich extracts from M. indica (Vimang) on inflammation and oxidative stress. A study completed by Pardo-Andreu et al. explored the effect of Vimang in elderly people (aged over 78). Drinking of Vimang extract increased the activity of extracellular SOD and the total antioxidant status in serum (ABTS test). The levels of oxidized glutathione and lipoperoxidation were also reduced (measured using TBARS)98). However, it is necessary to say that Vimang extract also contains other phenols which may contribute to its effects.
Cancer patients who used mangiferin-rich extract from M. indica showed improved quality of life (appetite, increase in body weight, self-sufficiency in everyday life etc.). From a clinical point of view, they showed evident reduction of inflammation and pain manifestations, including biochemical markers, as e.g. transaminase levels99).
Peripheral mononuclears (white blood cells) obtained from the blood of a person using oral mangiferin were partially protected from radiation impairment. However, it has been described that the protective effect of mangiferin was significant only after higher radiation doses and was missing in cells stimulated to proliferation100).
Practical use
Practical use of curcumin
Relatively poor absorption and biodistribution, extensive metabolism and quick elimination of curcumin suggested problems with bioavailability of this pharmacologically noteworthy substance. However, there are methods for elimination of bioavailability problems. Adjuvants, which block the metabolic processes of curcumin, combined in an orally administered formulation, pharmaceutical forms as e.g. nanoparticles, liposomes, micelles and phospholipide complexes, may increase absorption, prolong the elimination half-life of curcumin and increase the plasmatic concentrations of this compound37).
One of the options being explored is concomitant administration of piperine as an inhibitor of intestinal and hepatic glucuronidation. The tests of concomitant administration of curcumin and piperine on healthy volunteers found 2000% increase in bioavailability of curcumin. Similarly to other studies, the effect of a similar combination was only mild if tested on rats. Another study in humans showed doubled absorption of curcumin after the administration in combination with piperine41).
Other substances have also been tested in connection with the potential improvement in bioavailability or effects of curcumin. The combination of curcumin and quercetin proved efficient in the treatment of intestinal polyps101), a synergistic effect of the combination of curcumin and genistein against pesticide-induced growth of estrogen-dependent cells of breast cancer (MCF-7) has been described102). The combination of curcumin with eugenol and terpineol caused augmentation of the transdermal passage of curcumin103).
Another option being tested is the application of curcumin in the form of nanoparticles. Nanoparticles represent a promising method of administration with a potential for improving the absorption of lipophilic compounds like curcumin, which demonstrate poor water solubility. So far, only a few studies have been performed which describe the application of curcumin nanoparticles104). It has been ascertained that curcumin nanoparticles and “classic” curcumin exhibit the same effect on pancreatic cell line in vitro. The effect of curcumin nanoparticles on the activation of NF-κB and inhibition of pro-inflammatory cytokine production has been confirmed. The formulation with curcumin nanoparticles prevents oxidative degradation of curcumin. Local administration of curcumin nanoparticles increases efficacy.
Effective substances can be advantageously administered in formulations with liposomes, micelles and phospholipide complexes because these complexes may integrate both hydrophilic and lipophilic compounds. Liposomal curcumin in combination with oxaliplatine proved efficient both in growth inhibition and apoptosis stimulation in vitro and in vivo. In general, micelles and phospholipide complexes can improve gastrointestinal absorption of natural substances and consequently increase their plasmatic levels, slow down the elimination, and improve bioavailability. Various studies confirmed this effect also for curcumin105).
Practical use of resveratrol
Resveratrol is practically nontoxic even in relatively high doses. A single dose of 5 g did not cause any side effects to healthy volunteers and long-term administration of 2000 mg twice daily was well-tolerated, although occasional occurrence of diarrhoea, nausea and headache have been reported.
Bioavailability of resveratrol is probably significantly influenced by the method of administration and amount and type of administered food. As already mentioned, high levels of glucuronides and sulphates in plasma and urine after the administration of pure resveratrol have been observed. The administration of resveratrol glycosides, e.g. in grapefruit juice, tended to reduce bioavailability, although there is a study which showed the opposite. Bioavailability of resveratrol was not significantly influenced by the presence of other phenols in white wine or in vegetable juice. On the other hand, the ratio of food components, e.g. of proteins, saccharides, fats, fiber, and/or alcohol intake, may remarkably affect the bioavailability of resveratrol. The bioavailability of resveratrol has circadian rhythm (the best time for administration is the morning).
To slow down the quick metabolism of resveratrol, it is possible e.g. to encapsulate the substance in the liposomes. Another option could be piceid (resveratrol glycoside) administration, which is hydrolyzed only in the colon or in enterocytes, and therefore increases the bioavailability of aglycone75, 106).
Practical use of mangiferin
Toxicity of mangiferin is probably very low (e.g. after i.p. administration in rats it is LD50 365 mg/kg)107); various data suggest that the safe dose of mangiferin in mice is 25 g/kg108). Furthermore, plants containing mangiferin have been used as medicinal plants without problems for centuries.
The bioavailability of mangiferin can be a problem, currently there are no data available which could confirm sufficient concentrations in blood after oral administration. Therefore, it is questionable if concentrations achieved in vivo are sufficient to reach therapeutic effects observed in vitro.
Conclusion
Phenolic compounds found in plants belong to natural substances with an interesting pharmacological potential. They show a broad-spectrum action, which comprises also anti-inflammatory, analgesic and neuroprotective effects. An unquestionable advantage of these plant phenolic compounds is their very low toxicity. The substances described herein, i.e. curcumin, resveratrol, and mangiferin, are examples of such compounds. Curcumin, resveratrol, and mangiferin are components of many medicinal plants, drugs and plant formulations with biological activity. Their effects have been explored and documented in vitro; curcumin and resveratrol were, although to a limited extent, tested in vivo and in several clinical studies. The mechanisms of the anti-inflammatory and neuroprotective effects of these substances involve action on the expression of pro-inflammatory and anti-inflammatory cytokines mediated via the interaction of these substances with the NF-κB pathway. Furthermore, there are reports concerning the influence on the production of excessive amounts of free radicals (ROS and RNS) and their direct uptake. The problems connected with the administration of curcumin, resveratrol and mangiferin could be their relatively low bioavailability and intensive metabolism. It seems that the problems with bioavailability could be solved by administration in combinations or in suitable formulations. Application of nanoparticles, liposomal particles, or use of adjuvants affecting (slowing) the metabolism of these phenolic compounds have been described.
Some studies suggest the suitability of combining several natural substances. In tests of antioxidant activity in vitro, curcumin and resveratrol showed a significantly synergistic effect. It is assumed that resveratrol might regenerate curcumin, or that the antioxidant activity of resveratrol might be potentiated by the presence of curcumin, which acts as a chelating agent on metal ionts109). Synergistic effects of curcumin and resveratrol were observed also in another in vitro study on Hepa1-6 cells of hepatocellular carcinoma, in which the combination lead to synergistic antiproliferative effect and activation of caspases, and increased the production of ROS (signs of apoptosis)110). Similar effects have been also observed in vitro, in other types of cancer cell lines111, 112). Further in vitro studies showed positive influence of the curcumin-resveratrol combination on the modulation of inflammation in human chondrocytes; in this setting, the NF-κB-regulated expression of genes for inflammation-related substances (e.g. COX-2, MMP-3, and MMP-9 and VGEF) was successfully suppressed, and apoptosis was inhibited (Bcl-2, Bcl-xL, and TNF-α-receptor-associated factor 1). The administration of a mixture of curcumin and resveratrol suppressed IL-1β-induced activation of NF-κB via the inhibition of I and proteasome activation, inhibition of IκBα phosphorylation and degradation, and inhibition of the translocation of NF-κB into the nucleus113). Therefore, the combination of curcumin and resveratrol has a scientific rationale; further potentiation of the effects might be possible by adding mangiferin to the combination.
Data summarized in this short overview show that curcumin, resveratrol, or mangiferin might be useful in the treatment of various diseases with an inflammatory component, including peripheral neuropathies.
Conflicts of interes: none.
Received 25 Februar 2014
Accepted 6 March 2014
doc. PharmDr. Karel Šmejkal, PhD. (∗)
University of Veterinary and Pharmaceutical Sciences Brno
Faculty of Pharmacy, Department of Natural Drugs
Palackého 1/3, 612 42 Brno, Czech Republic
e-mail: karel.mejkal@post.cz
Sources
1. Höke A. Animal Models of Peripheral Neuropathies. Neurotherapeutics 2012; 9, 262–269.
2. Iyer S, Tanenberg R. J. Pharmacologic management of diabetic peripheral neuropathic pain. Expert Opin. Pharmacother. 2013; 14, 1765–1775.
3. Finkel T. Oxygen radicals and signaling. Curr. Opin. Cell Biol 1998; 10, 248–253.
4. Garrido-Suárez B. B., Garrido G., Delgado R., Bosch F., Del C. Rabí M. A Mangifera indica L. Extract Could Be Used to Treat Neuropathic Pain and Implication of Mangiferin. Molecules 2010; 15, 9035–9045.
5. Carozzi V. A. Ceresa C. The Role of Glutamate in Diabetic and in Chemotherapy Induced Peripheral Neuropathies and its Regulation by Glutamate Carboxypeptidase II. Curr. Med. Chem. 2012; 19, 1261–1268.
6. Nickel F. T., Seifert F., Lanz S., Maihöfner C. Mechanisms of neuropathic pain. Eur. Neuropsychopharmacol. 2012; 22, 81–91.
7. Finnerup N. B., Sindrup S. H., Jensen T. S. The evidence for pharmacological treatment of neuropathic pain. Pain 2010; 150, 573–581.
8. Head K. A. Peripheral Neuropathy: Pathogenic Mechanisms and Alternative Therapies. Altern. Med. Rev. 2006; 11, 294–329.
9. He S. M., Chan E., Zhou S. F. ADME properties of herbal medicines in humans: evidence, challenges and strategies. Curr. Pharm. Des. 2011; 17, 357–407.
10. Jayaprakasha G. K., Jagan Mohan Rao L., Sakariah K. K. Chemistry and biological activities of C. longa. Trends Food Sci. & Technol. 2005; 16, 533–548.
11. Shishodia S., Sethi G., Aggarwal B. B. Curcumin: Getting Back to the Roots. Ann. N.Y. Acad. Sci. 2005; 1056, 206–217.
12. Lee W. H., Loo C. Y., Bebawy M., Luk F., Mason R. S., Rohanizadeh, R. Curcumin and its Derivatives: Their Application in Neuropharmacology and Neuroscience in the 21st Century. Curr. Neuropharmacol. 2013; 11, 338–378.
13. Maheshwari R. K., Singh A. K., Gaddipati J., Srimal R. C. Multiple biological activities of curcumin: A short review. Life Sci. 2006; 78, 2081–2087.
14. Esatbeyoglu T., Huebbe P., Insa E. M. A., Chin D., Wagner A. E., Rimbach G. Curcumin – From Molecule to Biological Function. Angew. Chem. Int. Ed. 2012; 51, 5308–5332.
15. Singh U., Barik, A., Singh, B. G., Priyadarsini, K. I. Reactions of reactive oxygen species (ROS) with curcumin analogues: Structure-activity relationship. Free Rad. Res. 2011; 45, 317–325.
16. Feng, J.-Y., Liu Z.-Q. Phenolic and Enolic Hydroxyl Groups in Curcumin: Which Plays the Major Role in Scavenging Radicals? J. Agric. Food Chem. 2009; 57, 11041–11046.
17. Ak T., Gulcin I. Antioxidant and radical scavenging properties of curcumin. Chem. Biol. Interact. 2008; 174, 27–37.
18. Calabrese V., Bates T. E., Mancuso C., Cornelius C., Ventimiglia B., Cambria M. T., Di Renzo L., De Lorenzo A., Dinkova-Kostova A. T. Curcumin and the cellular stress response in free radical-related diseases. Mol. Nutr. & Food Res. 2008; 52, 1062–1073.
19. Zhang H.-Y., Yang D.-P., Tang G.-Y. Multipotent antioxidants: from screening to design. Drug Discov. Today 2006; 11, 749–754.
20. Mattson M. P. Dietary factors, hormesis and health. Ageing Res. Rev. 2008; 7, 43–48.
21. Ray P. D., Huang B.-W., Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal 2012; 24, 981–990.
22. Appiah-Opong R., Commandeur J. N. M., Istyastono E., Bogaards J. J., Vermeulen N. P. Inhibition of human glutathione S-transferases by curcumin and analogues. Xenobiotica 2009; 39, 302–311.
23. Sharma R. A., McLelland H. R., Hill K. A., Ireson C. R., Euden S. A., Manson M. M., Pirmohamed M., Marnett L. J., Gescher A. J., Steward W. P. Pharmacodynamic and pharmacokinetic study of oral Curcuma extract in patients with colorectal cancer. Clin. Cancer Res. 2001; 7, 1894–1900.
24. Balogun E., Hoque M., Gong P., Killeen E., Green C. J., Foresti R., Alam J., Motterlini R. Curcumin activates the haem oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. Biochem. J. 2003; 371, 887–905.
25. Schrader C., Schiborr C., Frank J., Rimbach G. Curcumin induces paraoxonase 1 in cultured hepatocytes in vitro but not in mouse liver in vivo. Br. J. Nutr. 2010; 105, 167–170.
26. Naik S. R., Thakare V. N., Path S. R. Protective effect of curcumin on experimentally induced inflammation, hepatotoxicity and cardiotoxicity in rats: Evidence of its antioxidant property. Exp. Toxicol. Pathol. 2011; 63, 419–431.
27. Jena S., Chainy G. B. N., Dandapat J. Expression of antioxidant genes in renal cortex of PTU-induced hypothyroid rats: effect of vitamin E and curcumin. Mo. Biol. Rep. 2012; 39, 1193–1203.
28. Dickinson D. A., Moellering D. R., Iles K. E., Patel R. P., Levonen A.-L., Wigley A., Darley-Usmar V. M., Forman H. J. Cytoprotection against Oxidative Stress and the Regulation of Glutathione Synthesis. Biol. Chem. 2005; 384, 527–537.
29. Wu J., Li Q., Wang X., Yu S., Li L., Wu X., Chen Y., Zhao J., Zhao Y. Neuroprotection by Curcumin in Ischemic Brain Injury Involves the Akt/Nrf2 Pathway. PLoS One 2013; 8, e59843.
30. He L. F., Chen H. J., Qian L. H., Chen G. Y., Buzby J. S. Curcumin protects pre-oligodendrocytes from activated microglia in vitro and in vivo. Brain Res. 2010; 1339, 60–69.
31. Zhu Y. G., Chen X. C., Chen Z. Z., Zeng Y. Q., Shi G. B., Su Y. H., Peng X. Curcumin protects mitochondria from oxidative damage and attenuates apoptosis in cortical neurons. Acta Pharmacol. Sin. 2004; 25, 1606–1612.
32. Darvesh A. S., Carroll R. T., Bishayee A., Novotny N., Geldenhuys W. J., Van der Schyf C. J. Curcumin and neurodegenerative diseases: a perspective. Exp. Opin. Inv. Drug. 2012; 21, 1123–1140.
33. Balasubramanyam M., Koteswari A. A., Kumar R. S., Monickaraj S. F., Maheswari J. U., Mohan V. Curcumin-induced inhibition of cellular reactive oxygen species generation: Novel therapeutic implications. J. Biosci. 2003; 28, 715–721.
34. Bremner P., Heinrich M. Natural products as targeted modulators of the nuclear factor-kappa B pathway. J. Pharm. Pharmacol. 2002; 54, 453–472.
35. Sharma S., Kulkarni S. K., Agrewala J. N., and Chopra K. Curcumin attenuates thermal hyperalgesia in a diabetic mouse model of neuropathic pain. Eur. J. Pharmacol. 2006; 536, 256–261.
36. Zhao X., Xu Y, Zhao Q., Chen C. R., Liu A. M., Huang Z. L. Curcumin exerts antinociceptive effects in a mouse model of neuropathic pain: descending monoamine system and opioid receptors are differentially involved. Neuropharmacol. 2012; 62, 843–854.
37. Anand P., Kunnumakkara A. B., Newman R. A., Aggarwal B. B. Bioavailability of Curcumin: Problems and Promises. Mol. Pharm. 2007; 4, 807–818.
38. Wahlstrom B., Blennow G. A. study on the fate of curcumin in the rat. Acta Pharmacol. Toxicol. (Copenhagen) 1978; 43, 86–92.
39. Lao C. D., Ruffin M. T., Normolle D., Heath D. D., Murray S. I., Bailey J. M., Boggs M. E., Crowell J., Rock C. L., Brenner D. E. Dose escalation of a curcuminoid formulation. BMC Complement. Altern. Med. 2006; 6, 10. doi: 10.1186/1472-6882-6-10.
40. Cheng A. L., Hsu C. H., Lin J. K., Hsu M. M., Ho Y. F., Shen T. S., Ko J. Y., Lin J. T., Lin B. R., Ming-Shiang W., Yu H. S., Jee S. H., Chen G. S., Chen T. M., Chen C. A., Lai M. K., Pu Y. S., Pan M. H., Wang Y. J., Tsai C. C., Hsieh C. Y. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001; 21, 2895–900.
41. Shoba G., Joy D., Joseph T., Majeed M., Rajendran R., Srinivas P. S. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998; 64, 353–356.
42. Garcea G., Jones D. J., Singh R., Dennison A. R., Farmer P. B., Sharma R. A., Steward W. P., Gescher A. J., Berry D. P. Detection of curcumin and its metabolites in hepatic tissue and portal blood of patients following oral administration. Br. J. Cancer 2004; 90, 1011–1015.
43. Metzler M., Pfeiffer E., Schulz S. I., Dempe J. S. Curcumin uptake and metabolism. Biofactors 2013; 39, 14–20.
44. Wang K., Qiu F. Curcuminoid Metabolism and its Contribution to the Pharmacological Effects. Curr. Drug Metab. 2013; 14, 791–806.
45. Pari L., Tewas D., Eckel J. Role of curcumin in health and disease. Arch. Physiol. Biochem. 2008; 114, 127–149.
46. Goel A., Kunnumakkara A. B., Aggarwal B. B. Curcumin as ‘‘Curecumin’’: From kitchen to clinic. Biochem. Pharmacol. 2008; 75, 787–809.
47. Deodhar S. D., Sethi R., Srimal R. C. Preliminary study on antirheumatic activity of curcumin (diferuloyl methane). Indian J. Med. Res. 1980; 71, 632–634.
48. Satoskar R. R., Shah S. J., Shenoy S. G. Evaluation of antiinflammatory property of curcumin (diferuloyl methane) in patients with postoperative inflammation. Int. J. Clin. Pharmacol. Ther. Toxicol. 1986; 24, 651–654.
49. Lal B., Kapoor A. K., Asthana O. P., Agrawal P. K., Prasad R., Kumar P., Srimal R.C. Efficacy of curcumin in the management of chronic anterior uveitis. Phytother. Res. 1999; 13, 318–322.
50. Lal B., Kapoor A. K., Agrawal P. K., Asthana O. P., Srimal R. C. Role of curcumin in idiopathic inflammatory orbital pseudotumours. Phytother. Res. 2000; 14, 443–447.
51. Sharma R. A., Euden S. A., Platton S. L., Cooke D. N., Shafayat A., Hewitt H. R., Marczylo T. H., Morgan B., Hemingway D., Plummer S. M., Pirmohamed M., Gescher A. J., Steward W. P. Phase I clinical trial of oral curcumin: biomarkers of systemic activity and compliance. Clin. Cancer Res. 2004; 10, 6847–6854.
52. Bundy R., Walker A. F., Middleton R. W., Booth J. Turmeric extract may improve irritable bowel syndrome symptomology in otherwise healthy adults: a pilot study. J. Altern. Complement. Med. 2004; 10, 1015–1018.
53. Durgaprasad S., Pai C. G., Vasanthkumar ??, Alvres J. F., Namitha S. A pilot study of the antioxidant effect of curcumin in tropical pancreatitis. Indian J. Med. Res. 2005; 122, 315–318.
54. Holt P. R., Katz S., Kirshoff R. Curcumin therapy in inflammatory bowel disease: a pilot study. Dig. Dis. Sci. 2005; 50, 2191–2193.
55. Hanai H., Iida T., Takeuchi K., Watanabe F., Maruyama Y., Andoh A., Tsujikawa T., Fujiyama Y., Mitsuyama K., Sata M., Yamada M., Iwaoka Y., Kanke K., Hiraishi H., Hirayama K., Arai H., Yoshii S., Uchijima M., Nagata T., Koide Y. Curcumin maintenance therapy for ulcerative colitis: randomized, multicenter, double-blind, placebo-controlled trial. Clin. Gastroenterol. Hepatol. 2006; 4, 1502–1506.
56. Neves A. R., Lúcio M., Lima J. L. C., Reis S. Resveratrol in Medicinal Chemistry: A Critical Review of its Pharmacokinetics, Drug-Delivery, and Membrane Interactions. Curr. Med. Chem. 2012; 19, 1663–1681.
57. Pervaiz S. Resveratrol: from grapevines to mammalian biology. Faseb J. 2003; 17, 1975–1985.
58. Ector B. J., Magee J. B., Hegwood C. P., Coign M. J. Resveratrol concentration in muscadine berries, juice, pomace, purees, seeds, and wines. Am. J. Enol. Viticult. 1996; 47, 57–62.
59. Bavaresco L., Mattivi F., De Rosso M., Flamini R. Effects of Elicitors, Viticultural Factors, and Enological Practices on Resveratrol and Stilbenes in Grapevine and Wine. Mini-Rev. Med. Chem. 2012; 12, 1366–1381.
60. Hasan M. M., Cha M., Bajpai V. K., Baek K.-H. Production of a major stilbene phytoalexin, resveratrol in peanut (Arachis hypogaea) and peanut products: a mini review. Rev. Environ. Sci. Biotechnol. 2013; 12, 209–221.
61. Peng W, Qin R, Li X, Zhou H. Botany, phytochemistry, pharmacology, and potential application of Polygonum cuspidatum Sieb.et Zucc.: A review. J. Ethnopharmacol. 2013; 148, 729–745.
62. Harikumar K. B., Aggarwal B. B. Resveratrol – A multitargeted agent for age-associated chronic diseases. Cell Cycle 2008; 7, 1020–1035.
63. Yoo Y. J., Saliba A. J., Prenzler P. D. Should Red Wine Be Considered a Functional Food? Compr. Rev. Food Sci. F. 2010; 9, 530–551.
64. Catalgol B., Batirel S., Taga Y., Ozer N. K. Resveratrol: French paradox revisited. Front. Pharmacol. 2012; 3, 141. doi: 10.3389/fphar.2012.00141.
65. Singh N., Agrawal M., Doré S. Neuroprotective Properties and Mechanisms of Resveratrol in in Vitro and in Vivo Experimental Cerebral Stroke Models. ACS Chem. Neurosci. 2013; 4, 1151–1162.
66. Zhang F., Liu J., Shi J.-S. Anti-inflammatory activities of resveratrol in the brain: Role of resveratrol in microglial activation. Eur. J. Pharmacol. 2010; 636, 1–7.
67. Bandyopadhyay U., Das D., Banerjee R. K. Reactive oxygen species: Oxidative damage and pathogenesis. Curr. Sci. 1999; 77, 658–666.
68. King R. E., Bomser J. A., Min D. B. Bioactivity of Resveratrol. Compr. Rev. Food Sci. F. 2006; 5, 65–70.
69. Zhang F., Shi J. S., Zhou H., Wilson B., Hong J. S., Gao H. M. Resveratrol Protects Dopamine Neurons Against Lipopolysaccharide-Induced Neurotoxicity through Its Anti-Inflammatory Actions. Mol. Pharmacol. 2010; 78, 466–477.
70. Stünkel W., Campbell R. M. Sirtuin 1 (SIRT1): The Misunderstood HDAC. J. Biomol. Screen. 2011; 16, 1153–1169.
71. Donmez G. The neurobiology of sirtuins and their role in neurodegeneration. Trends Pharmacol. Sci. 2012; 33, 494–501.
72. Piotrowska H., Kucinska M., Murias M. Biological activity of piceatannol: Leaving the shadow of resveratrol. Mutat. Res. – Rev. Mutat. Res. 2011; 750, 60–82.
73. Storniolo C. E., Moreno J. J. Resveratrol metabolites have an antiproliferative effect on intestinal epithelial cancer cells. Food Chem. 2012; 134, 1385–1391.
74. Cottart C. H., Nivet-Antoine V., Laguillier-Morizot C., Beaudeux J. L. Resveratrol bioavailability and toxicity in humans. Mol. Nutr. Food Res. 2010; 54, 7–16.
75. Smoliga J. M., Baur J. A., Hausenblas H. A. Resveratrol and health – A comprehensive review of human clinical trials. Mol. Nutr. Food Res. 2011; 55, 1129–1141.
76. Kennedy D. O., Wightman E. L., Reay J, L., Lietz G., Okello E. J., Wilde A., Haskell C. F. Effects of resveratrol on cerebral blood flow variables and cognitive performance in humans: a double-blind, placebo-controlled, crossover investigation. Am. J. Clin. Nutr. 2010; 91, 1590–1597.
77. Ghanim H., Sia C. L., Korzeniewski K., Lohano T., Abuaysheh S., Marumganti A., Chaudhuri A., Dandona P. A Resveratrol and Polyphenol Preparation Suppresses Oxidative and Inflammatory Stress Response to a High-Fat, High-Carbohydrate Meal. J. Clin. Endocrinol. Metab. 2011; 96, 1409–1414.
78. Ghanim H., Sia C. L., Abuaysheh S., Korzeniewski K., Korzeniewski K., Patnaik P., Marumganti A., Chaudhuri A., Dandona P. An antiinflammatory and reactive oxygen species suppressive effects of an extract of Polygonum cuspidatum containing resveratrol. J. Clin. Endocrinol. Metab. 2010; 95, E1–E8.
79. Almeida L., Vaz-da-Silva M., Falcao A., Soares E., Soares E., Costa R., Loureiro A. I., Fernandes-Lopes C., Rocha J. F., Nunes T., Wright L., Soares-da-Silva P. Pharmacokinetic and safety profile of transresveratrol in a rising multiple-dose study in healthy volunteers. Mol. Nutr. Food Res. 2009; 53, S7–S15.
80. Boocock D. J., Faust G. E. S., Patel K. R., Schinas A. M., Brown V. A., Ducharme M. P., Booth T. D., Crowell J. A., Perloff M., Gescher A. J., Steward W. P., Brenner D. E. Phase I dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential cancer chemoprotective agent. Cancer Epidemiol. Biomarkers. Prev. 2007; 16, 1246–1252.
81. Matkowski A, Kuś P, Góralska E, Woźniak D. Mangiferin – a bioactive xanthonoid, not only from mango and not just antioxidant. Mini Rev. Med. Chem. 2013; 13, 439–455.
82. Qin J. P., Deng J. G., Feng X., Wang Q., Wang S. B. Quantitative RP-LC Analysis of Mangiferin and Homomangiferin in Mangifera indica L. Leaves and in Mangifera persiciforma CY Wu et TL Ming Leaves. Chromatographia 2008; 68, 955–960.
83. Mirza R. H., Chi N., Chi Y. Therapeutic Potential of the Natural Product Mangiferin in Metabolic Syndrome. J. Nutrit. Ther. 2013; 2, 74–79.
84. Denisova O. A., Glyzin V. I., Patudin A. V., Gavrilenko B. D. Determination of the Content of the Xanthone Glycoside Mangiferin in Iris, Gentiana and Hedysarum Plants. Khim-Farm. Z. 1980; 14, 76–77.
85. Islam M. N., Yoo H. H., Lee J., Nam J. W., Seo E. K., Jin C., Kim D. H. Simultaneous Determination of Bioactive Xanthone Glycosides and Norlignans from Ethanolic Extract of Anemarrhena asphodeloides by Liquid Chromatography. J. AOAC Int. 2008; 91, 1271–1277.
86. Vyas A., Syeda K., Ahmad A., Padhye S., Sarkar F. H. Perspectives on Medicinal Properties of Mangiferin. Mini Rev. Med. Chem 2012; 12, 412–425.
87. Masibo M., He Q. Major Mango Polyphenols and Their Potential Significance to Human Health. Compr. Rev. Food Sci. F. 2008; 7, 309–319.
88. Campos-Esparza M. R., Sanchez-Gomez M. V., Matute C. Molecular mechanisms of neuroprotection by two natural antioxidant polyphenols. Cell Calcium 2009; 49, 358–368.
89. Bhatia H. S., Candelario-Jalil E., Pinheiro de Oliveira A., Olajide O. A., Martínez-Sánchez G., Fiebich B. L. Mangiferin inhibits cyclooxygenase-2 expression and prostaglandin E2 production in activated rat microglial cells. Arch. Biochem. Biophys. 2008; 477, 253–258.
90. Lopes S. C., da Silva A. V., Arruda B. R., Morais T. C., Rios J. B., Trevisan M. T., Rao V. S., Santos F. A. Peripheral antinociceptive action of mangiferin in mouse models of experimental pain: Role of endogenous opioids, KATP-channels and adenosine. Pharmacol. Biochem. Behav. 2013; 110, 19–26.
91. Izquierdo T., Espinosa de Los Monteros-Zuñiga A., Cervantes-Durán C., Lozada M. C., Godínez-Chaparro B. Mechanisms underlying the antinociceptive effect of mangiferin in the formalin test. Eur. J. Pharmacol. 2013; 718, 393–400.
92. Yoshikawa M., Nishida N., Shimoda H., Takada M., Kawahara Y., Matsuda H. Polyphenol constituents from salacia species: quantitative analysis of mangiferin with glucosidase and aldose reductase inhibitory activities. Yakugaku Zasshi 2001; 121, 371–378.
93. Delarue J., Magnan C. Free fatty acids and insulin resistance. Curr. Opin. Clin. Nutr. Metab. Care 2007; 10, 142–148.
94. Hu H. G., Wang M. J., Zhao Q. J., Liao H. L., Cai L. Z., Song Y., Zhang J., Yu S. C., Chen W. S., Liu C. M., Wu Q. Y. Synthesis of mangiferin derivatives as protein tyrosine phosphatase 1B inhibitors. Chem. Nat. Comp. 2007; 43, 663–666.
95. Hou S., Wang F., Li Y., Li Y., Wang M., Sun D., Sun C. Pharmacokinetic study of mangiferin in human plasma after oral administration. Food Chem. 2012; 132, 289–294.
96. Hattori M., Shu Y.-Z., Tomimori T., Kobashi K., Namba T. A bacterial cleavage of the C-glucosyl bond of mangiferin and bergenin. Phytochemistry 1989; 28, 1289–1290.
97. Wang H., Ye G., Ma C.-H., Tang Y.-H., Fan M.-S., Li Z.-X., Huang C. G. Identification and determination of four metabolites of mangiferin in rat urine. J. Pharm. Biomed. Anal. 2007; 45, 793–798.
98. Pardo-Andreu G. L., Philip S. J., Riano A., Sanchez C., Viada C., Nunez-Selles A. J., Delgado R. Mangifera indica L. (Vimang) protection against serum oxidative stress in elderly humans. Arch. Med. Res. 2006; 37, 158–164.
99. Nunez-Selles A., Paez-Betancourt E., Amaro-Gonzalez D., Acosta-Esquijarosa J., Aguero-Aguero J., Capote-Hernandez R. Oficina Cubana De La Propiedad Industrial Patent 2002, Cuba patent No. 1814.
100. Menkovic N., Juranic Z., Stanojkovic T., Raonic-Stevanovic T., Savikin K., Zdunic G., Borojevic N. Radioprotective Activity of Gentiana lutea Extract and Mangiferin. Phytother. Res. 2010; 24, 1693–1696.
101. Cruz-Correa M., Shoskes D. A., Sanchez P., Zhao R., Hylind L. M., Wexner S. D., Giardiello F. M. Combination treatment with curcumin and quercetin of adenomas in familial adenomatous polyposis. Clin. Gastroenterol. Hepatol. 2006; 4, 1035–1038.
102. Verma S. P., Salamone E., Goldin B. Curcumin and genistein, plant natural products, show synergistic inhibitory effects on the growth of human breast cancer MCF-7 cells induced by estrogenic pesticides. Biochem. Biophys. Res. Commun. 1997; 233, 692–696.
103. Fang J. Y., Hung C. F., Chiu H. C., Wang J. J., Chan T. F. Efficacy and irritancy of enhancers on the in-vitro and in-vivo percutaneous absorption of curcumin. J. Pharm. Pharmacol. 2003; 55, 593–601.
104. Sun M., Su X., Ding B., He X., Liu X., Yu A., Lou H., Zhai G. Advances in nanotechnology-based delivery systems for curcumin. Nanomedicine 2012; 7, 1085–1100.
105. Bansal S. S., Goel M., Aqil F., Vadhanam M. V., Gupta R. C. Advanced Drug Delivery Systems of Curcumin for Cancer Chemoprevention. Cancer Prev. Res. 2011; 4, 1158–1171.
106. Amri A., Chaumeil J. C., Sfar S., Charrueau C. Administration of resveratrol: What formulation solutions to bioavailability limitations? J. Control. Release 2012; 158, 182–193.
107. Bhattacharya S., Sanyal A. K., Ghosal S. Monoamine Oxidase-Inhibiting Activity of Mangiferin Isolated from Canscora-Decussata. Naturwissenschaften 1972; 59, 651–651.
108. Niu Y., Lu W., Gao L., Lin H., Liu X., Li L. Reducing effect of mangiferin on serum uric acid levels in mice. Pharm. Biol. 2012; 50, 1177–1182.
109. Aftab N., Vieira A. Antioxidant Activities of Curcumin and combinations of this curcuminoid with other phytochemicals. Phytother. Res. 2010; 24, 500–502.
110. Du Q., Hu B., An H.-M., Shen K.-P., DengS., Wei M.-M. Synergistic anticancer effects of curcumin and resveratrol in Hepa1-6 hepatocellular carcinoma cells. Oncol. Rep. 2013; 29, 1851–1858.
111. Majumdar A. P., Banerjee S., Nautiyal J., Patel B. B., Patel V., Du J., Yu Y., Elliott A. A., Levi E., Sarkar F. H. Curcumin synergizes with resveratrol to inhibit colon cancer. Nutr. Cancer 2009; 61, 544–553.
112. Masuelli L., Marzocchella L., Focaccetti C., Tresoldi I., Palumbo C., Izzi V., Benvenuto M., Fantini M., Lista F., Tarantino U., Modesti A., Galvano F., Bei B. Resveratrol and diallyl disulfide enhance curcumin-induced sarcoma cell apoptosis. Front. Biosci. 2012; 17, 498–508.
113. Csaki C., Mobasheri A., Shakibaei M. Synergistic chondroprotective effects of curcumin and resveratrol in human articular chondrocytes: inhibition of IL-1β-induced NF-κB-mediated inflammation and apoptosis. Arthritis Res. Ther. 2009; 11, R165. doi: 10.1186/ar2850
Labels
Pharmacy Clinical pharmacology
Article was published inCzech and Slovak Pharmacy

2014 Issue 2-
All articles in this issue
- Possibilities of influencing the drug content and encapsulation efficiency of chitosan microspheres prepared by ionic gelation process
- Medical and entrepreneurial character of the community pharmacy
- History of the development and production of drugs in the firm Lachema in Brno
- Selected natural phenolic compounds – potential treatment for peripheral neuropathy?
- Active pharmaceutical ingredients available as substances for extemporaneous preparation in veterinary medicine in the Czech Republic
- Czech and Slovak Pharmacy
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Active pharmaceutical ingredients available as substances for extemporaneous preparation in veterinary medicine in the Czech Republic
- History of the development and production of drugs in the firm Lachema in Brno
- Possibilities of influencing the drug content and encapsulation efficiency of chitosan microspheres prepared by ionic gelation process
- Selected natural phenolic compounds – potential treatment for peripheral neuropathy?
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career


