-
Medical journals
- Career
HPLC determination of saccharides after pre-column derivatization in honey samples
Authors: Katarína Hroboňová • Jozef Lehotay • Jozef Čižmárik • Pavol Májek • Martin Tuharský
Authors‘ workplace: Institute of Analytical Chemistry, Faculty of Chemical and Food Technology Slovak University of Technology, Bratislava
Published in: Čes. slov. Farm., 2013; 62, 136-143
Category: Original Articles
Overview
The work is focused on the quality evaluation of Slovak honey samples on the basis of minor saccharide contents. The samples included unifloral and multifloral types of honeys. The saccharide contents were determined by HPLC with fluorescence detection. Derivatization of sugars with dansylhydrazine was used to increase detection sensitivity. The C18 type stationary phase was used for the separation of analytes and the mixture of acetonitrile and acetic acid was used as the mobile phase. The determined carbohydrates were saccharose, maltose, turanose, and also the main sugars glucose and fructose. Limits of detection were in the range from 15 to 100 μg.ml–1 and suggest that low quantities of all studied compounds can be detected. Differences in saccharide contents between Slovak samples and samples from different geographical regions (France, Croatia) for maltose, turanose and also for saccharose were obtained. Using multivariate statistical treatment, Slovak honeys can be distinguished from other tested honeys using the saccharide profiles.
Keywords:
saccharides • honey • derivatization • HPLCIntroduction
The saccharides have been a complicated target for analytical chemists because of difficulties in both their separation and detection. Traditional methods of saccharide analysis include chromatographic and non-chromatographic methods. Non-chromatographic methods such as enzymatic methods were found to be less suitable for rapid and routine applications. Honey is used as an ingredient in many pharmaceutical products such as cough syrups. It is also used as a palatable sweetening agent in general pharmaceuticals. The pharmacopoeias of many countries describe a honey-based preparation which can be prepared by pharmacists (honey rose water) which is used for topical application in infected throats and various ulcers of the mouth.
One of the methods for the total content saccharide determination is spectrophotometry1). The major disadvantage is the difficulty to simultaneously evaluate different saccharides. Other methods for separation and determination of monosaccharides and disaccharides include capillary electrophoresis2, 3) and chromatography. Earlier methods, such as paper chromatography and thin-layer chromatography, have been now replaced largely by gas chromatography and by high performance liquid chromatography (HPLC). In many studies, HPLC was used for its accuracy, separation ability and rapidity. The saccharides are very weak acids at high pH (12–14) and could be partially or totally ionized. They are mostly separated by ion-exchange chromatography4) or by using hydrophilic interaction chromatography (HILIC)5) on aminopropyl-modified silica columns. The liquid chromatography methods are commonly applied for the analysis of saccharides when combined with different types of detectors. The underivatized saccharides could be detected by spectrophotometric (UV, at about 195 nm), refractive index (RI)6), electrochemical mainly pulsed amperometric detection4, 7), and evaporative light-scattering detection8). The detection sensitivity of more frequently used UV and RI detection methods is similar and is not enough high for trace analysis. One way to improve spectrophotometric detection sensitivity of saccharide analysis is pre - or post-column derivatization. The reaction with the appropriate derivatization reagent changes their properties for their efficient resolution by RP HPLC. The derivatization reagents include 9-fluoromethyl hydrazine9), 2-aminobenzoic acid10), 1-(4-isopropyl)phenyl-3-methyl-5-pyrazolone11), phenylhydrazine12, 13), rodamine B14), benzamidine15), danzylhydrazine16), etc.
The purpose of this paper was to determine and characterize saccharides in several types of honey produced in Slovakia. The analysis used the reversed-phase high performance liquid chromatography method with pre-column derivatization and fluorescent detection. For the characterization of honey samples also statistical methods were applied.
Experimental part
Chemicals and samples
Acetic acid and trichloroacetic acid (for analysis grade, Microchem, Slovak Republic); acetonitrile (for liquid chromatography grade, Merck, Slovak Republic); dansylhydrazine (5-(dimethylamino)-1-naphtalenesulphonyc hydrazide, purity grade higher than 95.0%); fructose, glucose, saccharose, turanose, and maltose (purity grade higher than 99%) (Sigma-Aldrich, Slovak Republic).
The honey samples were harvested from the Slovak Republic, Croatia and France. The samples included unifloral (acacia, linden, rape, raspberry, sunflower, rosemary, lavender, salvia, fir tree and chestnut), multifloral and forest honeys. The samples were purchased from markets or obtained directly from beekeepers in Slovak Republic between the years 2005–2006 (Table 1). The samples were stored in a refrigerator in a screw-capped glass flask.
1. Table 1. Characteristics of honey 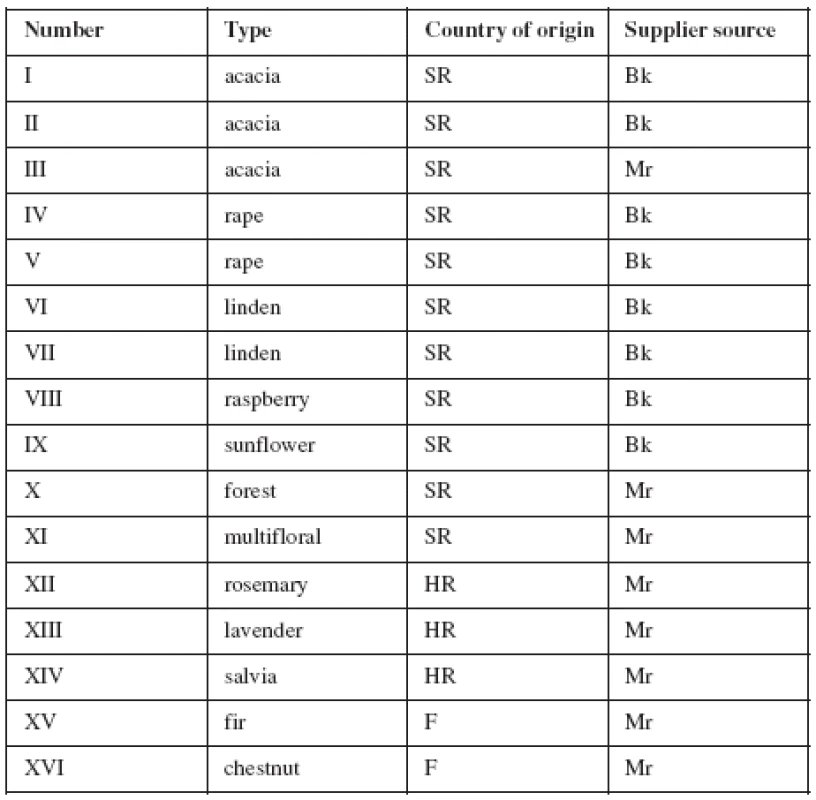
SR – Slovak Republic, HR – Croatia, F – France, Bk – Beekeepers’ honey, Mr – Market honey Solutions and sample preparation
The stock solutions of saccharose, turanose, maltose, fructose, and glucose were prepared in distilled water (3.0 mg.ml–1) and stored at –10°C. Working standard solutions were prepared by appropriate dilution of the stock solutions in distilled water. The solutions were filtered with a 0.45 μm filter.
One gram of honey sample was dissolved in 5 ml of distilled water and filtered with 0.45 μm filter.
The 100 μl of aqueous sample (or standard solution of analyte) was derivatized with dansylhydrazine16, 170) and analysed by HPLC.
HPLC analysis
Chromatographic separations were performed on an HPLC system (Agilent Technologies, Series 1100) consisting of a binary pump, a Rheodyne injection valve with a 20 μl injection loop, a thermostat, a fluorescence detector, and a refractive index detector. Saccharide dansylhydrazine derivatives were separated on a Nucleosil 120-5 C18 chromatographic column (250 × 4 mm I.D., 5 μm) (AZ Chrom, Slovak Republic) connected to a Separon SGX C18 guard column (10 × 4 mm I.D., 7 μm) (AZ Chrom, Slovak Republic). The mobile phase for separation consisted of acetonitrile and 80 mmol.ml–1 acetic acid (21/79, v/v). The flow rate was 1.0 ml.min–1 and the column temperature was kept at 23 oC. The fluorescence detector was operated at 360 nm (λex) and 470 nm (λem).
The separation of underivatized saccharides17) was performed on a Polymer IEX H form chromatographic column (250 × 8 mm I.D., 10 μm) (AZ Chrom, Slovak Republic). The mobile phase consisted of 9 mmol.ml–1 sulphuric acid. The flow rate was 0.5 ml.min–1 and the column temperature was kept at 23°C. The refractive index detector was used for detection.
Validation procedure
To assess linearity, five point calibration plots (from limit of quantification to 1 mg.ml–1) were used for each of the studied saccharides by plotting peak areas of dansylhydrazine derivatives against concentrations of saccharides. The limits of detection (LOD) and quantification (LOQ) were experimentally established as the concentration of saccharide, which produced a chromatographic peak with signal to noise ratio (S/N) higher than 3 and 10, respectively.
The accuracy of the method was determined for spiked honey sample (0.1 and 0.5 mg.ml–1). The intra-day and inter-day precision of the method was assessed as relative standard deviation (RSD, %) obtained on one day and on three different days at the saccharide concentration level of 0.1 mg.ml–1. The experiment was performed three times. The stability of the standard solutions (0.1 mg.ml–1) was also tested. They were stored in a refrigerator and analysed every day during the week.
Statistical analysis
The initial data matrix was constructed from analytical data with rows represented by honey samples (objects) and columns corresponding to chemical measurements (variables). Autoscaling was performed to produce variables with zero means and unit standard deviation. In Principal Component Analysis (PCA), varimax rotation was performed in each case to obtain maximal information from the extracted PCs. For Cluster Analysis (CA), Manhattan distances between objects and Ward’s aggregation method were used. Standard linear discriminant analysis (LDA) was used to derive a classification rule whereby the honey samples were classified according to brand. All statistical data analysis was performed using STATISTICA, version 7.1 (Statsoft Inc., OK, USA).
Results and discussion
HPLC separation and detection
Firstly, suitable chromatographic conditions – stationary phase, mobile phase and type of detection were selected. Our previous paper17) described an ion-exchange HPLC method for the separation and determination of a group of saccharides including monosaccharides and disaccharides. The advantages of this method, combined with refractive index detection, were a simple composition of the mobile phase (solution of sulphuric acid) and a shorter time of analysis (to 12 min). The only poor resolution of the studied group of saccharides (Rs from 0.3 to 1.3) was obtained. The method was suitable for the separation of a simple group of analytes.
The aim of the present work was to determine minor saccharides in honey samples by HPLC method and to study the possibilities of increasing the sensitivity of detection and resolution of analytes. The HPLC method used was based on the pre-column derivatization of the analytes with dansylhydrazine. The reason of the selection of this derivatization reagent was a rapid reaction, aqueous reaction media compatible with reversed-phase separation system and the detection sensitivity was enough high for trace analysis17). The resulting derivatives were analysed by reversed-phase liquid chromatography with fluorescence detection (λex = 360 nm, λem = 470 nm). Based on the suitable separation conditions the chromatographic characteristics, retention factors from 1.78 to 4.29 and resolution values from 0.9 to 4.3 were obtained (Table 2). The derivatization reagent (dansylhydrazine) was separated from saccharide derivatives (values of retention factors 0.22 and 10.91). The run time of analysis was 35 min. The advantage of the presented method was good suitability for analysis of saccharides, especially for aqueous samples with complex matrices.
2. Chromatographic characteristics (retention factor (k), resolution (Rs)) of dansylhydrazine derivatives of studied saccharides and validation results 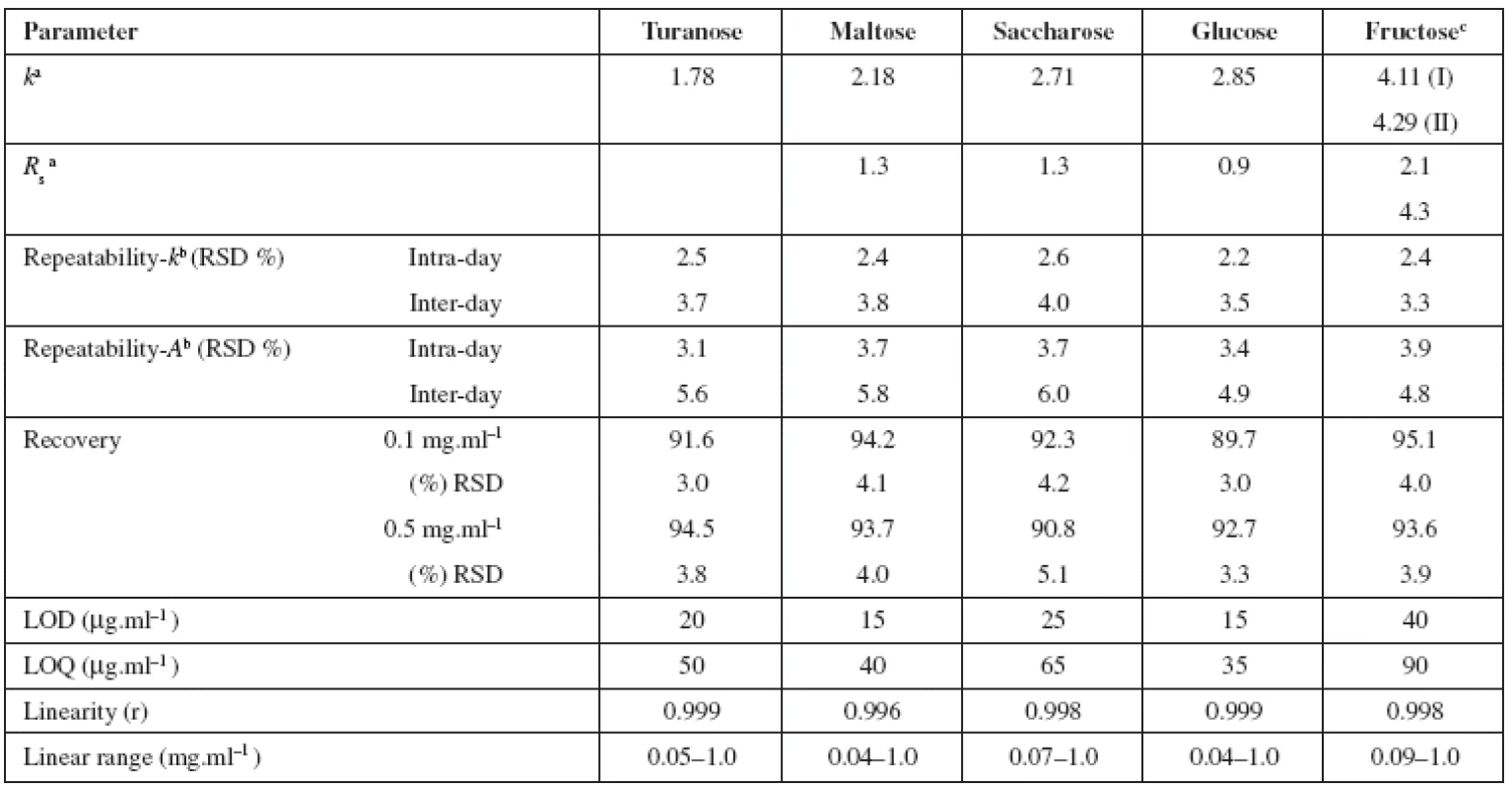
a tM = 2.67 min, n = 3 b values for concentration level 0.1 mg.ml-1 c calculated as fructose peak I The limit of detection was the decisive criterion of detector selection for HPLC method, since the assumed concentration of studied minor saccharides in honey samples was very low (<ppm). The limits of detection (LOD) and quantification (LOQ) obtained for fluorescence detection were lower than 0.04 mg.ml–1 and 0.09 mg.ml–1, respectively (Table 2). By using the method based on the precolumn derivatization with dansylhydrazine and fluorescence detection, an increase in detection sensitivity was achieved in comparison with the method without derivatization (LOD and LOQ obtained for refractive index detection were about 0.25 mg.ml–1 and 0.5 mg.ml–1, respectively18)).
Analytical characteristics
The suitability of the HPLC method for the analysis of saccharides was evaluated by validation studies including linearity, limit of detection and quantification, repeatability, accuracy and the stability of the solutions (Table 2).
At least five concentrations of the solutions were analysed in triplicate, and then the calibration curves were constructed by plotting the peak area versus the concentration of saccharide (concentration range from the limit of quantification to 1 mg.ml–1). The results indicated good correlation (r) between peak area of saccharide dansylhydrazine derivative and concentration. The resulting regression equations were used to calculate the concentration of saccharides in honey samples. The LODs and LOQs for target analytes were lower than 40 μg.ml–1 and 90 μg.ml–1, respectively. It suggests that low quantities of all compounds under study can be determined.
The method precision was obtained as RSD values for three successive determinations of target saccharides in honey (acacia, sample I) over 1 day (intra-day precision) and over three days (inter-day precision). The precisions were determined at the concentration level 0.1 mg.ml–1 of each compound under study. The intra-day and inter-day %RSD values were less than 3.9 % and 6.0%. The results showed good repeatability of retention times, which were less than 2.6% for intra-day, and 4.0% for inter-day assay. With regard to stability, the solutions of saccharides (concentration level of 0.1 mg.ml–1) were analysed after pre-column derivatization every day during 1 week and the peak areas obtained were compared. No decrease of the amount of saccharides could be observed. The accuracy of the method was determined by the recovery studies using the standard addition method (added concentration 0.1 and 0.5 mg.ml–1 of each compound). The results were in the range from 89.7% to 95.1% with RSDs less than 5.1% (Table 2) and they indicated no significant differences between the amount of saccharides added to the sample and the amount recovered.
Analysis of samples
The honey is one of the products whose production is fully natural. It is a source of saccharides, mineral compounds, proteins (including amino acids), enzymes, and vitamins. Saccharides represent the largest portion of its composition and due to this honey is very important energy food. Fructose and glucose are the most abundant monosaccharides found (26–38%), but others are usually also mentioned, namely saccharose (6–10%) and other saccharides (maltose, maltulose, isomaltose, raffinose, erlose, panose, maltotriose, trehalose, melezitose, and so on) (1–10%). Their contents depend on the botanical origin, as well as regional climatic conditions18). Honey saccharides, major monosaccharides and minor oligosaccharides, are responsible for some of its key functional properties. The separation and detection of the minor saccharides in honey by HPLC are difficult for two main reasons. The first problem is due to oligosaccharides with structural similarities (predominant disaccharides which are either glucose-glucose or glucose-fructose linked). The other problem is relating to the low concentration of the minor oligosaccharides in the honey.
The purpose of this work was to determine the saccharides in honey samples and also to evaluate the quantitative differences between the various types of honeys. Comparisons between different brands of the honey samples from Slovakia and the honey samples from different geographical and botanical regions were made on the basis of saccharose, maltose, turanose, glucose, fructose contents and fructose/glucose ratio. Seven types of Slovak honey samples (acacia, rape, linden, raspberry, sunflower, forest and multifloral), five honey samples from other localities (rosemary, lavender, salvia obtained from Croatia, fir tree and chestnut obtained from France) were analysed by a HPLC method with pre-column derivatization. For the detection of the saccharide derivatives, sensitive fluorescence detection was used. The concentrations of saccharose and glucose in honey samples were verified also by the HPLC method with a refractive index detector.
The results concerning the sugar contents of tested honey samples are compiled in Figures 1 and 2. The HPLC chromatograms (Fig. 1) of Slovak honey samples have similar profiles in contrast to the samples from other areas. Depending on the type of the honey, turanose (peaks 2) and maltose (peaks 3) were detected in the honey samples besides saccharose, glucose (peaks 4) and fructose (peaks 5 and 6). Saccharides were characterized by using the standard additional method and by comparison of retention factors and FL spectra of saccharide dansylhydrazine derivatives and sample components. Quantitative analysis was performed by the corresponding calibration curve. The content of analytes in samples is summarized in Figure 2. Differences in saccharide contents done by comparison of Slovak samples vs. other samples were obtained for maltose, turanose and also for saccharose. The total sugars content of the honey samples varied between 69.8% and 75.0% for Slovak samples and between 67.5% and 71.0% for other samples. The quantitative analysis results showed that the maltose concentration range varied from 0.8% to 5.3%, respectively. The mean maltose content for the Slovak sample group was 2.8% while for other samples group it was 5.2%. The values of maltose content in the case of the Slovak honey samples (I–XI) were moderately about twice as lower as those for other samples (XII–XVI). The saccharose content values in the honey samples I–XI varied from 1.6% to 3.9% and for the samples XII–XVI they varied from 3.6% to 6.7%. It is moderately about 30% higher. The content of turanose in Slovak honeys varied from 0.2% to 0.9% and from 0.3% to 0.6% (average content 0.5) in other samples. The total disaccharide content (saccharose, maltose and turanose) was lower in the samples obtained from the Slovak Republic (I–XI) than in the samples from Croatia (IX–XI) and France (XII, XIII). Small variations of the disaccharide composition of the honey samples from the Slovak Republic may be attributed to different floral sources. The large variation of the tested saccharide contents was related to the different geographical and the climatic conditions from which the honey samples originated. The fructose to glucose ratios of Slovak honey samples were distributed from 1.1 to 1.4 and indicated the variety of floral sources from which the honey samples originated. The fructose to glucose ratio was moderately lower in the samples from France and Croatia.
Fig. 1. HPLC chromatographic profile of dasylhydrazine derivatives of honey saccharides. Chromatographic conditions: see experimental part. 1 – dansylhydrazine, 2 – turanose, 3 – maltose, 4 – saccharose, glucose, 5 – fructose I, 6 – fructose II, 7, 8 – dansylhydrazine 
Fig. 2. Saccharide content in honey samples 
Multivariate statistical evaluation of results
The results of HPLC analysis of various kinds of honey (the selected set of 16 analysed honey samples) are processed by the application of statistical methods such as principal component analysis (PCA), cluster analysis (CA) and standard linear discrimination analysis (LDA). They provided the possibility to classify honey samples in a complex manner. The PCA is used for reduction in the number of variables while losing only a small amount of information and presentation of data in just two dimensions corresponding to individual variable and type of honey19). The PCA results are presented in Table 3. PCA showed that the first four latent variables (principal components, selected on eigenvalue one criterion) account for 96% of the variation in the saccharides data. Factor 1 (63.3% of the total variance) correlates mainly with glucose and monosaccharides. Factor 2 (13.7%) has correlation with turanose, factor 3 (11.8%) with maltose, saccharose and disaccharides, and factor 4 (7.8%) is presented by fructose. The cumulative variance suggests that the samples are well distinguished by their saccharide content.
3. Saturation of variables for four principal components as determined by principal component analysis content: Slovak honey, Croatia honey, France honey 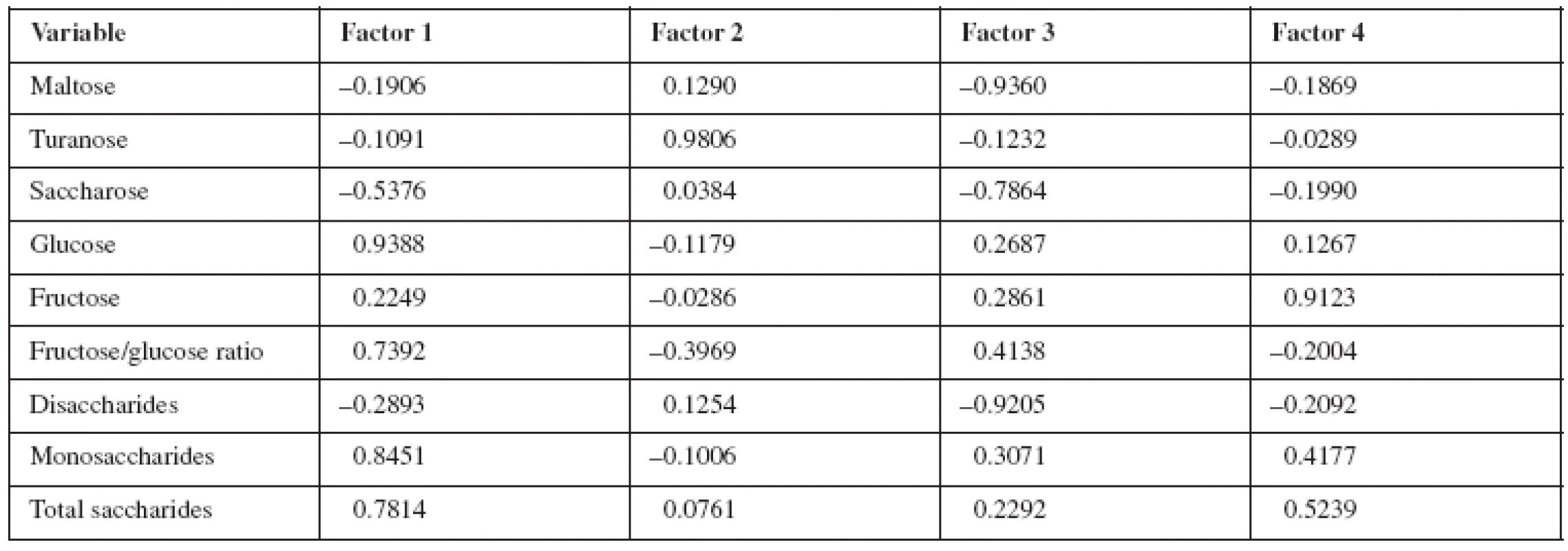
CA is a method for the study of similarity of multidimensional objects or a group of objects into clusters19). Figure 3 represents hierarchical cluster analysis of saccharides and a group of saccharides of honey samples. The dendrogram shows two predominant clusters. The first subcluster is formed mainly by monosaccharides (there is not a great difference between the levels of fructose and glucose) with the inner similarity 22% according to the honey nature and the second subcluster by disaccharides with 24% inner similarity.
Fig. 3. Dendrogram of different honey components according to cluster analysis 
Discriminant analysis is used to choose the parameters with higher discriminant power19). The LDA was performed on a set of 16 analysed honey samples. Two classification functions were able to discriminate studied honey dataset consisting of three groups of origin according to the types of saccharide correctly with 93.8%. The only one sample from France was misclassified as honey from Slovakia (Fig. 4).
Fig. 4. Linear discrimination score plot of honey samples based on saccharide content: Slovak honey, Croatia honey, France honey 
Conclusion
The purpose of the work was to test the suitability of pre-column derivatization of saccharides with dansylhydrazine and HPLC separation of derivatives for analysis of honey samples. The obtained results showed that by using the developed method, minor saccharides were determined in honey samples from different geographical regions. The method is sensitive with low LOD and LOQ values allowing the determination of saccharides in the honey, suggesting that the use of this technique could be extended to the analysis of other types of samples.
The saccharides are the main constituents of honey, and vary with its type. Reducing saccharides, mainly fructose and glucose, represent the largest portion of honey composition, but small quantities of other saccharides are also present such as maltose, turanose, and saccharose. Using multivariate statistical treatment, Slovak honeys can be separated from other tested honeys using the saccharide profiles.
This study was supported by the research project of the Scientific Grant Agency of the Ministry of Education of the Slovak Republic and Slovak Academy of Sciences (VEGA grant no. 1/0164/11).
Conflicts of interest: none.
Received 14 March 2013 / Accepted 24 April 2013
Assoc. Prof. Dr. Katarína Hroboňová, PhD. (∗) • J. Lehotay • P. Májek • M. Tuharský
Institute of Analytical Chemistry, Faculty of Chemical and Food Technology
Slovak University of Technology
Radlinského 9, 812 37 Bratislava, Slovak Republic
e-mail: katarina.hrobonova@stuba.sk
J. Čižmárik
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Comenius University
Odbojárov 10, 832 32 Bratislava, Slovak Republic
Sources
1. Pham P. J., Hernandez R., French W. T., Estill B. G., Mondala A. H. A spectrophotometric method for quantitative determination of xylose in fermentation medium. Biomass and Bioenergy 2011; 35, 2814–2821.
2. Tůma P., Málková K., Samcová E., Štulík K. Rapid monitoring of mono - and disaccharides in drinks, foodstuffs and foodstuff additives by capillary electrophoresis with contactless conductivity detection. Anal. Chim. Acta 2011; 698, 1–5.
3. El Rassi Z., Nashabeh W. Carbohydrate analysis: High performance liquid chromatography and capillary electrophoresis. Amsterdam: Elsevier 1995; 267–360.
4. Caseiro A., Marr I. L., Claeys M., Kasper-Giebl A., Puxbaum H., Pio, C. A. Determination of saccharides in atmospheric aerosol using anion-exchange high-performance liquid chromatography and pulsed-amperometric detection. J. Chromatogr. A 2007; 1171, 37–45.
5. Hemström P., Irgum K. Hydrophilic interaction chromatography. J. Sep. Sci. 2006; 29, 1784–1821.
6. Molnar-Perl I. Role of chromatography in the analysis of sugars, carboxylic acids and amino acids in food. J. Chromatogr. 2000; 91, 1–32.
7. Liang L., Zhang P., Cai Y., Mou, S. High-performance anion exchange chromatography with pulsed amperometric detection for simultaneous determination of monosaccharides and uronic acids. Chinese J. Anal. Chem. 2006; 34, 1371–1374.
8. Takeuchi T., Aspanut Z., Yamada T., Inui A., Lim L. W. Light-scattering detection with a fluorimetric detector in high-performance liquid chromatography. J. Chromatogr. A 2007; 1147, 42–45.
9. Rooyakkers D. R., Van Eijk H. M. H., Deutz N. E. P. Simple and sensitive multi sugar probe gut permeability test using HPLC with fluorescence labelling. J. Chromatogr. A 1996; 730, 99–105.
10. He L., Sato K., Abo M., Okubo A., Yamazaki S. Separation of saccharides derivatized with 2-aminobenzoic acid by capillary electrophoresis and their structural consideration by nuclear magnetic resonance. Anal. Biochem. 2003; 314, 128–134.
11. Zhang P., Wang Z., Xie M., Nie W., Huang L. Detection of carbohydrates using a pre-column derivatization reagent 1-(4-isopropyl) phenyl-3-methyl-5-pyrazolone by high-performance liquid chromatography coupled with electrospray ionization mass spectrometry. J. Chromatogr. B 2010; 878, 1135–1144.
12. Yamamoto K., Hamase K., Zaitsu K. 2-Amino-3-phenylpyrazine, a sensitive fluorescence prelabeling reagent for the chromatographic or electrophoretic determination of saccharides. J. Chromatogr. A 2003; 1004, 99–106.
13. Lattova E., Perreault H. Labelling saccharides with phenylhydrazine for electrospray and matrix-assisted laser desorption-ionization mass spectrometry. J. Chromatogr. B 2003; 793, 167–179.
14. Todoroki K., Hayama T., Ijiri S., Kazuta A., Yoshida H., Nohta H., Yamaguchi M. Rhodamine B amine as a highly sensitive fluorescence derivatization reagent for saccharides in reversed-phase liquid chromatography. J. Chromatogr. A 2004; 1038, 113–120.
15. Kakita H., Kamishima H., Komiya K., Kato Y. Simultaneous analysis of monosaccharides and oligosaccharides by high-performance liquid chromatography with post-column fluorescence derivatization. J. Chromatogr. A 2002; 961, 77–82.
16. Mopper K., Jonhnson L. Reversed-phase liquid chromatographic analysis of Dns-sugars. Optimization of derivatization and chromatographic procedures and applications to natural samples. J. Chromatogr. 1983; 256, 27–38.
17. Hroboňová K., Lehotay J., Jablonský M., Katuščák S. HPLC determination of saccharides in the process of accelerated ageing of paper. Chemické listy 2009; 103, 744–751.
18. White J. W. Honey. Advances in food research 1978; 24, 287–374.
19. Otto M. Chemometrics, 2nd Edition. New York: Wiley-VCH 2007.
Labels
Pharmacy Clinical pharmacology
Article was published inCzech and Slovak Pharmacy

2013 Issue 3-
All articles in this issue
- Warfarin – its synthesis and properties in a twenty-year retrospective*
- Use of human medicinal preparations in veterinary medicine
- Influence of formulation and process parameters on the characteristics of PLGA-based microparticles with controlled drug release
- A study of a new co-processed dry binder based on spray-dried lactose and microcrystalline cellulose
-
Study of local anaesthetics: Part 201*
Determination of the critical micellar concentration of pentacaine hydrochloride from the measurements of UV absorption of pyrene in methanol solutions - HPLC determination of saccharides after pre-column derivatization in honey samples
- Analysis of the consumption of sedative and hypnotic drugs in Yemen and the Czech Republic
- Czech and Slovak Pharmacy
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Warfarin – its synthesis and properties in a twenty-year retrospective*
- Use of human medicinal preparations in veterinary medicine
- Influence of formulation and process parameters on the characteristics of PLGA-based microparticles with controlled drug release
- HPLC determination of saccharides after pre-column derivatization in honey samples
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

