-
Medical journals
- Career
A study of a new co-processed dry binder based on spray-dried lactose and microcrystalline cellulose
Authors: Jitka Mužíková • Pavla Šináglová
Authors‘ workplace: Department of Pharmaceutical Technology, Faculty of Pharmacy Charles University in Prague, Hradec Králové
Published in: Čes. slov. Farm., 2013; 62, 127-131
Category: Original Articles
Overview
The paper studies the compressibility and disintegration time of tablets from the co-processed dry binder DisintequikTM MCC in combination with two lubricants at two concentrations in dependence on compression force. It also compares identical parameters in the physical mixtures of the spray-dried lactose Flowlac® 100 and the microcrystalline cellulose Microcel® MC-102 in the ratios of 9 : 1, 8 : 2 and 7 : 3, again in combination with two lubricants of two concentrations at one compression force. The lubricants employed are magnesium stearate and poloxamer 407 in concentrations of 1% and 2%. Compressibility is evaluated by means of energy balance of compression and tensile strength of tablets. DisintequikTM MCC shows higher values of total energy of compression due to higher values of the energy accumulated by the tablet, higher plasticity, higher strength and a longer disintegration time of tablets than the physical mixture of spray-dried lactose and microcrystalline cellulose of a corresponding content.
Keywords:
DisintequikTM MCC • magnesium stearate • poloxamer 407 • energy profile of compression • tensile strength of tablets • disintegration time of tabletsIntroduction
Co-processed dry binders are perspective auxiliary substances for direct compression of tablets. Their advantage is primarily their multifunctionality, which decreases the number of excipients in tableting, thus removing several steps of mixing and accelerating the process of production1). Co-processed dry binders, often prepared by spray-drying, usually exert better properties for tableting than the physical mixtures of their ingredients. In their preparation physical changes take place without their chemical change1, 2). In co-processed products for direct compression we can find, for example, two dry binders (MicroceLac® 100 – 75% αlactose monohydrate and 25% microcrystalline cellulose)3), a dry binder and a glidant (Prosolv® SMCC – 98% microcrystalline cellulose and 2% colloidal silicon dioxide)4), more recently a dry binder and a lubricant (LubriToseTM SD – 96% spray-dried lactose and 4% glyceryl monostearate)5) or even a larger number of auxiliary substances (Prosolv® EasyTab – 95–98% microcrystalline cellulose, 1.5–2.5% colloidal silicon dioxide, 0.5–2% sodium starch glycolate and 0.3–1% sodium stearylfumarate)6). This paper aimed to study the compressibility and disintegration time of tablets from the novel co-processed dry binder DisintequickTM MCC, which contains 90% of spray-dried lactose and 10% microcrystalline cellulose7), and this combination of excipients should positively influence the strength and disintegration time of tablets.
Experimental part
Materials
DisintequikTM MCC – co-processed dry binder of spray-dried lactose (90%) and microcrystalline cellulose (10%) (Kerry, USA);
Flowlac® 100 – spray-dried lactose (Meggle-Pharma, Germany);
Microcel® MC-102 – microcrystalline cellulose (Blanver, Brazil);
L∝trol® micro 127 – poloxamer 407 (BASF, Germany);
Magnesium stearate (Acros Organics, USA);
Preparation of tableting compositions
Altogether 16 tableting materials of the following compositions were prepared:
- DisintequikTM MCC with 1 and 2% magnesium stearate and poloxamer 407,
- Flowlac® 100 + Microcel® MC-102 9 : 1 with 1 and 2% magnesium stearate and poloxamer 407,
- Flowlac® 100 + Microcel® MC-102 8 : 2 with 1 and 2% magnesium stearate and poloxamer 407,
- Flowlac® 100 + Microcel® MC-102 7 : 3 with 1 and 2% magnesium stearate and poloxamer 407.
The mixtures were prepared by mixing in a mixing cube KB 15S (Erweka GmbH, Hausenstamm, Germany), rotated at a speed of 17 revolutions per minute. DisintequikTM MCC with lubricants was mixed for 5 minutes. Dry binders were mixed also for 5 minutes and subsequently a lubricant was added for another 5 minutes. The weight of the tableting materials under preparation was 30 g.
Preparation of tablets and energy evaluation of compacting process
Tablets were compacted on a material testing equipment T1-FRO 50 TH.A1K Zwick/Roell (Zwick GmbH & Co, Germany) by means of a special die with a lower and an upper punch. The rate of compaction was 40 mm/min, pre-load was 2 N, and the rate of pre-load 2 mm/s. Compression forces for DisintequikTM MCC with lubricants were 10, 12 and 14 kN, for the mixtures of dry binders with lubricants, 14 kN. Each compression force was employed to manufacture 16 tablets. The tablets were of cylindrical shape without facets, diameter of 13 mm, weight 0.5 ± 0.0010 g. In the first 10 tablets of each group, the computer programme testXpert V 9.01 recorded always the compression process by means of the “force-displacement” record and numerically evaluated the energy balance of compression, i.e., the energy consumed for friction E1, energy accumulated by the tablet after compression E2 and the energy released during decompression E3, total energy Emax, which is the sum total of all energies, and plasticity8).
Measurement of tensile strength of tablets
Tensile strength of tablets was always measured in 10 tablets no sooner than 24 hours after compression. Measurements were performed using a Schleuniger apparatus, which measures the diameter and height of tablets with a precision of 0.01 mm and destruction force in N. Tensile strength of tablets was subsequently calculated according to the equation [1]9):
P = 2 . F / (π . d . h), [1]
where P is tensile strength of tablets in MPa, F is destruction force in N, d is the diameter of tablets in mm, h is the height of tablets in mm.
Measurement of the disintegration time of tablets
Disintegration time was always measured in 6 tablets at each compression force at least 24 hours after compaction. The measurements were made on a device for testing the disintegration time of tablets Erweka ZT 301 (Erweka GmbH, Hausenstamm, Germany) using the method described in the Czech Pharmacopoeia 2009 – Supplement 201010). The test was carried out without discs in the medium of purified water tempered for 37 ± 1 °C. The tablets were considered disintegrated at the moment when on the net of the tube there was no remainder.
Statistical processing of results
Experimentally obtained values of tensile strength of tablets and disintegration time were statistically processed using the computer programmes Excel and Qcexpert. The values of energies and plasticity were statistically processed by the computer programme testXpert V 9.01 directly during processing. The average values with standard deviations of strength and disintegration time of tablets were processed graphically; the average values with the standard deviations of energies and plasticity were tabulated. In the case of unclear differences in the values the unpaired t-test at the level of significance of 0.05 was employed.
Results and discussion
This paper aimed to study the compressibility and disintegration time of tablets from the co-processed dry binder DisintequikTM MCC in combination with two lubricants in two concentrations in dependence on compression force. Another aim was to compare the identical parameters in physical mixtures of spray-dried lactose Flowlac® 100 and microcrystalline cellulose Microcel® MC-102 in the ratios of 1 : 9, 2 : 8 and 3 : 7, again in combination with two lubricants in two concentrations at one compression force. The lubricants employed were magnesium stearate and poloxamer 407 in concentrations of 1% and 2%. Compressibility was evaluated by means of energy balance of compression and tensile strength of tablets. The employed compression forces for DisintequikTM MCC with lubricants were selected in such a way as to have at least one of them usable for the comparison of the substance with the physical mixtures of dry binders, where it was assumed that tensile strength of tablets would be markedly lower. Thus the selected compression forces were 10, 12 and 14 kN, the compression force of 14 kN being used for the comparison of the co-processed dry binder with physical mixtures of spray-dried lactose and microcrystalline cellulose, even though the strength of tablets from the mixtures of DisintequikTM MCC with lubricants was above the limit of the optimal strength (0.56–1.12 MPa)11).
The results of the study are summarized in two Tables and four Figures. Table 1 shows the values of the energy balance of compression and plasticity for DisintequikTM MCC with lubricants. It is evident for the results that total energy of compression Emax increases with the compression force and is lower in the case of mixtures with magnesium stearate. This result is due mainly to lower values of energy for friction E1, as within the range of the lubricants employed there are no marked differences between the values of energy accumulated by the tablet after compression E2 and the values of energy of decompression E3. Within the employed concentration of both lubricants, no statistically significant difference was found in the values of total energy, energy for friction, and energy of decompression. Only in the energy accumulated by the tablet, there are slightly lower values for 2% of both lubricants at the compression force of 10 kN and for poloxamer 407 at the compression forces of 12 and 14 kN. Plasticity decreases with compression force, which is logical due to decreasing porosity of tablets12), and it decreases with increased concentration of lubricants. The type of the lubricant used does not influence plasticity. Table 2 lists the energies of compression and plasticity for physical mixtures of Flowlac® 100 with Microcel® MC-102. Dry binders were mixed in three ratios, the ratio of 9 : 1 corresponding as to the content to the substance DisintequikTM MCC. If the values of the energies of these two tableting materials are compared first, then the values of total energy Emax are higher for the substance DisintequikTM MCC, which is due to the higher values of energy E2, i.e. the energy accumulated by the tablet. Energy for friction does not exert greater differences in the values, and the energy of decompression is even in the corresponding physical mixture higher than in DisintequikTM MCC. In these two tableting materials there are lower values for Emax for the lubricant magnesium stearate, this time due to lower values of energy for friction, and independence of the employed concentration for both lubricants is observed here. In the case of the other physical mixtures of spry-dried lactose and microcrystalline cellulose, the Emax values are slightly increased with an increasing addition of microcrystalline cellulose due to increasing energy for friction and energy accumulated by the tablet after compaction. Values of Emax are lower for magnesium stearate due to decreased energy for friction and lower values are exerted by its higher concentration; in the case of poloxamer 407 there is no statistically significant difference in the range of the concentrations employed. The values of energy of decompression are, on the other hand, higher in the mixtures with magnesium stearate, because it decreases friction more and thus causes also higher back relaxation of tablets. DisntequikTM MCC exerts higher values of plasticity than the corresponding physical mixture of the individual components, which is due to the technological method of its preparation, which is probably spray-drying. Plasticity of this substance is not markedly influenced by the type or concentration of the lubricant. In the case of physical mixtures of dry binders, plasticity is increased with an increasing share of microcrystalline cellulose, which is plastically deformable. Lower values of plasticity are found in the mixtures with magnesium stearate, the effect of a higher concentration on the decrease of plasticity decreases with a higher representation of microcrystalline cellulose. When poloxamer 407 is used, there is no statistically significant difference between the values of plasticity in the range of the concentration employed.
1. Values of energies of compression and plasticity – Disintequik<sup>TM</sup> MCC with lubricants 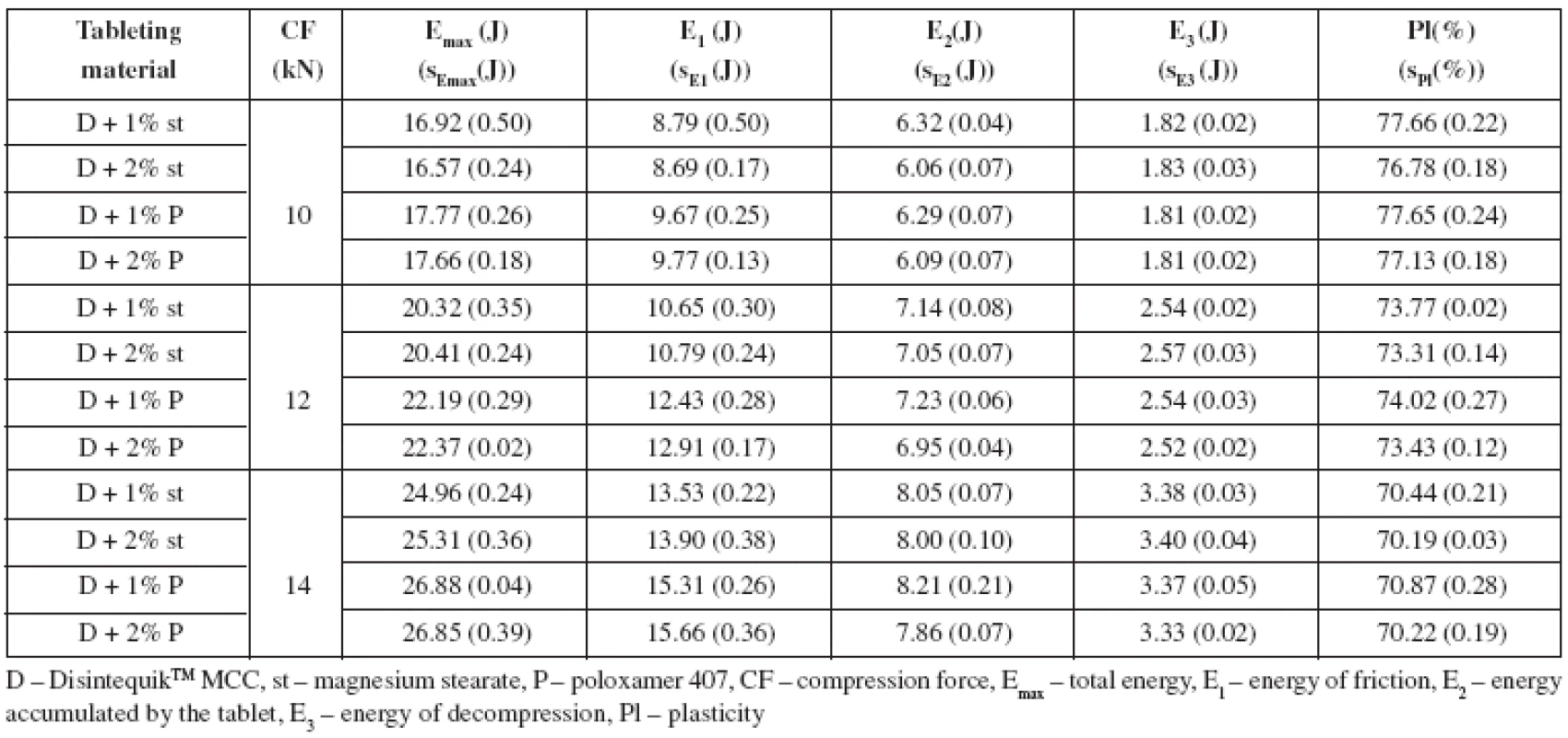
2. Values of energies of compression and plasticity at the compression force of 14 kN: Mixtures of Flowlac<sup>®</sup> 100 and Microcel<sup>®</sup> MC-102 with lubricants 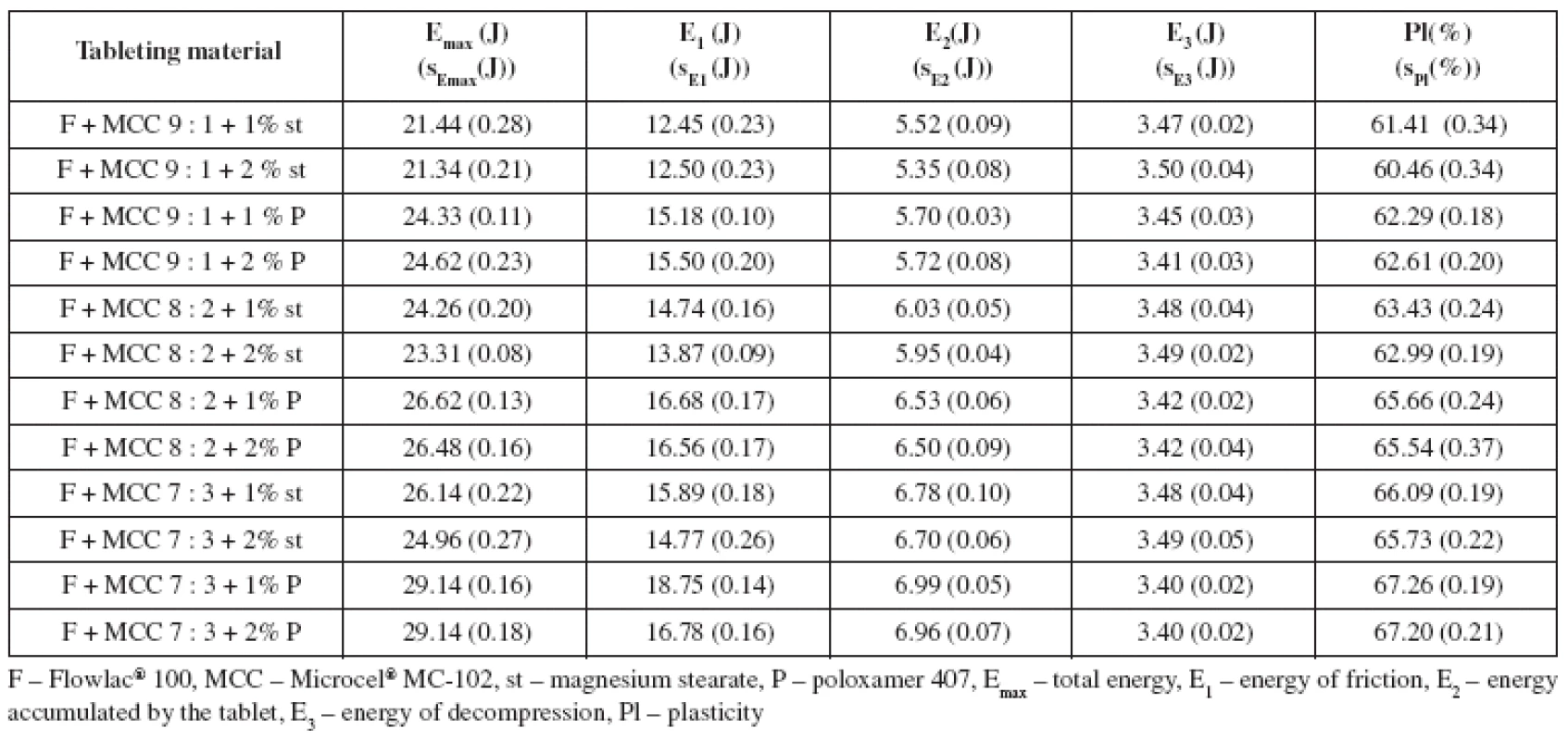
Figures 1–4 summarize the results of the strength and disintegration time of tablets. Figure 1 represents the dependence of tensile strength of tablets on compression force for DisintequikTM MCC with lubricants. The strength of tablets increases with compression force and depends on the employed concentration of lubricants. Increased concentration of magnesium stearate decreases the strength of tablets due to the presence of plastically deformable microcrystalline celulose13), whereas in poloxamer 407 there an increase in strength takes place apparently due to its positive binding properties. The strength of tablets is represented also in Figure 2, this time it is a comparison of the values of strength of DisitequikTM MCC and physical mixtures of spray-dried lactose and microcrystalline cellulose. A physical mixture of dry binders in a corresponding ratio as in DisintequikTM MCC provides tablets with a markedly lower strength. With increasing representation of microcrystalline cellulose in the mixtures, the strength of tablets is increased, being higher with the use of poloxamer 407 as the lubricant. An increase in the concentration of magnesium stearate does not change the strength in the mixture of a corresponding content, in the other mixtures it is decreased as the result of a higher representation of microcrystalline celulose13). On the other hand, an increase in the concentration of poloxamer 407 produces an increase in the strength, excepting the mixture with the highest representation of microcrystalline cellulose. Figure 3 describes dependence of the disintegration time of tablets for DisintequikTM MCC with lubricants. Disintegration time increases with compression force and increased concentrations of both lubricants. It is understandable for magnesium stearate due to it hydrophobicity, poloxamer 407 is water-soluble, but as a poloxamer with a higher molecular weight it possesses a tendency to become a gel and the tablet then disintegrates more slowly14). The effect of 1% concentration of lubricants depends on compression force, as at 10 kN tablets with 1% poloxamer 407 disintegrate more quickly, at 12 kN the values for both lubricants are balanced, and at 14 kN tablets with 1% magnesium stearate disintegrate more quickly. Figure 4 shows a comparison of disintegration times of tablets for DisintequikTM MCC and physical mixtures of dry binders at the compression force of 14 kN. The corresponding mixture of dry binders as well as other mixtures show multiple times shorter disintegration times of tablets than DisintequikTM MCC, and the effect of the type and employed concentration of the lubricant is not significant in them, excepting the mixtures of 9 : 1 and 8 : 2, where there is a slightly higher value for 2% of magnesium stearate.
Fig. 1. Tensile strength of tablets in function of compression force – Disintequik<sup>TM</sup> MCC with lubricants st – magnesium stearate, P – poloxamer 407 
Fig. 2. Tensile strength of tablets at the compression force of 14 kN D – Disintequik<sup>TM</sup> MCC, F – Flowlac<sup>®</sup> 100, MCC – Microcel<sup>®</sup> MC-102, st – magnesium stearate, P – poloxamer 407 
Fig. 3. Disintegration time of tablets in function of compression force – Disintequik<sup>TM</sup> MCC with lubricants st – magnesium stearate, P – poloxamer 407 
Fig. 4. Disintegration time of tablets at the compression force of 14 kN D – Disintequik<sup>TM</sup> MCC, F – Flowlac<sup>®</sup> 100, MCC – Microcel<sup>®</sup> MC-102, st – magnesium stearate, P – poloxamer 407 
Acknowledgment
The study was supported by the firm Kerry Bio-Science, Meggle-Pharma and Blanver, which supplied the samples of the dry binders tested.
Conflicts of interest: none.
Received 13 March 2013 / Accepted 26 March 2013
PharmDr. Jitka Mužíková, Ph.D. (∗) • P. Šináglová
Department of Pharmaceutical Technology, Faculty of Pharmacy
Charles University in Prague
Heyrovského 1203, 500 05 Hradec Králové
e-mail: muzikova@faf.cuni.cz
Sources
1. Nachaegari S. K., Bansal A. K. Coprocessed excipients for solid dosage forms. Pharm Technol 2004; 28, 52–64.
2. Saha S., Shahiwala A. F. Multifunctional coprocessed excipients for improved tableting performance. Expert Opin Drug Deliv. 2009; 6, 197–208.
3. Bolhuis G. K., Armstrong N. A. Excipients for direct compaction – an update. Pharm Dev Technol. 2006; 11, 111–124.
4. Gohel M. C., Jogani P. D. A review of co-processed directly compressible excipients. J Pharm Pharmaceut Sci 2005; 8, 76–93.
5. Kerry: Self lubricating excipients. Firm. lit. 2012 http://www.sheffieldbioscience.com/LubriTose/
6. JRS Pharma: Prosolv® Easytab. Firm. Lit. 2010 http://www. jrspharma.de/Pharma/wEnglisch/produktinfo/productinfo_prosolv_easytab.shtml
7. Kerry: Disintequik MCC. Firm. Lit. 2012 http://www. sheffieldbioscience.com/disintequik_mcc/
8. Ragnarsson G. Force-displacement and network measurements. In: Alderborn G., Nystrőm Ch. eds. Pharmaceutical Powder Compaction Technology New York: Marcel Dekker, Inc. 1996; 77–97.
9. Fell J. T., Newton, J. M. Determination of tablet strength by diametral-compression test. J. Pharm. Sci. 1970; 59, 688–691.
10. Czech Pharmacopoeia 2009 – Supplement 2010. Praha: Grada Publishing 2010; 4082.
11. Belousov V. A. K voprosu o vybore optimalnikh davlenii pressovania pri tabletirovanii lekarstvennykh poroshkov. Khim. Farm. Zh. 1976; 10, 105–111.
12. Picker-Freyer K. M. Tablet production systems. In: Gad S. C. (ed.) Pharmaceutical Manufacturing handbook production and processes. New Jersey: John Wiley and Sons, Inc. 2008; 1053–1098.
13. Bolhuis G. K., Hőlzer A. W. Lubrication Issues in direct compaction. In: Alderborn G., Nyström Ch. (eds.) Pharmaceutical powder compaction technology. London: Informa Healthcare, 2011; 205–234.
14. Dumortier G., Grossiord J. L., Agnely F., Chaumeil J. C. A review of poloxamer 407. Pharmaceutical and Pharmacological Characteristics. Pharm. Research 2006; 23, 2709–2728.
Labels
Pharmacy Clinical pharmacology
Article was published inCzech and Slovak Pharmacy

2013 Issue 3-
All articles in this issue
- Warfarin – its synthesis and properties in a twenty-year retrospective*
- Use of human medicinal preparations in veterinary medicine
- Influence of formulation and process parameters on the characteristics of PLGA-based microparticles with controlled drug release
- A study of a new co-processed dry binder based on spray-dried lactose and microcrystalline cellulose
-
Study of local anaesthetics: Part 201*
Determination of the critical micellar concentration of pentacaine hydrochloride from the measurements of UV absorption of pyrene in methanol solutions - HPLC determination of saccharides after pre-column derivatization in honey samples
- Analysis of the consumption of sedative and hypnotic drugs in Yemen and the Czech Republic
- Czech and Slovak Pharmacy
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Warfarin – its synthesis and properties in a twenty-year retrospective*
- Use of human medicinal preparations in veterinary medicine
- Influence of formulation and process parameters on the characteristics of PLGA-based microparticles with controlled drug release
- HPLC determination of saccharides after pre-column derivatization in honey samples
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

